Affiliation:
1Molecular Pharma Pvt. Ltd. 102A Windsor Palace, 6A, Iron Side Road, Kolkata 700019, West Bengal, India
Email: swapan1chatterjee@gmail.com
ORCID: https://orcid.org/0000-0002-5582-3069
Affiliation:
1Molecular Pharma Pvt. Ltd. 102A Windsor Palace, 6A, Iron Side Road, Kolkata 700019, West Bengal, India
Affiliation:
2Cagayan State University, Tuguegarao City & De La Salle University, Manila 0900, Philippines
ORCID: https://orcid.org/0000-0001-7939-9664
Explor Immunol. 2021;1:374–397 DOI: https://doi.org/10.37349/ei.2021.00025
Received: June 15, 2021 Accepted: November 01, 2021 Published: December 31, 2021
Academic Editor: Lorenzo Cosmi, University of Florence, Italy; Wangxue Chen, National Research Council Canada, Canada
The article belongs to the special issue Vaccine-induced Immune Responses Against SARS-CoV-2 Infections
Coronavirus disease 2019 (COVID-19) emerges as an expeditiously growing pandemic, in the human population caused by the highly transmissible RNA virus severe acute respiratory syndrome of coronavirus 2 (SARS-CoV-2). Prognosis of SARS-CoV-2 infection predominantly occurs at the angiotensin-converting enzyme 2 receptor and transmembrane protease serine type 2 positive (ACE2 + TMPRSS2)+ epithelial cells of the mucosal surface like nasal, oral mucosae, and/or the conjunctival surface of the eye where it has interacted along with the immune system. The primary host response towards the pathogen starts from an immune microenvironment of nasopharynx-associated lymphoid tissue (NALT) and mucosa-associated lymphoid tissue (MALT). The presence of exhausted lymphocytes, lymphopenia, pneumonia and cytokine storm is the hallmark of COVID-19. The multifaceted nature of co-morbidity factors like obesity and type 2 diabetes and its effects on immunity can alter the pathogenesis of SARS-CoV-2 infection. Adipose tissue is a crucial endocrine organ that secretes a plethora of factors like adipokines, cytokines, and chemokines that have a profound impact on metabolism and augments the expression of mucosal pro-inflammatory cytokines, like tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), and the interleukin-12 (IL-12)/IL-23. Mucosal immunization could be a superior approach to activate mucosal and systemic immune responses against pathogenic invasion at mucosal surface entry ports. Mucosal vaccines are also able to generate strong systemic humoral immunity—required to neutralize any virus particle that dodges the primary immune response. To develop an efficient vaccine against mucosal pathogens, considering the designing of the delivery route, immunomodulatory features, and adjuvants are very important. In this article, we further provide evidence to understand the significant role of mucosal immunity, along with secretory and circulating immunoglobulin A (IgA) antibodies in generating a novel mucosal vaccine against COVID-19. Moreover, along with mucosal vaccines, we should look for combination treatment strategies with plant bioactive molecules. Glycan-binding lectins against viral proteins for targeted activation of mucosal immune response are one of such examples. This might play a promising role to halt this emerging virus.
Coronavirus disease 2019 (COVID-19) is a looming, expeditiously growing pandemic, carried out by the enveloped positive-sense single-stranded RNA viruses severe acute respiratory syndrome of coronavirus 2 (SARS-CoV-2; βCoVs). It has created an associate degree of unprecedented threat to public health, and prosperity globally since December 2019. As over 128 million COVID-19 cases and 2.82 million deaths till June, 2021 have been reported globally with re-imposed lockdowns and/or curfews, indicates the onset of the second wave and also a continued threat to society [1]. As this virus infects different mammals, humans, and avian species, together with companion animals and livestock, are not solely a public health but also a veterinary as well as an economic concern. Coronaviruses, like human coronavirus OC43 (HCoV-OC43) and HCoV-229E, along with recently discovered HCoV-HKU1 and HCoV-NL63 have been circulated in the human population for a considerable time and are known to cause mild respiratory tract infections associated with the seasonal “common cold” symptoms. Contrary to this, Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV, and SARS-CoV-2, are highly pathogenic [2]. Infectious source, route of transmission, and a susceptible population—are three vital steps for contagious disease development. Due to the fabrication of higher quantities of virus in the upper respiratory tract (URT) amidst the pre-symptomatic period, persons with SARS-CoV-2-infection act as the major source of virus transmission. As the viral load detected in asymptomatic individuals and symptomatic patients is similar, indicating that asymptomatic infections have also the potentiality for transmission [3]. Transmission of SARS-CoV-2 infections are caused by person-to-person close contact, aerosol droplets, and potentially fecal-oral transmission. Although the predominance and transmissibility of SARS-CoV-2 are much greater than MERS and SARS, the severity extent and mortality rates among patients with SARS-CoV-2 infections are comparatively lesser than those with MERS and SARS [4].
In humans, by infecting the mucosal surface, mainly the lungs (pneumocytes, URT cells, and bronchial epithelial cell) or the intestine, SARS-CoV-2 infections can develop into severe lung injuries and life-threatening respiratory pathologies. This pathogenesis is driven by an exaggerated immune response for which no specific therapeutic, as well as a prophylactic treatment, has been approved to date. So far, the immunological analysis of this disease has focused mainly on the systemic immune response found in the blood, but gaining insights into the local mucosal response will help to understand viral pathogenesis along with vaccine efficacy. As the COVID-19 is new to mankind, it is crucial to develop not only a safe but also an effective vaccine strategy to successfully manage the pandemic situation and reinstate normalcy [3, 5].
The largest integrant of the entire immune system is the mucosal immune system which is augmented to provide a defense mechanism against various environmental pathogens at the mucosae. In humans by causing the infection through mucosal surfaces of the mouth, respiratory tract, or digestive tract, the conjunctival surface of the eye and at the pneumocytes, bronchial epithelial cells, and URT cells, SARS-CoV, SARS-CoV-2, and MERS-CoV are able to develop acute, life-threatening respiratory pathologies and/or lung injuries. Upon any viral infection nasopharynx-associated lymphoid tissue (NALT) and mucosa-associated lymphoid tissue (MALT) act as the primary-line defenses. To induce the immune response against invading microorganism, NALT plays a significant role in activation of immune cells like dendritic cells, T helper1 (Th1) and Th2 cells, macrophages, innate lymphoid cells, resident M cells, B cells, immunoglobulin A (IgA) as well as immune mediators such as cytokines, beta-defensins, collectins, etc. Gut-associated lymphoreticular tissues (GALT) encompass the similar activity as NALT. Extensive research and clinical studies have been manifest COVID-19 as a “multisystem disease” instead of localized “respiratory infection”. It is also apparent that the progression of this disease involves a complex interplay between inflammatory, coagulative, and immunological cascades. So far, no therapeutic or specific prophylactic treatment has been approved for such conditions [1–3].
Initial steps of viral infection depend upon the distinct binding of the viral protein to the host cellular entry receptors, such as SARS-CoV-2, SARS-CoV, and HCoV-NL63 binds with angiotensin-converting enzyme 2 (ACE2), HCoV-229E with human aminopeptidase N (APN), and MERS-CoV interacts with dipeptidyl peptidase 4 (DPP4). The tissue distribution and expression of entry receptors play a major role in controlling pathogenicity as well as viral tropism. Generous expression of ACE2 is found on the lung and intestine epithelial cells, especially secretory goblet cells (nasal mucosa), type II pneumocytes (lung), and absorptive enterocytes (small intestine) which allows the spike (S) proteins to bind with it and establish the host tropism. Along with receptor binding, proteolytic cleavage by the host cell-derived proteases such as transmembrane serine protease 2 (TMPRSS2; cell-surface serine protease), cysteine proteases cathepsin L (CatL), and endosomal CatB is essential by the coronavirus S proteins for priming and entry, within the host cell. Further studies also have shown that TMPRSS2 is profusely expressed in the human respiratory tract, and plays a major role in the transmission and pathogenesis of SARS-CoV-2. So, inhibition of TMPRSS2 by inhibitors such as nafamostat mesylate and camostat mesylate was sufficient to prohibit the SARS-CoV-2 entry in primary lung cells and lung cell lines [5]. Moreover, the presence of a polybasic cleavage site (PRRAR) at the S1-S2 boundary of the SARS-CoV-2 S protein also allows the efficient cleavage by the proprotein convertase called Furin. Such proteolytic cleavage leads to increased infection transmissibility, zoonotic potential, and cell tropism [1, 3]. Inside the host cell coronaviruses integrate and replicate their large (> 30 kb) genomic RNA and produce full-length copies of the genomic RNA, later on, which are integrated within the newly produced viral particles.
In addition to ACE2, other different attachment and entry factors, such as integrins, cellular glycans, and/or neuropilin 1 (NRP-1), have a great impact on the establishment of zoonotic potential. CD147 or extracellular matrix metalloproteinase inducer (EMMPRIN) or Basigin is a transmembrane glycoprotein belongs to the immunoglobulin superfamily, and act as the binding site for SARS-CoV-2. CD147 was identified as red blood cell (RBC) receptor for the parasite Plasmodium (Malaria causing) infection in humans [6, 7]. It also plays a major role in Plasmodium invasion, tumor development, bacterial and viral infection. A recent study by Wang and co-worker [6] demonstrated that SARS-CoV-2 S protein is capable to binds to CD147 along with ACE2 and help in viral invasion and dissemination within the host cell. A phase II clinical trial process is underway for the development of anti-CD147 humanized Meplazumab for injection against SARS-CoV-2 [7]. CD147 is proposed to be a marker protein of untransformed lung stem and progenitor cells, undifferentiated embryonic stem cells, mesenchymal stem cells and act as stimulators of matrix metalloproteinases (MMPs) [6, 7]. Cellular tropism of SARS-CoV in ACE2 expressing residing lung stem cells suggests that failing in repair of the lung injury mainly involves the immobilization as well as un-differentiation of lung stem and progenitor cells. Additionally, during SARS-CoV-2 infection upon viral invasion and loss of lung stem cells, CD147 expressing regenerative cells in lung deposit excessive extracellular matrix (M) proteins and leads to fibrotic diseases such as fibrous stripes and pulmonary fibrosis due to aberrant expression of transforming growth factor-beta1 (TGF-β1). In fact, current clinical pieces of evidence consider the pulmonary fibrosis and/or fibrous stripes in the injured alveolar zone as COVID-19 related complications [8, 9]. Presence of acquired along with genetic disparity in the host immune system further aggravates the host repertoire verging to wide heterogeneity in the clinical outcome.
Prognosis of SARS-CoV-2 infection predominantly occurs at the respiratory and/or oral mucosae where it has interacted along with the immune system, at both inductive as well as effector phases. The mucosal immune system is the biggest integrant of the entire immune system which imparts protection at the main sites of infectious threat—the mucosae. Moreover, glycosylation present on the coronavirus S proteins helps in shielding epitopes, allows the immune evasion from viral-induced neutralizing antibodies within the host cell [10]. Uncontrolled and rapid SARS-CoV-2 replication plays a pivotal role in evading the host’s innate immune activation results in aberrant and enhanced pro-inflammatory responses, infiltration of immune cells in the lungs followed by a severe form of tissue damage and other clinical manifestations. Studies also revealed that coherent presence of SARS-CoV-2 infection modulates the transcriptional landscape of infected cells and induces inflammatory cytokine and chemokine that triggers the inflammation and immunopathologies [11, 12]. IgA is the most heterogeneous immunoglobulin isotypes derived from bone marrow found in three molecular forms (polymeric, secretory, and monomeric), two subclasses such as IgA1 and IgA2, along with numerous glycoforms. IgA-producing mucosal B cells are generated by mucosal inductive site tissues that help in differentiation in polymeric IgA (pIgA)-secreting plasma cells at the mucosal effector sites [13, 14]. In addition to this in the tonsil (home to peripheral lymphoid tissues) IgG-producing B cells are differentiated and secrete IgG for circulation. In the sub-epithelial spaces of the mucosae, pIgA is selectively transported [polymeric Ig receptor (pIgR) mediated pathway] and being released as secretory IgA (sIgA). In nasal passages, trachea, and bronchi, the environmental virus encounters a mucosal immune system-mediated sIgA-dominated environment and sustains a non-inflammatory milieu [15]. However, the terminal airways and alveoli are controlled by circulation-derived IgG. Moreover, plasma-derived IgG appeared in the URT and the lower respiratory tract (LRT), where it helps in the induction of effector mechanisms such as the confrontation of phagocytes [neutrophils and macrophages along with natural killer (NK) cells]; activation of the complement system, etc [16]. The extreme pathological condition related to COVID-19 is enhanced in the terminal airways of the lungs, where circulating IgG intensifies the inflammation by encompassing the cells recruited by virus-induced chemo-attractants. Cytotoxic CD8+ T cells and CD4+ (a cellular arm of the adaptive immune response), are delivered through the circulation to curtail further propagation of the infection by destroying the infected cells [16, 17]. Although, ongoing researches on the development of an effective vaccine against COVID-19 extensively concentrate on systemic injection that is poorly competent at generating mucosal immune responses. Systemic injections are able to activate the production of cytotoxic T cells along with circulatory IgG antibodies whereas mucosal routes of immunization (partly compartmentalized) such as NALT in the URT, GALT in the gastrointestinal tract are able to induce mucosal immune responses [17, 18].
The alveolar epithelial cells, vascular endothelial cells along lymphocytes, act as the main destination for the virions [19]. Upon replication by RNA polymerase enzyme system SARS-CoV-2 virus releases a large number of virions (infect the neighboring target cells) and viremia (cause embellished systemic and pulmonary inflammatory response) [20]. The production of interferons (part of cellular defense mechanisms) gets interrupted by the virus which results in shock, acute respiratory distress syndrome (ARDS), and coagulopathy, i.e., the clinical presentation of severe COVID-19. The complement pathway through C3a and C5a (potent pro-inflammatory component) also plays a pivotal role in the hyper inflammation by triggering inflammatory cell recruitment along with activation of neutrophils. Crosstalk between several pathways, such as Janus kinase/signal transducer and activator of transcription (JAK/STAT), nuclear factor kappa B (NF-κB), and the macrophage activation pathway leads to the release of TNF-α, IL-6 along with other cytokines and chemokines [21, 22]. Such hyper-inflammatory condition contributes to the “cytokine storm”, the hallmark of SARS-CoV2 infection [23]. The extensive production of unregulated interleukins, especially IL-6 (a key player in the cytokine storm), form “positive feedback loops” that stimulate other downstream pathways, and increase the reactants such as C-reactive protein (CRP) production. The persistence of such conditions leads to severe immunopathological conditions of COVID-19 [24].
Loss of smell considers as early symptoms or only symptoms of COVID-19 infection in the case of the non-hospitalized population. Though, chronic or acute olfactory dysfunction (OD; loss of smell) is associated with other conditions such as chronic rhinosinusitis, head trauma, neurological disorders, exposure to toxic agents, and/or viral infections [25]. In the case of viral infections, different mechanisms such as alteration in mucus composition, and production; swelling of the mucosa in the olfactory cleft; along changes in olfactory signaling by local cytokines or injury of the olfactory neuroepithelium is related to OD. The olfactory epithelium is a complex cytological architecture consist of olfactory neurons (ON); the basal globose; multiple non-neural cell types like Bowman’s gland, sustentacular, and microvillar cells; and horizontal cells (reservoir of ONs stem cells) [26, 27]. According to a prior study, horizontal stem cells present in the vascular pericytes and olfactory cleft along with supporting cells express ACE2 receptor as well as TMPRSS2. As per a previous study, NRP-1 and NRP-2 are considered as two other possible receptors for viral entry in mice, which allow SARS-CoV-2 infection in neuronal cells of the olfactory bulb [27, 28]. According to a recent study local infection of these cells by SARS-CoV-2 might cause “inflammatory” conditions such as mucosal architecture disruption, hamper of processing, and transmission of a signal to the brain which results in a block of olfactory function. Furthermore, according to another study, neuroinvasion of SARS-CoV-2 occurs at the neural–mucosal interface via regional nervous structures by the transmucosal entry of the virus [29, 30]. Later on, this invasion gets transported along the olfactory tract of the central nervous system (CNS), resulting in alterations of smell and taste perception in COVID-19. In a small population of patients, extensive degeneration of stem cells takes place, as a result, epithelial regeneration gets hamper and causes a worse prognosis of the disease. Although, most of the COVID-19 patients with OD show an improvement within a few weeks in severe cases, olfactory rehabilitation along with intranasal glucocorticosteroids considered to be useful for those with a poor prognosis [31].
In clinical settings, SARS-CoV-2 infection can be elucidated on the basis of amplification of viral RNA from nasopharyngeal swab samples, and saliva tests (less invasive in nature), but sometimes feces test show the presence of SARS-CoV-2 RNA even prior to symptoms appear and also long after a patient has tested negative from a conventional swab. Diagnosis of COVID-19 is affirmed by polymerase chain reaction (PCR) test of nucleic acids of SARS-CoV-2 found in respiratory tract specimens. Although clinical assessment is indispensable, identification of laboratory markers, or biomarkers, are able to provide additional information such as early suspicion of disease, confirmation, and classification of disease severity, identification of high-risk cohort, and framing hospital admission criteria, management, and disposition of patients and many more [32]. Each of these components might have a crucial effect on the healthcare system as well as patient care. Biomarker panels might provide more authentic information rather than a single biomarker.
Studies have revealed that anemia and modified iron homeostasis were connected with elevated mortality rate and higher level of ferritin/transferrin ratio in hospitalized COVID-19 patients. It is also clear that the severity of COVID-19 disease is directly associated with a remarkable increase of neutrophils, leukocytes, infection biomarkers like ferritin, CRP, and procalcitonin (PCT), and cytokines such as IL-6, IL-2R, IL-8, TNF-α, IL-10 level and decreased lymphocyte counts [23]. IL-6 cytokines are significantly increased in COVID-19 patients and act as good markers to check therapeutic responses. Moreover, an elevated expression of IL-6 along with other pro-inflammatory cytokines such as IL-17, IL-1b, IL-8, IL-2, granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GMCSF), interferon-gamma-inducible protein 10 (IP-10), C-C motif chemokine ligand 3 (CCL3), monocyte chemotactic protein-1 (MCP-1), and TNF-α are significantly increased in severe disease conditions patients [33, 34]. Furthermore, another study revealed that the levels of cytokines in COVID-19 patients with ARDS were insignificant than in those with trauma, septic shock, or out-of-hospital cardiac arrest. Coagulopathy, in COVID-19, is differed from the disseminated intravascular coagulation, in terms of high fibrinogen level, platelet count, and altered prothrombin time as well as thromboplastin time. Elevated levels of D-dimer are very frequently seen in COVID-19 patients and considered as an early marker in managing COVID-19 patients [34]. Elevated expression of different cardiac biomarkers such as N-terminal of the prohormone brain natriuretic peptide (NT-proBNP), creatine kinase (CK), cardiac troponin I (cTnI), myoglobin (Mb), creatinine kinase-muscle/-brain activity (CK-MB), lactate dehydrogenase (LDH), alpha-hydroxybutyrate dehydrogenase (α-HBDH), and aspartate aminotransferase (AST), have been observed in patients with SARS-CoV-2 infection. Though, the expression of CK, LDH, AST, and α-HBDH are associated with injury in kidneys, lungs, and liver rather than myocardial one [35]. In the case of critically ill COVID-19 patients, hypoalbuminemia is attributed to decreased protein synthesis and serum albumin total mass, increased capillary permeability, and expression of vascular endothelial growth factor.
Assessment of hematological as well as immunological parameters in severe/critical disease and non-survivors has shown that T-cell subsets, eosinophils, lymphocytes, and platelets were very low at the time of admission. Expression of eosinophils, lymphocytes, and platelets are increased in survivors whereas, a significant drop along with progressive increases in neutrophils, basophils, and IL-6 were associated with fatal outcomes in non-survivors [34]. So, understanding of biomarkers and pathogenesis related to COVID-19 and its impact on the mucosal immune system has profound implications on diagnosis, treatment, and development of an effective mucosal vaccine [34, 35].
By highlighting speedily emerging evidence, the World Health Organization (WHO) recognizes obesity and type 2 diabetes as co-morbidity factors for becoming severely ill with COVID-19. Obesity is considered an immense healthcare concern as it is correlated with several chronic diseases including cardiovascular diseases, renal insufficiency, stroke, various types of cancers, and a significant degree of endothelial dysfunction. Such conditions are closely associated with disease severity, hospitalization, and increased mortality rate in COVID-19 [36–38]. In obese patients, alterations in the chest wall physiology and respiratory system due to fat deposition in the mediastinum and abdomen are considered to be associated with ARDS, acute lung injury and other respiratory system diseases. Obesity is related to impaired gas exchange, increased airway resistance, surfactant dysfunction, and positional gas trapping [37, 38]. These functions as well as physiological changes incline them to pulmonary hypertension, hypoventilation-associated pneumonia, and cardiac stress [39]. This establishes obesity as one of the prominent causes of death, worldwide.
It is assumed that the initial viral load and increased expression of ACE-2 on cells in the gut, nose lining, the lungs, kidneys, pancreas, adipose tissues (ATs), and in the heart muscle, and in the lining of blood vessels are widely accepted primary factors which cause the severity of SARS-CoV-2 infection with worse clinical outcomes [39]. In case of obesity, AT is altered, and expression of ACE2 is enhanced, either by high-fat induction (or by high sucrose or high fructose diet) and act as a big reservoir where the virus can remain for extended periods of time [40, 41]. A study by Higham et al. [42] has illustrated that expression of ACE2 in the bronchial epithelium of chronic obstructive pulmonary disease (COPD) patients got increased in overweight or obese persons than to lean ones. Additionally, SARS-CoV-2 infections are able to alter the gene expression associated with lipid metabolism in lung epithelial cells and also play a crucial role in impairment of immune system [43].
In human, AT is perceived as a crucial endocrine organ that secretes a plethora of factors like adipokines, cytokines, and chemokines that has a profound impact on metabolism as well as the immune system [44, 45]. A comprehensive set of immune cells are present within the normal lean AT and play a major role in maintaining a balance between anti-inflammatory and pro-inflammatory environments [46]. Whereas, changes found in the resident immune cell composition of AT are associated with the presence of enlarged adipocytes, which has a key role in the disruption of the balance between pro-inflammatory and anti-inflammatory immune cells. Such obesity-induced expansion changes the architecture as well as the function of AT and forms inflammatory adipose by attracting the macrophages and/or other immune cells [46, 47].
The function of normal AT is associated with the presence of three negative regulators of inflammation—Th2 cells, M-2 macrophages, and regulatory T (Treg) cells. Significant alteration along with a marked decrease in Th2 cells, M-2 macrophages, and Treg cells and abundance of pro-inflammatory cells like M-1 macrophages and CD8+ T cells are associated with obesity [48–50]. Abundance of > 40% M-1 macrophages within the obese inflamed AT are a major source of pro-inflammatory cytokines which results in a state of chronic inflammation at a local or a systemic level. Other immune cells like dendritic cells, neutrophils, and mast cells release a plethora of pro-inflammatory factors like IL-6, TNF, and CRP along with the inappropriate secretion of adipokines by adipocytes contribute to the onset of inflammation. According to a recent study, plant bioactive isolated from Bignay can work as steroid and able to control the neutrophilic infiltration, IL-6 expression, the hallmark of ARDS [51–53]. An early study has revealed that in hypoxic conditions, the innate inflammatory response of the visceral fat depots is one of the major sources of upregulated IL-6 secretion which are capable to act as an independent risk factor for developing severe COVID-19 [52, 54, 55]. In this pro-inflammatory state, the establishment of an “auto-regenerating inflammation loop” by recruiting different immune cells (T cells, macrophages, and B cells) impedes the immune system [56]. Moreover, deposition of excess lipid modifies the architecture and integrity of primary lymphoid tissues thereby reforming the development as well as activation of immune cells. This chronic inflammation status is augmented by acute inflammation emerging out of COVID-19 could likely begin an impaired immune response, followed by a cytokine storm due to pro-inflammatory cytokines overproduction. The cytokine storm could lead the way to more severe disease phenotype like vascular hyperpermeability as well as multiorgan failure in severe cases of COVID-19 [56, 57]. Moreover, obesity-associated metabolic changes like leptin and insulin resistance are the negative regulator of immune cell function. Such metabolic changes have a significant influence on the glucose metabolism, and proliferation of the immune cells, and activation of T cells which eventually results in the detriment of host immune defense [57, 58].
Diabetes is one of the chronic, non-communicable pandemic diseases worldwide signalized by unusually high blood glucose levels due to disablement in insulin secretion and/or insulin action. Over a period of time, a high level of blood glucose can desecrate tiny to large blood vessels, resulting in increased risk for macrovascular or microvascular complications [59]. Destruction of insulin-producing β-cells by the autoimmune system is associated with type 1 diabetes (T1D), whereas, a combination of a defect in insulin secretion from β-cell, and insulin resistance results in T2D. Overweight/obesity, hyperlipidemia, hypertension, cardiovascular disease and chronic kidney disease are the most frequent conditions in patients with T2D. In the ongoing pandemic, late diabetes conditions like ischemic heart disease and diabetic kidney disease might play a major role by making individuals with diabetes frailer along with increasing the severity of COVID-19 disease [60, 61].
Studies have shown that in patient with T2D insulin deficiency and hyperglycemia is associated with disrupted host immune response, altered cellular immunity and the damaged natural barrier at the mucosal site (due to neuropathy). Diabetes-related impairment of host’s defense against pathogens include various alter expression of immune cells like defects in phagocytosis, suppression of cytokine production, failure to kill microbial proteins, altered neutrophil chemotaxis and dysfunction of other immune cells. In patients with T2D, infection with SARS-CoV-2 can lead to aggravated expression of inflammatory mediators in the blood, such as inflammatory cytokines toxic metabolites, and lipopolysaccharide. Infection with SARS-CoV-2 accentuates the apoptosis of lymphocytes such as CD3+, CD4+, and CD8+ T cells infect circulating immune cells, delays activation of Th1/Th17 modulates the activity of NK cell and IFN-γ production finally leads to lymphocytopenia. Moreover, the SARS-CoV-2 infection leads to increased production of reactive oxygen species (ROS) that are related to vascular endothelial damage, lung fibrosis, and ARDS (Figure 1). Moreover, COVID-19 patients with associated T2D show altered NALT (CD4+ and CD8+ T cell reduction), aggravate OD and increased rate of susceptibility and severity of the disease [62]. Dysfunction of NALT can impair the development of B cell as well as the production of antibody for clearance of SARS-CoV-2 pathogen [63, 64].
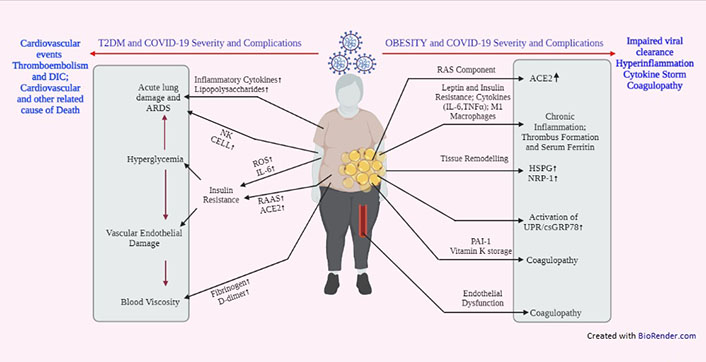
Schematic representation showing different mechanisms involved in T2D mellitus (T2DM) and/or obesity related COVID-19 severity and complications. DIC: disseminated intravascular coagulation; RAS: renin-angiotensin system; RAAS: renin-angiotensin-aldosterone system; PAL-1: plasminogen activator inhibitor 1; HSPG: heparan sulfate proteoglycan; UPR: unfolded protein response; csGRP78: cell surface glucose related protein 78
In T2D patients, SARS-CoV-2 infection initiates a crosstalk between different signaling systems which in turn control the molecular activity of innate immunity. In hyperglycemic patients the complex signaling system of advanced glycation end products (AGE)-receptor for AGE (RAGE) provides the first barrier against pathogens and plays a pivotal role in chronic vascular complications. Proteins, high mobility group box-1 (HMGB1) and S100 acts as the ligand for RAGE signaling system contribute to the inflammatory response by immune cells, like NK cells, macrophages, and dendritic cells, or passively released from damaged cells [65, 66]. In short, due to coronavirus infection in T2D patient crosstalk between different signaling molecules might arbitrate an injurious pulmonary inflammatory response including derangement of the epithelial barrier, lung oedema, neutrophil infiltration that contributes to respiratory failure as well as the death of the patient [67].
The common mucosal immune system (CMIS), which is described as an integrated network of tissues, cells, and effectors molecules that protect the host against infection and environmental harm at mucous membrane surfaces, has received a lot of attention (Figure 2). Mucosal surfaces are immunologically distinctive in that they serve as the principal interface between the host and the physical environment while also serving as an important barrier against pathogens. The mucosal immune system appears to be a system-wide organ, according to a growing body of research. The stimulation of one compartment of the mucosal immune system has been shown to cause changes in distal locations, according to studies [68]. Understanding the connectivity across mucosal locations is critical for disease characterization and vaccine development in the following phase. Finding out what elements link one section of the mucosal immune system to another, as well as the complexities of this communication, will help us appreciate the mucosal immune system as a global organ. This is an issue that must be addressed as soon as possible. Based on their functional and anatomical properties, CMIS can be divided into initiation sites and effector sites. Mucosal vaccination extorts the immune responses not only at the delivery site of antigens but also at the distant, multiple mucosal effector sites.
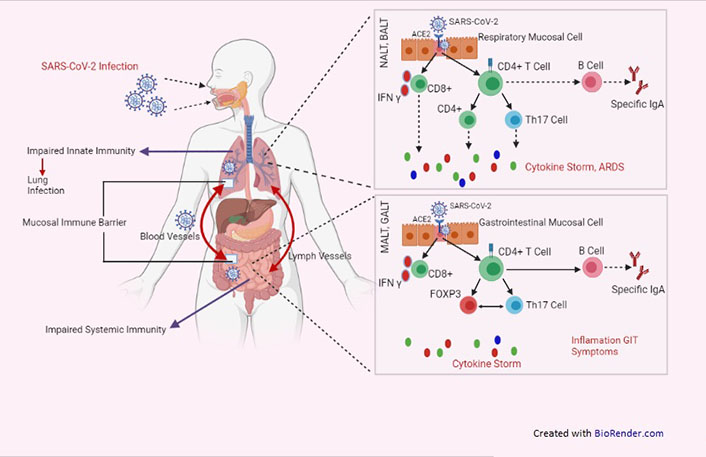
Schematic representation showing NALT, bronchus-associated lymphoid tissue (BALT), MALT, GALT provided first line of immunity against SARS-CoV-2 infection. GIT: gastrointestinal tract; FOXP3: forkhead box P3
Upon SARS-CoV-2 entry within the host cell, the host immune system triggers the adaptive immune responses by initiating specific virus antigen recognition and presentation processes to control viral reproduction and tissue damage. Tertiary lymphoid tissue, i.e., NALT via dendritic cells and macrophages can initiate innate immunity response, as the first line of mucosal host defense against SARS-CoV-2 along with the prevention of OD and recovery of olfactory function (OF). They help in capture as well as the internalization of virus-specific antigens for T cells presentation at secondary or tertiary lymphoid organs [68, 69]. Additionally, priming of naive T cell maturation (differentiate into antigen-specific effector T cells) and B cell activation which strives the effects at virus infection sites are mainly dependent upon maturing dendritic cells (DCs) migration from the mucosal infection site to NALT. Furthermore, induction of CD4+ Th1 cells and CD4+ Th2 cells are required for amplification of the immune response via macrophages activation and IFN-γ secretion, and the antigen-specific IgA-producing cells generation in NALT respectively. SARS-CoV-2 specific CD8+ T lymphocytes (CTLs) are able to generate high levels of cytotoxic molecules (perforin and granzyme B) and effective cytokines (IFN-γ and TNF-α). More importantly, SARS-CoV-2 neutralization takes place with the help of specific plasma cells that are able to secrete atopic monoclonal antibodies (e.g., IgM, IgA, and IgG) and thereby completely cure the COVID-19 [68–70]. Most B cells within NALT are sIgM+ IgD+ phenotyped naive B cells which depend upon antigen presenting cells (APCs) for their differentiation into committed B cells for the immune response against the anti-viral antigen. Therefore, an impairment of B cell development and/or reduction in B cell quantity could result in antibody production deficiencies, results in failed SARS-CoV-2 clearance [64].
On the other hand, mucosal inductive sites, such as GALT encompass a MALT. The GALT is organized into three compartments: beneath the intestinal epithelium in the lamina propria, within the epithelial compartment itself, and in organized lymphoid follicles like Peyer patches. It helps guard against external stimuli that pass the luminal mechanical barrier. The MALT consists of B cell-enriched areas that contain a plethora of surface IgA+ B cells, T-cell zones, and a subepithelial area with APCs (site for specific immune responses initiation) act as a continuous source of memory T and B cells to mucosal effector sites. A follicle-associated epithelium comprises differentiated microfold epithelial cells, lymphoid cells, and columnar epithelial cells. The hallmark of microfold cells is the lack of secretion of mucus as well as the glycocalyx, which helps them in enabling endocytosis activity and also makes them a suitable vehicle for transferring antigens from luminal to inductive MALT sites. Initiation of mucosal immune response takes place in microfold cells by taking up the viral antigen from the nasal mucosal or intestinal lumen. Later on, these microfold cells transport them to the underlying APCs, like DCs via transcytosis and activate T cells [71–73]. However, relocation of these primed T cells to the germinal centers helps in the secretion of cytokines which in turn promote the B-cell isotype switching for the production of IgA antibodies.
The lungs are the predominant infection site for SARS-CoV-2, it is critical to developing a vaccine that may elicit mucosal protection. Enhancement of mucosal immunity might intensify the production of effective specific IgA at the mucosal site, specific memory T-cell response, and neutralizing IgG. The local mucosal immune response especially the presence of tissue-specific memory T-cells in nasal epithelial and lungs is essential to mitigate the replication of the SARS-CoV-2 virus and also a chance of secondary infection in the host. So, in the case of respiratory infections like COVID-19, the nasal route is more appropriate for the direct administration of vaccines [73, 74]. Although mucosal administration is not contemplating as the usual route for vaccination, certain pharmaceutical companies and research institutes have considered the mucosal formulations of the COVID-19 vaccine as a better alternative (Figure 3).
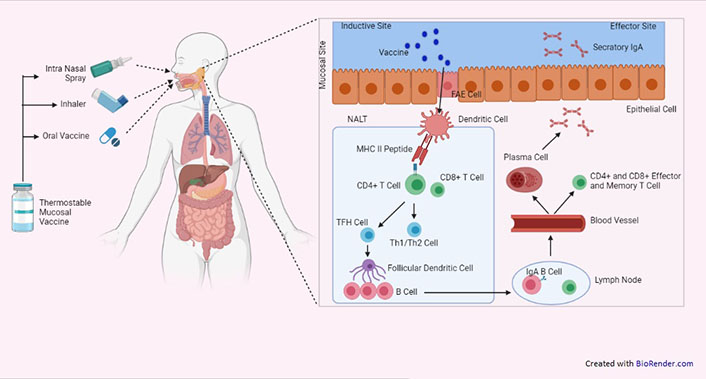
Schematic representation showing targeted activation of mucosal immune system by mucosal vaccine. FAE: follicle associated epithelium; TFH: follicular helper T cell
According to Brandtzaeg [74], a Norwegian immunologist intranasal administration of vaccines would elicit the regional immune effect on the upper airways by producing a plethora of both mucosal and systemic IgA and systemic IgG immunoglobulins. Furthermore, the adenoids and tonsils are important components of the NALT or Waldeyer’s ring, which is a CMIS. This mucosal-related lymphatic tissue is found under the nasal mucosa’s lamina propria. They act as the primary induction site for the secretory immune system by producing memory-type IgA+ B cells and sIgA by plasma cells. Additionally, current studies have shown the presence of isolated lymphoid follicles (ILFs) in the mouse small intestine, which has been recognized as a part of GALT especially mucosal inductive tissue [75]. These ILFs primarily consist of microfold cells, DCs, and B cells in the overlying epithelium. Conjunctiva-associated lymphoreticular tissue (CALT), Larynx-associated lymphoreticular tissue (LALT), Salivary duct-associated lymphoreticular tissue (SDALT), and Tear duct-associated lymphoreticular tissue (TALT) are all mucosal inductive tissues, according to other recent investigations. IgA-producing plasma cells and B and T cells, i.e., antigens-specific mucosal effector cells, are found in mucosal effector locations such as the upper respiratory (UR), lamina propria areas of the gastrointestinal (GI), secretory glandular tissues, reproductive tracts, and intestinal intraepithelial lymphocytes [76, 77].
Mucosal addressin cell adhesion molecule-1 (MAdCAM-1) is a mucosal vascular addressin that is expressed predominantly by venular endothelial cells at lymphocyte extravasation sites in mouse mucosal lymphoid organs and lamina propria. Activated T lymphocytes express CC chemokine receptor 9 (CCR9) and 47 as gut-homing receptors in Peyer’s patches (PPs) for migration into the intestinal lamina propria. In this regard, MAdCAM-1 acts as ligand that aids in T-cell recruitment into the intestinal endothelium. Furthermore, recent studies also demonstrated the role of the mesenteric lymph nodes (MLNs) and retinoic acid-producing DCs in PPs as the enhancer of α4β7 and CCR9 expression by antigens-specific effector CD4+ T cells. In addition to the mucosal T-cell homing, retinoic acid-producing DCs in PPs also take part in the regulation of T cell-independent IgA class switching and expression of gut-homing receptor on B cells [78, 79]. Such findings confirm that CMIS has a distinct role in induction as well as regulation of sIgA antibody responses in mucosal effector tissue.
Both monomeric serum IgA and polymeric (pIgA) (dimmers, tetramers, pentamers, and multimers), are produced by plasma B cells. This multivalency of the IgA antibody results in greater avidity for viral peptides compared to IgG and also significantly prevents the intrusion of pathogens by immune exclusion. The secretory component (SC) of the pIgR helps in the active transportation of pIgA as a secretory (sIgA) complex across the cell membrane of the secretory epithelium. Upon reaching the surface of the uninfected cell, the secretory (sIgA) complex gets separated from SC and gets them diffuse into the mucus layer and impart the protective mechanisms. Studies in vitro demonstrated that free SC is able to bind and inhibit IL-8-mediated recruitment of neutrophils [80]. It also helps in the prevention of neutrophil-dependent extracellular traps in the upper airways. During the presence of any viral infection, sIgA plays a role in the neutralization of the pathogens or toxins in the mucosal environment by following three mechanisms such as—excretion, immune exclusion, and intracellular neutralization. Upon systemic and mucosal vaccination, sIgA along with IgG, and transudate IgA are generated and contribute to local surface defense in the LRT and in the genitourinary mucosa as these areas are more permeable to serum-derived antibodies than to the intestine. For providing protection against pathogens, such antibodies deploy a distinct range of effector functions such as—the neutralization of toxins; antibody mediates opsonization and phagocytosis for internalization of invading pathogens. If sIgA-dependent eradication of pathogen is unsuccessful, transudated IgG as well as Serum IgG antibodies exert their immunopathological effect and take part in viral clearance from systemic circulation [80, 81].
Despite the fact that IgA is one of the most widely produced antibodies in the body, MALT tissue synthesis falls with age, which is a characteristic of immunosenescence. The elderly is the most vulnerable population in the current COVID-19 pandemic due to the immunosenescence of IgA antibodies. A study in mice has disclosed that the aging process affects the GI-associated lymphatic tissue to a greater degree than the NALT result in the retaining of immune-competent cells in the nasal mucosa to provide an effective immune response. Nonetheless, it is the need of the hour to design an appropriate, safe and effective mucosal vaccine strategy that uses the combination of proper mucosal adjuvants, delivery systems as well as optimization of the immunization schedule along with different routes of mucosal immunization [81, 82].
Mucosal immunization has the potential to be more effective than traditional parenteral vaccination in terms of triggering immune resistance in both mucosal and systemic tissue to protect mucosal surfaces from pathogen invasion. Mucosal vaccines have been developed to give the first line of defense at these entrance points, and they hold a lot of potential for decreasing the burden of infectious illnesses. Mucosal vaccines are a medical waste-free and needle-free vaccine method that can be given by intranasal or oral routes, as well as vaginal, rectal, sublingual, or ophthalmic routes. It has a condemning role in creating hindrance for viral or pathogenic bacteria entry either by oral route or respiratory tract. As the mucosal vaccines are capable to evoke a robust immune response at both mucosal sites as well as systemic circulation, show more advantage over systemic vaccines even in terms of cost and administration. Oral immunization elicits robust immune responses mostly in the GI tract, salivary glands, and mammary glands, whereas intranasal vaccination elicits a robust immune response in the respiratory, genital, and GI tracts [83].
The primary site of SARS-CoV-2 infection is the mucosal membranes of the nose, eyes, or mouth. As a result, the nasal and gastric mucosa along with conjunctiva plays an important role in both the transmission and clinical course of SARS-CoV-2 infection. In addition, inducing mucosal immunization with an intranasal or oral vaccination is proven to be a viable method for SARS-CoV-2 immune-prophylaxis. Vaccination at the mucosal sites not only induces a strong local immune response but also reduces the risk of antibody-dependent disease enhancement (ADE). Mucosal vaccines are also able to generate strong systemic humoral immunity—which is required to neutralize any virus particle that dodges the primary immune response [84].
Despite the challenges and obstacles of mucosal immunization, the vaccine has been made in case of some enteric diseases such as the Bacille Calmette-Guerin (BCG) vaccine against tuberculosis (TB), the oral polio vaccine (OPV) against poliomyelitis, typhoid, and rotavirus [84]. Polio viral vaccines are an effective example of the importance of mucosal responses—the live-attenuated oral vaccine (OPV) vs. inactivated poliovirus vaccine (IPV). OPV is capable to introduce sterilizing mucosal responses that can block the viral shedding in the stools and also prevent viral transmission. The efficacy of IPV is limited to the instigation of systemic immune responses and the prevention of severe disease [85]. Re-purposing of such pre-existing oral and mucosal vaccines can improve the effects of COVID-19 by inducing IFN and other innate immunity via broader protection against unrelated pathogens. Furthermore, more in-depth research by using novel approaches like eye drop delivery, sublingual immunization, plant-based delivery systems, and the nanomatrix need to be designed for an effective and safe vaccine.
Development of the mucosal vaccine mainly depends upon a wide range of vaccine platforms of COVID-19 candidate such as—a) viral vectors (replicating and non-replicating); b) virus-like particle (VLP)-based; c) nucleic acid-based (DNA/RNA plasmids); d) recombinant protein subunit vaccine; e) inactivated whole virus; and f) live-attenuated vaccine. Although each of these prospective vaccines has its own set of benefits and drawbacks, it is critical to evaluate the balance between humoral (neutralizing antibody) and T cell responses throughout vaccine development. Viral structural proteins that are capable of self-assembly are used in the case of VLP-based vaccines whereas the DNA vaccine consists of viral immunogens that are encoded by a recombinant plasmid which elicits the desired immune response. Both of these vaccine platforms, however, are safe, noninfectious, and maintain the fundamental antigenic structure of viral immunogens, although their immunogenicity is low. Vaccines based on viral vectors rely heavily on the antigenic protein being encoded and displayed on the surface of APCs in order to elicit a humoral and cellular immune response. Despite the fact that these vaccines are very effective, pre-existing immunity to the vector may trigger a detrimental immune response. Pathogens that are attenuated or inactivated by chemical treatment or heat might be considered as Live-attenuated vaccines or inactivated vaccines. So far intranasal influenza vaccine, OPV, and other mucosal vaccines that are licensed for human use are mainly of live-attenuated approach. Due to the possibility of incomplete inactivation of harmful pathogens safety concerns are often associated with attenuated vaccines. In addition, recombinant protein subunit vaccines contain specific viral antigenic fragments that are able to elicit strong cellular as well as antibody-mediated immune response. Lastly, inactivated whole virus vaccines are relatively inert, inexpensive, and nontoxic but confirmation related to immunogenicity needs to be addressed [84–86].
In general, the main goal of any vaccine is to induce the anti S protein–neutralizing antibodies that are able to inhibit attachment of virus with the host cell, and also infection capability. Few techniques, such as mRNA vaccines related to viral vaccines, have yet to be licensed for clinical use, but in the current situation, they require suitable evolution. Though in the case of COVID-19 the mechanism of actions of different vaccine platforms is unclear, the importance of activation of mucosal immunity via mucosal vaccine to reduce nasal shedding and also the presence of additional structural antigens to provide a broader immune protective response cannot be overlooked [85].
Designing a new-generation vaccine against the SARS-CoV-2 virus requires the proper selection of the target antigen based on their pathobiology and structural information. S protein, nucleocapsid (N) protein, M protein, and envelope (E) protein are the structural proteins that are present in the virion, among them the main antigenic component is S protein [87]. SARS-CoV-2 contains a single-stranded, positive-sense RNA as genome. The N protein plays the main role in coating the large positive-stranded RNA genome, and also encased within a host cell membrane-derived lipid E, the other three proteins, i.e., S, M, and E proteins are inserted into it. Studies have shown that only antibodies that are directed to the S protein of SARS-CoV are able to neutralize the virus as well as prevent further infection [88]. S proteins that are present in virus particles can bind to ACE2 on the host cell surface, and allow the receptor-mediated endocytosis of the virus. Crystallography studies have shown the presence of highly homologous binding patterns among ACE2 receptor and SARS-CoV-2 as well as SARS-CoV. Depend upon this data, during the development of SARS-CoV-2 vaccines, one should consider at least a portion of the S protein (plays a critical role in the virus life cycle) such as the S1 domain or the receptor-binding domain (RBD). Though the vaccines that are using S protein as antigen can inhibit viral infection and even able to evoke a potent immune response against it but the use of full length S protein as an antigen has raised few safety issues like ADE of viral infection in vaccinated subjects. Previous animal investigations using the full-length S protein as the vaccine antigen for the development of the SARS-CoV vaccine revealed the possibility of liver injury [87]. When designing the COVID-19 vaccine, it will be safe to employ a fragment of S protein such as the RBD of S protein because it is highly immunogenic and confers substantial neutralizing power. RBD lacks the non-neutralizing immunodominant region of S protein; it minimizes the risk of ADE upon exposure to the virus in vaccinated individuals. Other than RBD, S1 and S2 fragments can also be used as antigens for the development of vaccines against COVID-19. Furthermore, in mice, the N protein of a SARS-CoV-based mucosal vaccination elicited both cellular and humoral protection. Because the N-protein sequences of SARS-CoV-2 and SARS-CoV virus are so similar, it may be possible to develop a broad-spectrum coronavirus vaccine employing N-protein as the viral antigen [88, 89].
A higher dose of antigen is required in developing a mucosal vaccine than that of a parenteral vaccine as the antigenic preparation might get diluted (mucus in the nasal cavity) or get expelled (mucus and ciliary movement in the respiratory tract). Designing effective intranasal mucosal vaccination needs the antigen to have the property of—crossing the mucus layer, reaching mucosal sites, and also inducing IgA production. Whereas, before reaching the immunological sites an oral vaccine has to tolerate the low pH environment in the upper GI tract and the presence of different proteases and nucleases present in the digestive tract. In addition, to overcome these biochemical and physical barriers, improvement of mucosal and serum antibody responses, mucosal antigens vaccines need to be administered complemented with a specific inactivated adjuvant. Adjuvants are the natural or synthetic supplementary materials present in a vaccine formulation that elicit distinctive immunological profiles capacity of a vaccine. Along with that, adjuvant also plays a critical role in augmenting antigen bioavailability, enhancing antigenic stimulation, and maintaining the structural integrity of the antigen. There are two types of adjuvants: a) adjuvants for carrier systems—aid in antigen transport to immunological induction sites; b) adjuvants for immunostimulators—improve antigen internalization, presentation, and processing in APCs. Liposomes and emulsions are also among the other carriers frequently studied for the mucosal vaccine [90–92]. Due to their high affinity toward mucosal surfaces, different polymers such as poly lactico-glycolic acid (PLGA), chitosan, etc. have been used as carrier adjuvant in various vaccine formulations as immunostimulator. Microfold cells and DC present in mucus are considered as a major determinant of mucosal immune response induction and also an ideal site for antigen presentation due to the presence of surface markers for antigen delivery. The presence of receptor-like Toll-like receptor (TLR) agonists such as cytosine-phosphate-guanosine-oligodeoxynucleotides (CpG ODNs), pathogen recognition receptor allows the APCs to take up the vaccine antigen and potentiate the vaccine immunogenicity. Along with nanoparticles, plant lectins, immune-stimulating complexes (ISCOMs), cholera enterotoxin (CT) and heat-labile enterotoxin (LT) from E. coli might be used as adjuvant as they have the interacting capacity with GM1 gangliosides present on the surface of follicular DC. However, for the development of a successful and safe COVID-19 vaccine, a detailed investigation of the screening of various combinations of antigens with adjuvants, as well as the nature of immune responses, is required [92–93].
Mucosal vaccines are a recent attempt to deliver antigens to the aero-digestive, intestinal, and urogenital mucous membranes to elicit protective immune responses.
Mucosal surfaces are large patches of skin that can be infected by pathogenic bacteria. Antigens, infections, and vaccinations that enter the body through mucosal surfaces are distinguished from those that are injected or inhaled directly into tissues or the bloodstream by the adaptive immune system. The function of mucosal tissues and the interplay of innate and adaptive immune responses that results in immune protection at mucosal surfaces are currently being studied in innovative ways. These breakthroughs have the potential to speed up the development and testing of novel mucosal vaccines for a variety of human diseases, including SARS-CoV-2.
A mucosal vaccine that is administered as nasal powder or drops is able to evoke a better immune response in the upper as well as LRTs and also the lungs than any other route. COVI-VAC (single-dose intranasal lives attenuated) is being considered as the most promising intranasal vaccine against SARS-CoV-2 infection, which has proceeded to phase 1 trial. This vaccine provides protection against a range of SARS-CoV-2 strains as it can neutralize all the proteins no on viral S protein. This replicating viral vector-based RBD expressing vaccine is from Codagenix (USA) and Serum Institute (India) that is developed by the University of Hong Kong and Beijing Wantai Biological Pharmacy (China). Another promising candidate is AdCOVID from the University of Alabama (USA) and Altimmune (USA) which is now in Phase 1 trial. This vaccine candidate is a replication-deficient human adenovirus 5 (hAd5) vectored single-dose vaccine that encodes the RBD domain of the S protein of SARS-CoV-2, that is able to activate both systemic and mucosal immunity. According to a recent publication, AdCOVID remains stable over several months at room temperature and also able to evoke a strong T-cell response (CD4+ and CD8+ response), serum neutralizing antibodies, and mucosal IgA in the respiratory tract. Furthermore, another intranasal vaccine candidate which is now in the pre-clinical good manufacturing practice (GMP) manufacturing stage (by AttenuBlock™ proprietary technology) is the Meissa vaccine (USA). By following their previous research on respiratory syncytial virus intranasal vaccine (Phase 2 trial) this vaccine also has been designed based on codon optimization technique. Along with the above-mentioned vaccines, other vaccine candidates such as Razi Cov Pars by Razi Vaccine and Serum Research Institute (Iran), BBV154 from Bharat Biotech India (licensed from Washington University, School of Medicine in St. Louis, USA), CIGB-669 by Centre for Genetic Engineering and Biotechnology (Cuba), etc, are being considered as a promising intranasal vaccine against COVID19. A collaborative research work of Wageningen Bioveterinary Research University Netherlands, Utrecht University Netherlands, and Intravacc Netherlands on reverse genetics technology with Newcastle Disease Virus as the vector for expressing the S protein of SARS-CoV-2 is currently in progress for the development of an intranasal vaccine against SARS-CoV-2. Moreover, they are working on another nasal spray vaccine consisting of its proprietary outer membrane vesicle (OMV) click technology in which the OMV is coupled with the recombinant SARS-CoV-2 S protein. Except for viral vectors nanomaterial-based adjuvant containing viral antigen could be used as prospective candidates for the intranasal mucosal vaccine. A prime example of this technology is S-2P-NE-01 which is designed by using oil-in-water nanoemulsion vehicle adjuvant (400–500 nm size; NanoVax®, BlueWillow, USA) along with SARSCov-2 S-2P ectodomain (maintains the perfusion spike conformation). This nano-emulsion-based vaccine is developed by Medigen Vaccine Biologics Corporation, Taiwan, and BlueWillow Biologics, USA, which has shown strong IgA response both in broncheo-alveolar lavage and serum. These findings suggest that with an appropriate form and quantity of antigen with a properly targeted delivery system (viral vectors or nano-based or plant-based) the intranasal vaccine could be one of the most preferred vaccine candidates for inducing both systemic as well as mucosal immunity [93–96].
Most interestingly, non-conventional oral vaccine formulations against the SARS-CoV-2 infection are developing in two companies namely such as—Vaxart Inc. (USA) and iosBio Pharma (UK). Recently, an oral recombinant COVID-19 vaccine tablet has been designed by Vaxart that has moved to the Phase 1 trial. This vaccine is an enteric-coated tablet that contains genes for ‘S’ and the ‘N’ proteins of the SARS-CoV-2 encoded by adenoviral-vector. The enteric coating present on the tablet helps the active ingredient to skip the acidic environment of stomach coated and gets dissolved in the digestive tract. After getting dissolved they evoke the protective mucosal immunity against the SARS-CoV-2 infection. A pre-clinical study report published by Vaxart, shown that oral mucosal administration of the full-length wild-type (WT) ‘S’ and ‘N’ antigens, to hamsters, (two-dose regimen at 0 and 4 weeks) showed a significant increase in the production of neutralizing antibodies against SARS-CoV-2 than that of the non-vaccinated group. Even this vaccine was able to induce the activity of the antigen-specific CD4+ and CD8+ T cells. With this a new door has opened up in designing of oral mucosal vaccines against SARS-CoV-2. Furthermore, another oral vaccine candidate (similar to Zika virus vaccination) that is in phase 1 trial is OraPro-COVID-19™. A UK-based company named iosBio (previously known as Stabilitech) has started a collaboration with Therm-SB technology and ImmunityBio (US-based Biopharmaceutical Company) for the manufacture of this oral coronavirus vaccine. This thermally stable, encapsulated (enteric-coated) oral vaccine contains a replication-defective adenovirus-5 (Adv5) vector that encodes the modified S protein gene (S-fusion) and N protein gene of the SARS-CoV-2 with an enhanced T-cell stimulation domain (N-ETSD) gets dissolved in the intestinal lymphoid tissues. This oral vaccine is able to generate both cellular (CD4+ and CD8+ T cell-mediated) and humoral (antibody-mediated) immune responses. Upon successful completion of clinical phases of evaluation this self-administered capsulated vaccine, would be a great achievement to immunize millions of people around the globe without any assistance from a healthcare professional [96, 97].
A recent study in mice has shown that eye drop administration of viral antigen is able to induce CALT development and also increase the microfold cell-like cell numbers [98]. Although the potential adverse effects, of this eye drop immunization yet to be identified, the study has shown that administered antigen did not have any effect on the CNS. Altogether, these findings clearly suggest that this eye drop vaccine would be a novel strategy for the induction of mucosal immunity against a broad range of coronavirus.
A modified trans-dermal solid, hollow, degradable patch called the Microneedle patch contains countable optimized microscopic sharp projections that deliver drug(s) through the surface of the patch (part that contact with the skin) with minimal pain. Due to the presence of antigen-presenting cells in the dermis layer microneedle patches could prove more beneficial for administration vaccine. According to recently published data by Kim et al. [99], they have used the vaccine encapsulated microneedle patches on mice model to deliver the SARS-CoV-2 antigen for COVID-19 vaccine which was able to generate vaccine-induced antibodies within 2 weeks of administration. They were able to design the COVID-19 vaccine rapidly due to their prior experience with a similar study for the MERS virus by using carboxymethyl cellulose as an encapsulating and dissolvable biopolymer. In another study by Kuwentrai et al. [100], suggests the use of microneedle patches with hyaluronic acid (low molecular weight) along with the RBD domain of the SARS-CoV-2 S protein in the mouse model. This study demonstrates that this vaccine was able to evoke the significant generation of T-cell response and antibody. Although the commercial production of such vaccine remains a distant dream, this type of study provides a ray of hope to design the painless, fearless microneedle-based COVID-19 vaccine.
Although mucosal immunity strategies are superior and promising also have advantages over the parenteral one, but the presence of some critical dilemmas is creating hindrance in mass production or administration of mucosal vaccines. The mucosal barriers of the intestine and upper airways can absorb and reduce the amounts of antigen, and also pose different obstacles due to immunotolerance at the mucosal site. As there is a chance of exclusion of exogenous antigens, captured in mucus gels, getting diluted in mucosal secretions, epithelial barriers, and/or attacked by proteases and nucleases, so this type of vaccine required more elevated antigens than that of parenteral immunizations. The challenges in administration inhibit the effective delivery and also disorganize antigen presentation route results in immune tolerance that is not able to induce immunity at the mucosal site. Moreover, identification, screening, and evaluation of appropriate antigens and adjuvants are very time-consuming, and sometimes results are dissatisfactory.
The presence of mucosal physical, as well as chemical barriers diminishes the antigen presentation to avoid unwanted immune responses. The barriers present in the mucosal sites are classified into two types such as physical barriers (dwell with tight junctions and the goblet cells producing mucus in the GI, respiratory, and reproductive tracts) and chemical barriers (implemented by antibacterial peptides produced by PPs cells, components of innate immunity, i.e., innate immune cells and TLR). The mucosal vaccine antigens that are capable to tolerate the mucosal barriers can transport across organized lymphoid structures to elicit the immune reaction. For example, nasal mucosal vaccine needs to tolerate physiological defense mechanisms such as ciliary movements (quickly clear inoculated antigens) and mucus of the respiratory tract. On the other hand, an oral vaccine is in need to face the critical threat of a variety of proteases present in the digestive tract (protein antigens are sensitive to the GI tract pH) [101–103].
Although in normal physiological conditions, to avoid inflammatory responses (Treg cells dependent), immunotolerance is essential for harmless antigens or self-antigens but in the case of mucosal vaccines immunotolerance impairs the vaccinating effect. Studies have shown that in the lungs and GI tract immunotolerance acts as an active process to inhibit mucosal immunity and also microorganism introduced by memory. Antigen-induced mucosal immunotolerance depends upon several factors, like formulation, dose, and exposure frequency of antigens, pathogen-associated molecular patterns (PAMPs). Using antigens without an adjuvant, in mucosal vaccination is able to induce T- and B-cell tolerance. Along with immunotolerance, immunosenescence is another critical hurdle for designing a mucosal vaccine against SARS-CoV-2 in elderly people [101–103].
The complexity of vaccine components recognition mechanism for efficient antigen presentation impairs mucosal vaccine design. Mucosal immunizations depend upon induction of long-term T cell and B cell memory but memory cells (T and B lymphocytes) can detect a wide range of antigens only by binding antigen recognition receptors on T cells to major histocompatibility complex (MHC) molecules present on the cell surface of host cells. So, the main obstacle in mucosal vaccine designing is that antigens, especially recombinant proteins are unable to evoke sufficient immune responses [101–103].
The COVID-19 pandemic has caused significant public health issues, widespread psychological, economic, and sociological damage across the globe. In humans, SARS-CoV-2 infection is associated with ARDS, severe respiratory tract infections, multi-organ failure, and death. The primary infection site for SARS-CoV-2 is the mucosal surface, mainly the lungs and/or the intestine, where the epithelial cells get infected with the virus. The CMIS, NALT, BALT, MALT, and GALT provide the primary line of defense agonists viral infection. The tissue distribution and expression of entry receptors play a serious role in controlling pathogenicity as well as viral tropism. ACE2 has been considered as an entry receptor in coronavirus infection, followed by replication within the host cell. The SARS-CoV-2 entry into the cells favors the progression of inflammatory and thrombotic processes by down-regulates the expression of ACE2 receptors. The loss of ACE2 is directly associated with lung edema, enhanced vascular permeability, and severe lung injury induced by activating the renin-angiotensin system. So, treatment by blocking the ACE2 receptor probably increases the chance of a negative effect along with a poor druggable target. In addition to ACE2, EMMPRIN or CD147 acts as SARS-CoV-2 binding along with the host cell. Moreover, in COVID-19 patient’s pulmonary fibrosis can be observed that is driven by extensive differentiation of resident lung progenitor/stem are into myofibroblasts. The process of pulmonary fibrosis might be associated along with the direct invasion of SARS-CoV-2 into the progenitor/stem cells via CD147 or ACE2, which could decrease the cellular stocks result in failing lung repair. Therefore, a combination strategy of pharmacological along with MSC therapy will be more efficient against COVID-19 and other viral diseases.
An elevated level of cytokine IL-6 and CRP in blood serum considered as the biomarker of severe β-coronavirus infection. The extensive production of unregulated interleukins, especially IL-6 (a key player within the cytokine storm), forms “positive feedback loops” that are able to stimulate other downstream pathways. The persistence of such conditions results in severe immunopathological conditions of COVID-19. So, expression of the inflammation biomarker panels of IL-6, IL-1β, CRP, procalcitonin, D-dimer, and fibrinogen might be considered as more authentic in the prediction of disease severity. Since an appropriate immune response depends upon a balance between cytokines. As excessive enlargement of adipocytes due to lipid overloading promotes mechanical stress and inflammation, results in alteration of secretory function, and induction of necrotic cell death [104–107]. However, in obese patients, IL-6 has associated with the activation of multiple cytokine pathways in a pro-inflammatory state and shuffles this essential balance. In the case of obese patients, disruption of lymphoid tissue integrity and alterations in activity and development of leukocytes could lead to the incoordination of adaptive as well as innate immune responses [51]. Efficient differentiation of preadipocytes to mature adipocytes has a fundamental role in hyperplastic fatty tissue expansion and thus, successfully modulates the whole-body glucose and lipid homeostasis in obesity states. Higher expression of ACE2 receptor in AT might function as a SARS-CoV-2 reservoir. Moreover, obesity might cause hyperglycemia by inducing insulin resistance. In the case of T2D patients, in coagulation homeostasis, and innate immunity, the RAGE system act as a critical player, therefore, by targeting this system it is possible to halt the thrombotic manifestations and cytokine storm combined with dysregulated immune responses to SARS-CoV-2 infection. In COVID-19 patients with co-morbidity factors, dysfunction of NALT can impede the development of B cells along with the production of coherent antibodies for clearance of the SARS-CoV-2 virus. Therefore, reinforcement of NALT not only will help us to understand the antigen presentation process but also will shed light on the effects of nasal vaccination by improving the prognosis along with retrieval of olfactory function in COVID-19 [52, 62, 63].
SARS-CoV-2 virus infection affects adversely on the host lungs and intestine. From the primary attack site, they are transmitted through the respiratory tract or digestive tract and precisely infect the host pulmonary epithelial or intestinal epithelial cells. These act as a causative agent to lung and/or intestinal tissue damage and revoke a systemic immune response. After causing a serious injury to the lungs, SARS-CoV-2 can cross the mucosal immune barrier and move through the “gut-lung axis” and affect the intestine, or vice versa. Cross talk between these two mucosal sites of the host cell might take place through blood and/or lymphatic circulation. Persistent excessive inflammatory response, intestinal tissue damage, and/or dysfunctional immune response can cause disorder to the intestinal microbial flora. Although studies have suggested that use of the probiotics, personalized diet, beneficial metabolites, and elimination of harmful bacteria can provide resistance against such adverse effects. With an emerging number of data, it is very evident that the gut microbial flora plays a major role in blood proteomic biomarkers’ prediction and also in determining the severity of COVID-19 in individuals. Activation of mucosal immunity through the mucosal vaccine required detailed understanding of primary mucosal sites, gut microbial flora, cross-talk between “gut-lung axis”, and their effect on different varieties of coronavirus.
Understanding the connectivity across mucosal locations is critical for disease characterization and vaccine development in the following phase. Moreover, efficient and safe mucosal vaccine development depend upon several factors like the type of antigen, administration route, formulation, choice of adjuvant, and appropriate animal model (safety and efficacy evaluation), etc. A systematic and rational approach of vaccine formulations needs a great knowledge on biophysical characterization as well as immunogenicity of antigens, antigen-adjuvants (use of appropriate viral vectors or nano-based one) interactions, detailed assessment of safety and stability (especially thermostability especially for poor countries) measure. Mucosal immunization has the potential to be more effective than the traditional parenteral vaccination in terms of triggering immune resistance in both mucosal and systemic tissue to protect mucosal surfaces from pathogen invasion. The recent intuitiveness in the innate immune response and the highly vaccine-induced adaptive immune response should be considered. In the coming future, the clinical trial of new mucosal vaccines will be in need. So, the improved formulations along with modern and effective delivery technologies will be considered as the main part of the mucosal vaccine development platform [83].
In this review, we summarize the different aspect of mucosal immunization and their probable prospect against the COVID-19 pandemic. Finally, the most reliable and efficient options must be considered further in clinical trials against SARS-CoV-2 variants. It might consist of either monotherapy or combinational therapies consist of an efficient mucosal vaccine, and novel, broad-spectrum, antiviral drugs active against different variants of coronavirus. Plant-based lectins especially glycan-binding lectins have great potential to bind with SARS-CoV-2 S protein and are considered attractive vaccine adjuvant candidates [108]. Further study on the formulation of nasal spray or inhaler with such plant bioactive molecules or engineered antibodies will be a potent therapeutic and prophylactic strategy in the prevention of a larger array of SARS-CoV2 with reduced side effects.
ACE2: angiotensin-converting enzyme 2
ADE: antibody-dependent disease enhancement
APCs: antigen presenting cells
ARDS: acute respiratory distress syndrome
ATs: adipose tissues
BALT: bronchus-associated lymphoid tissue
CK: creatine kinase
CMIS: common mucosal immune system
COVID-19: coronavirus disease 2019
CRP: C-reactive protein
DCs: dendritic cells
E: envelope
GALT: gut-associated lymphoreticular tissues
GI: gastrointestinal
HCoV-OC43: human coronavirus OC43
IFN-γ: interferon-gamma
IL-12: interleukin-12
LRT: lower respiratory tract
MALT: mucosa-associated lymphoid tissue
MERS: Middle East respiratory syndrome
N: nucleocapsid
NALT: nasopharynx-associated lymphoid tissue
NK: natural killer
NRP-1: neuropilin-1
OD: olfactory dysfunction
OPV: oral polio vaccine
pIgA: polymeric immunoglobulin A
PPs: Peyer’s patches
RAGE: receptor for advanced glycation end products
RBD: receptor-binding domain
S: spike
SARS-CoV-2: severe acute respiratory syndrome of coronavirus 2
SC: secretory component
sIgA: secretory immunoglobulin A
T1D: type 1 diabetes
Th1: T helper1
TMPRSS2: transmembrane serine protease 2
TNF-α: tumor necrosis factor-alpha
Treg: regulatory T cell
URT: upper respiratory tract
SKC: conception and design of the study, editing and approval of final version of manuscript. SS: drafted and prepared the manuscript, and drawing figures. MNMM: read and editing the draft of manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2021.
Copyright: © The Author(s) 2021. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Kabeer Haneef ... Zainab Fatima
Eduardo Tosta
Krupanidhi Sreerama
Eknath D. Ahire, Sanjay J. Kshirsagar
Kenneth Lundstrom
Sevilay Hintistan, Hatice Demirağ
Mandeep Garg ... Suruchi Garg
Marileia Chaves Andrade ... Hellen Oliveira Rosa
Devlina Ghosh ... Abhishek Saxena
Yoanna Slabakova ... Tsvetelina Velikova
Balram Ji Omar ... Manju O. Pai
Basista Rabina Sharma, P. Veeranna Ravindra
