Vaccine-induced Immune Responses Against SARS-CoV-2 Infections
Guest Editor
Dr. Wangxue Chen E-Mail
PhD, Principal Research Officer, National Research Council Canada, Human Health Therapeutics Research Center, Ottawa, Canada
Research Keywords: Mucosal immunology, vaccination
About the Special lssue
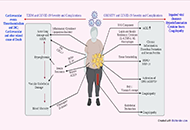
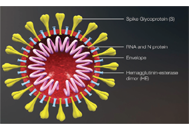
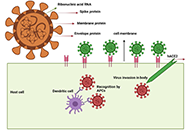
The pandemic of COVID-19, caused by coronavirus SARS-CoV-2, has created an unprecedented threat to global public health, security, and prosperity. Vaccination is highly cost-effective to prevent viral infection, stop its transmission, and develop herd immunity. With the strong global and state political leadership, active contribution of vaccine industries and research community, and significant financial investment, several COVID-19 vaccines have already been approved under special regulatory authorities and many more are in clinical trials or advanced preclinical development.
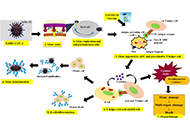
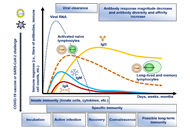
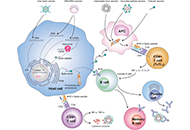
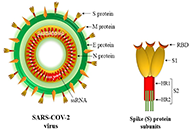
Development of safe and effective COVID-19 vaccines requires a better understanding of the vaccine-induced immune responses and the mechanisms of immune protection. Almost all SARS-CoV-2 vaccine candidates currently in late stages of development are targeted at the induction of neutralizing antibodies against the spike (S) protein or its receptor binding domain (RBD) of the virus. However, the recent emerging and rapid global spread of several SARS-CoV-2 variants have possessed new challenges to the efficacy of current vaccine candidates and to the development of future COVID-19 vaccines. Increasing evidence suggests the importance of vaccines to target other viral antigens and induce specific T cell responses. In addition, identification of immune correlates of protection will be critical for bridging the regulatory approval of next wave of vaccines against the current and future SARC-CoV-2 strains.
The aim of this Special Issue is to provide insight into our current knowledge and understanding of the immune responses induced by different COVID-19 vaccine candidates and their application in the development of new vaccines with long-lasting and broad protection against multiple SARS-CoV-2 strains and variants. More specifically, we wish to invite Original Research articles, Reviews, and Commentaries that address the following topics:
Humoral and cell mediated immune responses and innate memory responses;
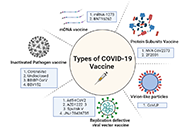
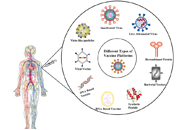
Immune responses induced by different vaccine platforms;
Mucosal vaccination and mucosa immune responses;
Immune correlates of protection;
Immune responses to non-spike proteins and emerging variants;
Systems immunology and vaccinology;
Novel in vitro and in vivo assays and models for assessing vaccine-induced immunity and predicting vaccine efficacies.
Keywords: SARS-CoV-2, vaccine, immune response
Published Articles