Affiliation:
1Medical University-Sofia; Blvd. “Akademik Ivan Evstratiev Geshov” 15, 1431 Sofia, Bulgaria
ORCID: https://orcid.org/0000-0003-3893-9307
Affiliation:
2Department of Pharmacovigilance, Blvd. “Tsarigradsko shoes” 115G, 1784 Sofia, Bulgaria
ORCID: https://orcid.org/0000-0003-4331-5072
Affiliation:
3Department of Clinical Immunology, University Hospital Alexandrovska, Sveti Georgi Sofiyski 1, 1431 Sofia, Bulgaria
4Medical University-Sofia, Sofia, Bulgaria
ORCID: https://orcid.org/0000-0002-7471-7368
Affiliation:
5Department of Clinical Immunology, University Hospital Lozenetz, Kozyak 1 str, 1407 Sofia, Bulgaria
6Sofia University St. Kliment Ohridski, Sofia, Bulgaria
Email: tsvelikova@medfac.mu-sofia.bg
ORCID: https://orcid.org/0000-0002-0593-1272
Explor Immunol. 2022;2:9–24 DOI: https://doi.org/10.37349/ei.2022.00033
Received: September 03, 2021 Accepted: January 04, 2022 Published: February 11, 2022
Academic Editor: Wangxue Chen, National Research Council Canada, Canada
The article belongs to the special issue Vaccine-induced Immune Responses Against SARS-CoV-2 Infections
A few pieces of research exist about the protective titer against severe acute respiratory syndrome (SARS) coronavirus 2 (CoV-2; SARS-CoV-2) in monkeys and humans in which the protection could be shown as dose-dependent. Early studies supposed that higher levels of pre-existing neutralizing antibodies (Nabs) against SARS-CoV-2 can potentially correlate with the protection to consequent infection. The data so far showed that cellular immunity is as essential as the humoral one. If needed, its presence can be beneficial if the titer of immunoglobulins is not optimal. It is also known that the immune response to the vaccine is similar to the one after natural infection with a production of very high naturalization titers antibodies. However, medical community is still unaware of the immunoglobulin titer needed for protection against the virus. The answers to the questions regarding correlates of protection are yet to be discovered. Still, no studies indicate a specific virus-Nab titer, so one can assume a patient is protected from being infected in the future. The evoked immunological response is indeed encouraging, but a future investigation is needed. Nonetheless, it remains a mystery how long the immunity lasts and whether it will be enough to shield the patients in the long run. Therefore, identifying immune protection correlations, including neutralization titer of antibodies and T cell immune response against SARS-CoV-2, could give a clue. Unfortunately, recent studies in the field have been more controversial than concise, and the data available is far from consensus.
The ongoing global coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome (SARS) coronavirus 2 (CoV-2; SARS-CoV-2) has already taken the lives of 5 million people and infected more than 255 million people worldwide [1]. Nevertheless, unfortunately, so far, no one has taken responsibility to pinpoint a specific protective titer of antibodies against SARS-CoV-2 in humans [2]. However, there have been a few papers about the protective titer against SARS-CoV-2 in monkeys (Macaca mulatta) in which the protection is dose-dependent. The study showed that cellular immunity is as essential as the humoral one. If needed, its presence can be beneficial if the titer of immunoglobulins is not optimal [3].
From vaccine trials in 2020, we know that the immune response to the vaccine is similar to the one after natural infection with very high naturalization titers. However, we are still unaware of the immunoglobulin titer needed for protection against the virus. The evoked immunological response is indeed encouraging, but a future investigation is needed [4]. Furthermore, recent articles noted that the correlates of protection are yet to be discovered [5]. Still, no studies indicate a certain virus-neutralizing antibodies (Nabs) titer, so we can assume a patient is protected from being infected in the future. Nonetheless, it remains a mystery how long the immunity lasts and whether it will be enough to shield the patients in the long run [5].
Coronaviruses are one of the leading causes of infections in the animal kingdom. Seven of them are known to affect humans. The CoV family has a lot of similarities between the members. All of them are enveloped RNA viruses and have spikes (S) on their surface. Four out of the seven are the main reason for the so-called “common cold” infections: human CoV 229E (HCoV-229E), HCoV-OC43, HCoV-NL63, and HCoV-HKU1 [6]. They are regularly seen (15%) in the pediatric and adult populations. Severe disease can be caused by other family members like Middle East respiratory syndrome CoV (MERS-CoV), SARS-CoV, and the current SARS-CoV-2, leading to pneumonia, severe complications, and death [6].
A systematic review of Huang et al. [7] focused on public-health interventions and COVID-19 model epidemic scenarios induced by SARS-CoV-2, presuming that the infection leads to an immune response that provides a specific period of protection against subsequent infections or diseases. However, infections and/or vaccinations may impact future transmission and disease severity or may not be protective immunity (if available). The authors reviewed the available scientific literature on CoV antibody immunity, including SARS-CoV-2 and associated SARS-CoV, MERS-CoV, and human endemic CoVs. They examined 2,452 abstracts and 491 handbooks and concluded that a correlation of protection needs to be identified before the known exposure or risk period in which infection or disease outcome is defined [7]. Generally, most long-term investigations have shown that SARS-CoV and MERS-CoV immunoglobulin G (IgG) has decreased with time (usually up to one year). In contrast, others have demonstrated measurable amounts of IgG 3 years after the infection. In addition, the intensity gradient of antibodies varied in kinetics, and more severe symptoms stayed evident after sickness [7].
Furthermore, the challenges of humans with HCoV demonstrate the probable correlated protective effects against infection and diseases in the serum and mucosal immune responses (serum IgG, IgA, Nabs titer and mucosal IgA) [7]. However, a repeated challenge with a single HCoV in human trials shows that people may get HCoV 1 year after the initial challenge, although it may be less severe. Cross-reactivity between alpha and beta CoVs is also possible. While endemic HCoVs seldom generate the cross-reaction of antibodies against emerging HCoVs, SARS-CoV and MERS-CoV activate pre-HCoV-induced antibodies [7].
We have to be aware that variants of concern (VOC) may reduce the efficacy of Nabs in both convalescents and vaccinated people [8]. For example, B.1.1.7 (alpha) reduced neutralization titer by 2.9 times, B.1.351 (beta)-13.3 times, and B.1.617.2 (delta) 2.6 times. Furthermore, reducing neutralization titers above 8 times leads to a 50% decrease in protection threshold by day 90 following COVID-19 [9]. Thus, it would be possible to predict the duration of protection after a previous SARS-CoV-2 infection. However, this hypothesis should be validated further.
Lau et al. [8] suggested that even protection by Nabs against the specific variant that caused the infection (especially after symptomatic disease) is likely to remain for almost 2 years [8]. However, VOC may disrupt this protection and make the individual susceptible to all new variants [10].
Identifying correlates of immune protection, including neutralization titer of antibodies and T cell immune response against SARS-CoV-2, is urgently needed. However, recent studies in the field have been more controversial than concise, and the data available is far from consensus.
When we discuss the correlate titers of protection, we have to start with the existing knowledge on other CoVs. A 2004 study proved that IgG antibodies were still found in the patients’ blood 60 days following SARS-CoV infection. In contrast, IgM levels dropped quickly and were not detectable after 77 days [11]. This research showed a significant drop (at least 5 times) in immunoglobulin titers in 9 months. In addition, asymptomatic patients seemed to have a lesser immune response to the virus, and their antibodies disappeared even faster [10]. Previous studies also found that antibodies against the S1 S protein have a virus-neutralizing effect by blocking the binding to receptors [12].
However, research regarding protective immunity was needed for vaccine development, so an animal model was used. Mice were injected with receptor-binding domain (RBD)–fragment crystallizable region (Fc) of immunoglobulin and boosted consecutively at different periods. The humoral response was explored, extending to a year after immunization. During the study, the mice were rechallenged with the same strain of SARS-CoV. It was found that RBD can be used in the making of subunit vaccines because of the robust protective humoral immunity it provokes [13].
A prospective cohort study with healthcare workers (HCWs) showed that people infected in 2003 demonstrated detectable IgG to CoVs 12 years later [14]. Moreover, the highest immunoglobulin titer was assessed in 2004. During the infection, the ones treated with corticosteroid drugs had lesser humoral response, and the IgG levels were lower. The study concluded that the existence of IgG antibodies against SARS-CoV could protect the survivors from re-infection with the primary or other beta CoVs as well [14].
Another study, while researching nidoviruses and the immunogenicity of SARS-CoV in mice and rabbits, showed potent inhibition of SARS-CoV after immunization with RBD-Fc protein [15]. The observed mean 50%-neutralization titers in antisera were estimated as 1:15,360 [15]. Another remarkable demonstration from the same study was that the titers of antibodies against the S protein needed for 50% inhibition after immunization were 1:7,393 and 1:2,060 in rabbits and mice, respectively [15].
Studies have implied possible immune cross-reactivity between SARS and betacoronavirus HCoV-EMC closely related to other zoonotic CoVs in Hong Kong. Around 2% of the tested individuals had antibody titer against the virus and SARS-CoV ≥ 1:20. The neutralizing immunoglobulins were < 1:10. The researchers detected that 60% of SARS survivors had detectable antibody titers, and 25% had a low HCoV-EMC titer with neutralizing activity [16]. However, further investigation proved that there is no cross-neutralization between the two viruses [17].
Another study performed in the UK proved the variability of neutralizing humoral response in participants. However, most antibodies persisted for a few months after infection [18]. In the first week after infection, all of the patients were negative for Nabs. However, the following week 64% of the participants tested positive. The mean titers were up to 40. Ranging from 1 to 200, all of the patients were positive in week 3. The highest titers were detected in week 4, with an inhibitory concentration of 90 and a mean titer from 28 to 640. In some patients, the humoral immunity endured for more than 200 days after initial symptoms [18].
Several monoclonal antibodies were isolated while searching for a cure during the SARS-CoV epidemic; they had virus-neutralizing activity. However, their concentrations peaked 2 months post-infection; the neutralizing immunoglobulins, on the other hand, remained at a stable concentration (1/128). All of them were IgG1 isotypes [19].
Research made by Gao et al. [20] that showed a robust immune response in monkeys following intramuscular immunization with the adenoviral vector vaccine was crucial for a vaccine against SARS development. Another animal model that used ferrets and chimpanzees examined and proved that adenoviral vaccines evoke a specific and safe immunity needed to protect and prevent pneumonia after being challenged with SARS-CoV and stimulated robust immune responses in macaques [21].
The humoral immune response to SARS-CoV was studied in a 2006 prospective study that took 2 years in China [22]. The serum samples were analyzed with enzyme-linked immunosorbent assay (ELISA), and the titers of neutralizing immunoglobulins were estimated by neutralization assays. Sixty-three participants satisfied the criteria and were included in the survey, and 56 of those gave 3 blood samples. The ones with comorbidities were 9, and 27 were male. Five of them were treated with prednisolone during the illness [22]. IgG was found in all follow-up visits except the last one (11.8% negative results). The maximum titer was assessed at the 4th month (mean IgG reciprocal titer 250). A massive decrease was noted after one year. Nabs were investigated and tested positive in all visits. Again, a peak was evidenced 4 months post-infection (mean Nabs reciprocal titer estimated between 1,000–1,500). Interestingly, sex seems to play an essential role in the duration of humoral response because the decline of immunoglobulins was more rapid in the male participants. Again, there was not a certain titer pointed as protective [22].
Rockx et al. [23] published a paper on monoclonal antibody cross-resistance between mutant strains of SARS-CoV. They demonstrated that the amino acid sequence is crucial for antibody activity, making immune escape impossible [23]. Therefore, when we try to pinpoint the protective titer against SARS-CoV-2, we should consider the type of antibody most effective for neutralizing other variants. Although escape mutants were a problem researched by Rockx et al. [23], in his article, it was noted that monoclonal antibodies were effective in protecting mice from heterologous SARS-CoV variants.
In 2012 there was another CoV outbreak-MERS-CoV, another zoonotic infection that came from camels and was the reason for the death of more than 800 patients in its first appearance in Saudi Arabia [24]. It spread in 27 countries. According to the World Health Organization (WHO), the mortality rate was 35%. In a study conducted with three groups of patients with mild, moderate, and severe MERS infection made in Saudi Arabia 6 years after infection, the serology results showed a correlation between the severity of the disease and the titer of virus-neutralizing titer (VNT) [24]. The median titer of VNT in the tested patients was 45 in the second year, 76 in the fourth, and 42 in the sixth year after infection, respectively. Six years after infection, VNT was detected in 100% of moderate and severe cases and 50% in mild cases [24].
A more recent study with rhesus macaques regarding vaccines against MERS found the protectivetiter 14 days after the vaccine [25]. Neither group was infected with MERS beforehand, and no immunoglobulins were present before vaccination. Thus, the vaccine not only decreased the severity of the disease significantly but limited the virus replication as well. Although the study was not meant to discover the correlates of protection, interestingly enough, it was mentioned that one of the animals was protected despite not having detectable antibodies [25]. Similarly, a clinical study including MERS survivors was carried out in 2017. A few of the patients did not have any detectable immunoglobulins. However, they had a robust CD8+ T-cell response [26].
Callow et al. [27] researched common cold CoVs with low severity. They were able to test the humoral immunity in a group of volunteers. The research team published in the Journal of Hygiene in 1985 demonstrated that IgA was associated with less virus shedding, reduction of symptoms, and protection against re-infection. The local secretory immunoglobulins were high for a few weeks after infection. Interestingly enough, there was proof that a more recent infection meant that the volunteers were less susceptible to the virus. They had little to no symptoms, and the viral shedding period was shortened [27].
HCoVs with low severity have been used in human studies over the years, measuring the antibody response after infection and the protection they guaranteed. An article published in Cambridge University Press displayed volunteers’ immune response to common cold CoV 229E [28]. Although there was still antibody titer detectable one year after infection, re-infections with the same virus occurred. The virus shedding period was shorter, and some resistance was noted. In 3 weeks after inoculation, the immunoglobulins raised significantly. Unfortunately, by the 12th week, the concentration decreased strikingly. The immune response showed individuality and divergence. Most of the volunteers’ titers (especially IgG) returned to normal 1 year later. During the rechallenge, the volunteers from the uninfected group became infected. Furthermore, the majority of participants from the infected group were re-infected but did not progress to a cold. In both groups, the duration of virus shedding was shorter [28].
Article published in 2021 indicated that perhaps the immunity after infection with SARS-CoV-2 is not as short-lived as we believed [29]. Although there was a decrease in immunoglobulins post-infection, plasmatic cells were found in aspirates of bone marrow in 15 of the 19 volunteers who had the infection prior. Furthermore, the antibody responses were tested in 77 patients, from whom the blood samples were consecutively taken at first, the fourth, the seventh, and eleventh month and showed a decline in anti-S IgG antibodies (from 6.3 to 5.3 mean loge-transformed half-maximal dilution) but were still detectable [29]. That decline correlated and depended on the conversion of plasma cells residing in the bone marrow. The plasmablasts (reliant on T-cells) detected in the early stages of recovery secrete antibodies that decline rapidly. However, a more stable titer is observed once they convert into long-lived plasmatic cells in the bone marrow. This study implies that the persistence of antibodies is not the only line of protection. Except for the noted bone marrow plasma cells (BMPCs), there are B-memory cells that quickly adapt in the presence of antigen and are fundamental factors in the long-lasting immune defense against SARS-CoV-2 [29].
From April to June 2020, a study was conducted in the UK. It involved 78 medical practitioners who previously suffered from COVID-19. Exceedingly inconsistent immune response was observed among the volunteers, especially regarding the T cell response and N-specific antibodies. However, their humoral immunity grouped them into two groups—high and low responders (who produce antibodies). The study implied that protection from re-infection with different strains might be possible [30].
Humoral immunity seems to be a key player in the immune arsenal of protection against SARS-CoV-2. Its main targets are the S protein and the internal nucleocapsid antigen. Okba et al. [31] report that in a most polymerase chain reaction (PCR)-confirmed SARS-CoV-2 cases, seroconversion is observed around two weeks after disease onset.
Additionally, a complex multiparametric study by Zohar et al. [32] describes a plethora of exciting associations of antibody specificities, subclasses, and Fc receptor (FcR) binding profiles and the disease outcome. According to the study comparing humoral immune response in different categories of patients (moderate, severe, and patients who eventually died due to COVID-19), IgM and IgA evolved similarly and were a common finding. However, a proper IgG response was observed only in the survivors’ group, which, authors hypothesized, was probably due to appropriate FcR function utilization [32].
The dynamics of immune responses during infection with SARS-CoV-2, similar to vaccine challenge, are presented in Figure 1.
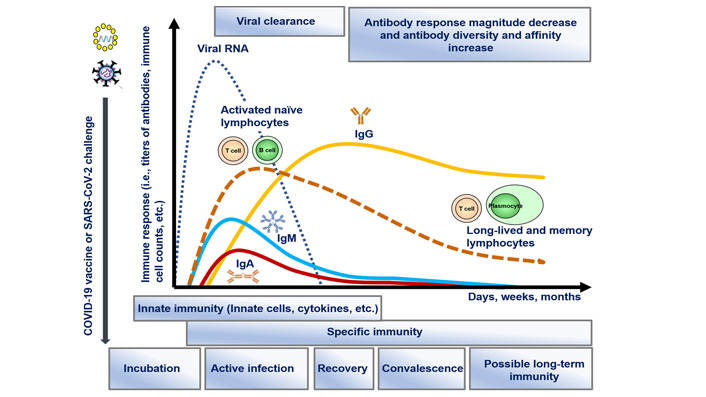
Dynamics of immune response development after encountering SARS-CoV-2, including innate immunity involvement and specific (adaptive) immune mechanisms (activation of T and B cells and production of antibodies), leading to viral clearance possible development of long-lived and memory lymphocytes together with matured and more affinity and diverse antibodies in the post-infection and convalescent period. The same immune response is activated after vaccination (on the figure—RNA vaccine), without the infection periods and viral load in the organism
Nevertheless, significant heterogeneity was observed in patients who survived the infection, including both fast IgG-converters and individuals who converted to IgG slowly but steadily. Other curious findings with the potential for being correlates of protection were steady IgG1 class-switching and early S2-specific antibody elevation [33]. While Nabs are the main factor of protection from subsequent re-infection, antibodies specific for S and RBD with other functionalities such as antibody-dependent complement deposition (ADCD) and antibody-dependent cellular phagocytosis (ADCP) and other Fc-dependent functions seem to play a critical role in viral clearance itself after the infection has already been established [33]. Thus, we can argue that some antibodies (e.g., neutralizing) are protective per se and defend us from subsequent re-infection with SARS-CoV-2 while others are, but more precisely, curative. Their titers may be crucial for the current disease outcome (e.g., antibodies with primary Fc-dependent functions).
On the other hand, autoantibodies with certain specificities generated during the infection (anti-type I interferon antibodies) are rendered as pathogenic or aggravating [33]. Zhu et al. [34] proposed that the kinetics of immune response should be measured to decide on vaccination. However, the official medical consensus on this topic is different.
Furthermore, S2 is the most conserved part of the S-protein, and antibodies to S2 seem to be cross-reactive with other beta CoVs [35]. RBD- and S2-specific FcɣR2B and FcɣR3B binding antibodies started to elevate steadily from the first week of infection in the severe survivors’ group. In contrast, in the individuals whose infection ultimately ended up fatal, their titers were low and elevated poorly. Thus, pre-existing cross-reactive immunity to S2 seems to facilitate the initial viral control, has a positive predictive value [33], and maybe a good strategy for utilization in future pan-β-CoV vaccines [36].
A study regarding protection against symptomatic disease (with B.1.177 and B.1.1.7 variants) after vaccination showed a correlation between serological assay and infection [37]. However, significant variability between participants was noted. The likelihood of infection was lower in patients with a more robust antibody response, but it was absent. This study mentioned a similar correlation in respiratory syncytial viru (RSV), once again emphasizing that categorical titer is still unknown [37].
European Centre for Disease Prevention and Control (ECDC) states that antibody titer is evidential for some protection, but it’s still unknown how long the immunity will last and whether the protection is sufficient for prevention for future infection. We also fully support ECDC’s statement that the presence of antibodies accounts for some protection. However, it is still unknown how long the immunity is going to last and whether or not the titer is sufficient for preventing future SARS-CoV-2 infections [38].
The dynamic in antibody titer after natural infection with SARS-CoV-2 was examined in Wuhan, China, one year after symptoms. Although the highest titer was found 3–4 months after disease onset (above 1:160), there was a slow decline up to the 6th month. Thus, after one year, the Nabs titers were low and unable to counteract the beta variant [39].
That is why amplifying the natural immunity with a single dose can be beneficial for previously infected individuals. However, for the pandemic to stop, we need an unsusceptible population worldwide. If the previous statement is not fulfilled, the prognosis will continue to be pessimistic.
The wide distribution of different types of vaccines around the globe is a crucial step towards ending the SARS-CoV-2 pandemic on an international level. As the number of vaccines administered rises, more data becomes available regarding their safety, efficacy, and tolerability among humans [40, 41].
However, correlates of protection after immunization with SARS-CoV-2 vaccines are yet to be clarified [41]. Recently published research about correlates of protection against SARS-CoV-2 in rhesus macaques provides information about the antibodies’ levels (including virus-Nabs) and the correlation with protection against SARS-CoV-2 [3, 42]. It indicates that relatively low levels of antibodies are required for defense in the upper and low respiratory tracts. The titers of Nab, which are found to be effective in protecting against SARS-CoV-2, are also considered to be reasonably low. Cellular immunity might play an important role when the level of antibodies starts to decline [42]. Therefore, the licensed vaccines against SARS-CoV-2 must stimulate sufficient humoral and cellular immune responses [43].
Vaccines based on two major technologies are the ones currently in most accelerated use. AZD1222 (AstraZeneca) uses a non-replicant chimpanzee adenoviral vector (ChAdOx1), encoding and transports the S-protein [44]. Data about the immune response in the vaccine recipients (during phase 1/2 clinical trial in the UK) show that anti-S IgG antibodies are detected 28 days after the first dose. It is additionally estimated that antibodies titers rise after the second dose. Evidently, Nab is found in 91% of the vaccinees after prime dose and in 100% of them after the boost immunization [41].
Correlates of protection were investigated while researching vector vaccine—Ad26.COV2.S, after immunization and infected later on with SARS-CoV-2, lung tissue was collected 4 days later. Antibody titer and VNT titer were measured. The hamsters before inoculation and were randomized to two groups–infected and noninfected. Viral load beneath 102 tissue culture infectious dose (TCID50)/g was established as a sign of preventing disease. Interestingly enough, the protected group had antibody binding titers above 3.5 using ELISA and virus-Nabs above 64. Perhaps animal models could shed some light on the matter with protective titer [45].
Tostanoski et al. [46] team researched the susceptibility for SARS-CoV-2 in hamsters as well. About 50 Syrian golden hamsters were previously immunized with vector vaccine. The challenge was made in week 4 after vaccination. The ELISA titer at week 2 correlated with the viral load oppositely (negatively) with the virus found in lung tissue on the 4th day after exposition [46].
The efficacy of current vaccines was researched, and antibody titers were shown to be predictive of immunity. Geometric IgG mean titers using ELISA corresponded with the antibodies found in vaccinated individuals after 1 to 4 weeks following immunization. The highest efficacy was correlating with titersabove 1 × 104 binding antibody units (BAU/mL). Geometric mean titers of Nab corresponded with over 95% vaccine efficacy. A titer above 400 was associated with high efficacy, although the effectiveness remained high with even lower antibody titer [47]. Perhaps not everything is as black and white as we hoped for.
The behavior of SARS-CoV-2 immunity was predicted in analysis using data from 7 vaccine studies and one regarding convalescent patients. The prediction was that although the neutralization declined over time and booster doses were needed, the protection from severe infection lasted longer than expected. Furthermore, the highest vaccine protective efficacy corresponded with mean neutralization titer above 4 [10].
An article published in the Journal of Infection showed an intriguing correlation between antibody titer and the possibility of future infection in more than 8,758 HCWs with a median age of 40 and over 50% females [48]. The study was conducted in France starting in July of 2020 and finishing in April 2021. When the authors analyzed the data, they speculate that we should have in mind the epidemic conditions in the county itself in the same timeframe. The study itself did not examine T-cell immunity [48]. The methods used were ELISA and assay in Vero cell with the B.1.160 strain for neutralization titers. A significant advantage in this study was the use of BAU/mL as an international standard unit. This study shows that having Nab above 256 and total antibody measured with ELISA above 1,700 BAU/mL predicted 100% protection against future SARS-CoV-2 infection [48]. However, among the limitations of this study was the lack of data for disease progression in the HCWs with positive tests and how that correlated with the previously existing antibody titer. Perhaps routine antibody screening might be helpful for addressing the need for boosting immunity.
Another vector-based vaccine, Ad26.COV2.S (Janssen), is administered as a single dose. Results from the placebo-controlled 1/2a trial indicate that Nabs against SARS-CoV-2 are found in 90% of vaccinees on day 29 and in 100% of them on the 57th day. There is a strong correlation between the quantity of the S-binding antibodies and the Nab. This is mainly observed in the younger vaccine recipients [41].
The RNA vaccines consist of lipid nanoparticle nucleoside-modified mRNAs that encode and deliver the SARS-CoV-2 S protein to the cell. Following the first dose, I of BNT162b2 (BioNTech/Pfizer) binding IgG antibodies against the S protein are detected. It is noticeable that virus-Nabs are found in most vaccine recipients on day 28, seven days after the second administration [41]. Moderna’s vaccine (mRNA1273) demonstrated immunization after the first dose. The booster dose led to rising quantities of both binding and Nab in all vaccinees during the phase 1 clinical trial [41].
Additionally, one dose of either mRNA vaccine in previously infected with SARS-CoV-2 leads to a prompt immune response. The antibody titers following vaccination are almost the same or even higher than those who are seronegative and received a complete vaccination cycle. This finding should be a subject of further research [43].
The Nab titer post-vaccination is related to the vaccine’s protection efficacy. Considering that, Nab could be labeled as an immune correlate of protection. However, additional binding and functional antibodies also might have a part in the protection process [49–51]. Considering the data, further investigation regarding the protective titers of antibodies against SARS-CoV-2 and their dynamics is required.
Khoury et al. [10] reported on data from currently vaccinated people to analyze the association of in vitro neutralization level with the observable protection SARS-CoV-2 infection [10]. They calculated the neutralization threshold to be 20.2% of average convalescent [95% confidence interval (CI) = 14.4% to 28.4%], at 50% protection against detectable SARS-CoV-2 infection. The estimated level of neutralization necessary to protect against severe infection was substantially lower (3% of average recovery level; 95% CI = 0.7–13%, P = 0.0004) [10]. Modeling of the degradation in the titer of neutralization in the first 250 days after vaccination indicates that the protection from SARS-CoV-2 infection will be significantly decreased while it is important to maintain protection against severe conditions. Neutralization titers for certain variations of SARS-CoV-2 are lowered in relation to the vaccination strain, and our model predicts the link between neutralization and viral variant effectiveness. Here, the authors show that the neutralization level of immune protection is highly predictive and presents an evidentiary model of immune protection against SARS-CoV-2 that helps establish vaccination policies to monitor a future trajectory [10].
Dimeglio et al. [48] also show that immunization increases the immune response of infected HCWs. The most remarkable conclusion is that those HCWs immunized 9–12 months following their SARS-CoV-2 first illness were not re-infected. The rate for infected but unvaccinated HCWs is in favorable contrast.
This is possible owing to the extraordinarily high quantities of Nabs seen in individuals vaccinated with one or two doses. Thus, excellent protection against SARS-CoV-2 re-infection seems to be available in persons who have been infected and vaccinated [48]. However, there were no data of Nab titers in vaccinated HCW but not previously infected. The results now indicated that the Nab titers of the infected persons were lower than those that were infected and vaccinated. However, they found no difference in Nabs and re-infection rates between those HCWs infected before July 2020, who got a single dose of vaccination and HCWs with two doses. This supports the recent advice to administer a single dose of the vaccination to patients who are already infected with SARS-CoV-2, even if the illness is “old” (over 9 months) [48]. Finally, the authors found that antibodies that attach and neutralize S protein continue to present up to a year after infection. Furthermore, their findings show that vaccination for those already sick generates a high level of protection considerably higher than that after infection alone. This is very significant for HCWs that stay exposed to SARS-CoV-2 more than most people [48].
However, at this point of gained knowledge, there is still a debate on the differences and durability of natural versus vaccine-induced antibodies, including their correlates titer of protection [52].
A summary of existing data on the correlates titer of protection is presented in Table 1.
Correlates titer of protection against SARS-CoV-2 proposed by different study groups
| Authors, reference | Type of study | Design | Subjects, participants | Methods for anti-SARS-CoV-2 antibodies detection, units | Proposed correlate titer of protection (units) |
|---|---|---|---|---|---|
| Corbett et al. [53] | Animal model | mRNA-1273 vaccine | Non-human primates | IU/mL | No animal with S-specific IgG > 336 IU/mL had BAL subgenomic RNA (sgRNA) > 10,000 copies/mL, and no animal with S-specific IgG > 645 IU/mL had NS sgRNA > 100,000 copies/swab, so these were chosen as the thresholds for protection |
| Sui et al. [50] | Animal model | VaccineChAdOx1nCov-19Ad26COV2.SmRNA-1273BBIBP-CorVPiCovaccDNA | Non-human primates | Ab-pseudo/live Nab | 10-160/N/A408/1131862/3481N/A/200N/A/10–100N/A/74 |
| Feng et al. [37] | Randomized single-blind vaccine efficacy trial | ChAdOx1 nCoV-19 vaccine | Humans | For 90% VE: anti-S IgG: 899 (369, NC), BAU/mL; Anti-RBD IgG: 2360 (723, NC) BAU/mLNormalized live-virus neutralization assay: 938 (294, NC) NF50Pseudovirus neutralization assay: 140 (43, NC) IU/mLA vaccine efficacy of 80% against symptomatic infection with majority alpha (B.1.1.7) variant of SARS-CoV-2 was achieved with 264 BAU/mL: and 506 BAU/mL for anti-S and anti-RBD antibodies, and 26 IU/mL and 247 normalized neutralization titers (NF50) for pseudovirus and live-virus neutralization, respectively | For 80% VE against primary symptomatic COVID-19, for anti-S IgG, was a level of 264 BAU/mLFor anti-RBD IgG, 80% efficacy was achieved with a median antibody level of 506 BAU/mL (95% CI: 135, NC) |
| Lau et al. [8] | Cohort study | Immunity 90 days after infection with SARS-COV-2/convalescence | Human | N/ANeutralizing antibody is clearly one major correlate of protection, but the titers associated with protection from re-infection are not precisely defined | The threshold for 50% protection from re-infection for PRNT50 and PRNT90 were 1:25.9 (95% CI 1:24.7–1:27.6) and 1:8.9 (95% CI 1:8.6–1:9.4) respectively50% of patients who recover from symptomatic SARS-CoV would be protected from re-infection for 701 days based on PRNT90 titers or 990 days as estimated by PRNT50 titers |
| Meschi et al. [54] | Observational study | Antibody response to an mRNA vaccine | Human, 120 vaccinated HCW and 94 previously infected | N/AQuantitative anti-RBD and virus microneutralization test for robust (≥ 1:80) MNT titerA threshold of 2,000 BAU/mL is highly predictive of strong MNT response in vaccinated individuals and may represent a good surrogate marker of a protective response | Correlation between BAU and MNT titers (MNT titer (≥ 1:80) was reached at 1,814 and 3,564 BAU/mL) |
| Bates et al. [55] | Cohort study | Vaccine/convalescence | Human, vaccinated and previously infected | N/A | Escape of the emerging SARS-CoV-2 variants from neutralization by serum antibodies, which may lead to reduced protection from re-infection or increased risk of vaccine breakthrough |
| Earle et al. [47] | Statistical analysis | Vaccine | Human | N/A | Antibody titers correlated with efficacy across seven different vaccines; where higher titers correlated with higher vaccine efficacy, despite other uncontrolled variables |
| Khoury et al. [10] | Statistical analysis | Vaccine | Human | N/A | Despite the known inconsistencies between studies, a comparison of normalized neutralization levels and vaccine efficacy demonstrated a remarkably strong non-linear relationship between mean neutralization level and the reported protection across different vaccines |
| Dimeglio et al. [48] | Cohort study | Vaccinated/unvaccinated/convalescence | Humans, 8758 HCWs | NAb titer ≥ 256 was associated with full (100%) protectionELISA-concentration of 1,700 BAU/mL and above provided full protection | The thresholds of protection should be confirmed in further studies on other populations.Antibody’s reduced neutralizing capacity against new emerging VOC should be tested |
| van der Lubbeet al. [45] | Animal model | Vaccine | Syrian hamsters | Nab protective titer between 64–128ELISA log10 between 3.5–4 | Hamsters were classified either as infected or protected from SARS-CoV-2, defined as a lung viral load of either above or below 102 TCID50/g, respectively |
| Tostanoski et al. [46] | Animal model | Vaccine | 70 Syrian golden hamsters | RBD-specific binding antibodies were assessed by ELISA | A significant correlation was found between ab titer and lower viral load |
| Yu et al. [50] | Animal model | Vaccine | Rhesus macaques, non-human primates | Log Nab titer | Log Nab titer above 2.0 provided complete protection |
| McMahan et al. [3] | Animal model | Convalescent | Non-human primates (31 rhesus macaques) | NAb titres | NAb titers of approximately 500 fully protected macaques, and titers of approximately 50 partially protected macaques. These titers should be readily achievable by vaccination in humans. These data demonstrate that relatively low NAb titers are sufficient to protect against SARS-CoV-2 in rhesus macaques |
| Haveri et al. [56] | Observational study | Convalescense | Humans | Nab | NAb against the WT virus persisted in 89% and S-IgG in 97% of subjects for at least 13 months after infection. Only 36% had N-IgG by 13 months. The mean S-IgG concentrations declined from 8 to 13 months by less than one-third; N-IgG concentrations declined by two-thirds |
| Xiang et al. [39] | Observational study | Convalescent COVID-19 patients | Humans | Virus neutralizing Ab | After one year, approximately 90% of recovered patients still had detectable SARS-CoV-2-specific IgG antibodies against nucleocapsid antigen and RBD-S. Neutralizing activity was detectable in ~43% of patients with a reduction of virus-neutralizing capacity of 22.6% to VOC |
NC: not computed; BAL: broncho-alveolar lavage; Ab: antobodies; VE: vaccine efficacies; NF50: normalized live-virus neutralization titer; N/A: not applicable; MNT: minimal neutralizing titer; WT: wild type; PRNT50: plaque reduction neutralization at 50% neutralization endpoint; PRNT90: plaque reduction neutralization at 90% neutralization endpoint; NS: non-significant
The authorized SARS-CoV-2 vaccines generate anti-infection neutralization antibodies. However, the underlying protective immune responses remain unknown. T follicular helper (Tfh) cells—a subset of CD4 T cells—are necessary for producing Nabs, and some of them [circulating Tfh (cTfh)] are examined in the blood [57]. Nevertheless, there is not much known about SARS-CoV-2 infection and mounted Tfh responses so far. It is challenging to examine lymphoid tissues in people directly. However, the cTfh or Tfh in the blood is essential for understanding Tfh reactions in germinal centers. SARS-CoV-2-specific cTfh are seen in convalescent people. Boppana et al. [58] investigated cTfh’s reactions in people retrieved from COVID-19 to three critical structural proteins. They show that SARS-CoV-2-specific cTfh numbers correlate with antibody neutralizing responses [58]. Furthermore, cTfh reactions to proteins other than S protein can help Nabs development and delay the establishment of cTfh reactions in the SARS-CoV-2 infection [57].
T-cell immunity will probably play a role in SARS-CoV-2 protection by aiding the generation of Nabs. In 26 convalescent patients, Turner et al. [57]. have longitudinally investigated CD4 T cell responsiveness to SARS-CoV-2 membrane (M), nucleocapsid (N), and S structural proteins. During the first 2 months following the beginning of the illness, at least one CD4 T cell response was mounted by a majority (81%). The cTfh cells [programmed cell death 1 (PD1); CXCR5+PD1+ CD4 T cell] were mountable by 48% in people. SARS-CoV-2-specific cTfh responses were observed across all antibody neutralization and S protein-specific correlation. When reviewed over time, a second convalescence, median 38 days after symptoms, cTfh responses were reported, in particular against M protein, enhanced convalescence antibodies and strong cTfh responses higher than 5%. Three and six months after the symptoms, CD4 T cell reactions decreased but remained at modest magnitudes. These results extend our knowledge of the cTfh-specific antigen response in SARS-CoV-2 infections, showing that cTfh may also help create Nabs, in addition to S, M, and N proteins, that are protein-specific, and that cTfh response formation in SARS-CoV-2 may be delayed. Late recovery lowers the amplitude of SARS-CoV-2-specific cTfh response [57].
Additionally, Turner et al. [57] demonstrated that plasma secretion of IgG and IgA aimed against the S protein peaked one week after the second vaccination and decreased after three weeks. These plasma reactions preceded maximum levels of serum anti-S and Nabs for an early SARS-CoV-2 strain as well as new variations, particularly in patients previously SARS-CoV-2 infected (who produced the most robust serological responses). They have also discovered active germinal centers consisting of B cells producing antibodies against S protein in each participant following vaccination by analyzing fine nose aspirates of draining axillary lymph nodes [57]. High frequencies of S-specific B cells and plasmablasts were sustained in these draining lymph nodes for at least 12 weeks following booster vaccination. Antibodies produced by these B cells mainly targeted the S protein RBD and fewer N-terminal clones or shared epitopes with human beta-CoVs S-protein OC43 or HKU1. These later cross-reactive B-cell clones reveal the origin of B-cell memory. The authors concluded that immunizations based on SARS-CoV-2 mRNA cause a permanent germinal center B reaction that generates substantial humoral immunity [57].
Identifying correlates of protection in support of future vaccine deployment is urgently needed for predictive models of COVID-19 immune protection. On the other hand, it would be necessary to have specific serological testing to allow those deemed immunes to rejoin the workplace when social distance restrictions are lifted. Evidence from prior new HCoVs indicates that cross-reactivity with endemic HCoVs gives little false-positive results. Antibody titers may not necessarily translate into immunity, however. Still, there is a knowledge gap in protective correlating and durability of immunity against SARS-CoV-2.
BAU/mL: binding antibody units
CI: confidence interval
CoV-2: coronavirus 2
COVID-19: coronavirus disease 2019
cTfh: circulating T follicular helper
ELISA: enzyme-linked immunosorbent assay
Fc: fragment crystallizable region
HCoV: human coronavirus
HCWs: healthcare workers
IgG: immunoglobulin G
M: membrane
MERS: Middle East respiratory syndrome
Nabs: neutralizing antibodies
RBD: receptor-binding domain
S: spikes
SARS: severe acute respiratory syndrome
Tfh: T follicular helper
VNT: virus-neutralizing titer
VOC: variants of concern
YS and TV contributed conception and design of the study; DG and NI searched and organized the existing papers on the topic in a database; YS organized the table; TV crafted the figure; YS wrote the first draft of the manuscript; DG, NI, and TV wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Kabeer Haneef ... Zainab Fatima
Eduardo Tosta
Krupanidhi Sreerama
Eknath D. Ahire, Sanjay J. Kshirsagar
Kenneth Lundstrom
Sevilay Hintistan, Hatice Demirağ
Mandeep Garg ... Suruchi Garg
Swapan K. Chatterjee ... Maria Nilda M. Munoz
Marileia Chaves Andrade ... Hellen Oliveira Rosa
Devlina Ghosh ... Abhishek Saxena
Balram Ji Omar ... Manju O. Pai
Basista Rabina Sharma, P. Veeranna Ravindra
