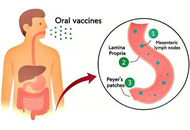


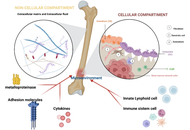

For decades, vaccines have been a key tool against microbial infections. However, the high cost of production and purification renders vaccines largely inaccessible to many developing countries. The limitations of conventional vaccines can be overcome by edible vaccines. To produce an oral vaccine, favourable vectors, such as plants and probiotics, are used. Recent studies have revealed the immunomodulatory effects of probiotics. To improve the efficacy of these vaccines, several adjuvant approaches have been employed. Postbiotics can be used as promising therapy for preventing infections and enhancing the host immune system due to their unique biochemical and microbial-derived properties. In this review, we discuss the feasibility of postbiotics as adjuvants for oral vaccines, highlighting their mechanisms of action, safety profile, and potential to enhance both mucosal and systemic immune responses.
For decades, vaccines have been a key tool against microbial infections. However, the high cost of production and purification renders vaccines largely inaccessible to many developing countries. The limitations of conventional vaccines can be overcome by edible vaccines. To produce an oral vaccine, favourable vectors, such as plants and probiotics, are used. Recent studies have revealed the immunomodulatory effects of probiotics. To improve the efficacy of these vaccines, several adjuvant approaches have been employed. Postbiotics can be used as promising therapy for preventing infections and enhancing the host immune system due to their unique biochemical and microbial-derived properties. In this review, we discuss the feasibility of postbiotics as adjuvants for oral vaccines, highlighting their mechanisms of action, safety profile, and potential to enhance both mucosal and systemic immune responses.
DOI: https://doi.org/10.37349/ei.2026.1003237

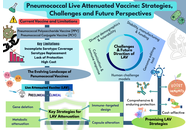
Pneumococcal disease remains a major global health challenge despite the availability of polysaccharide and conjugate vaccines. Although these platforms have reduced invasive disease, their limitations, such as poor immunogenicity in infants, lack of durable protection, and restricted coverage, highlight the need to explore innovative preventive strategies. Next-generation vaccines that provide comprehensive protection, sustained immunity, and cost-effectiveness are urgently required. Live attenuated vaccines (LAVs) represent a promising frontier in this effort, with recent advances focused on overcoming developmental and safety challenges. This review highlights the evolving pneumococcal vaccine landscape, with emphasis on LAV strategies. We summarize the strengths and shortcomings of current vaccines, examine recent advances in LAV development, including key aspects of attenuation, immune-protective mechanisms, and delivery approaches. LAVs demonstrate potential to induce balanced mucosal, humoral, and cellular immunity, addressing critical gaps left by existing platforms. Key challenges related to genetic stability, safety, and translational applicability are also discussed. By synthesizing established knowledge and highlighting advancements, this review underscores the promise of LAVs as next-generation candidates that can provide broader, longer-lasting protection against pneumococcal disease.
Pneumococcal disease remains a major global health challenge despite the availability of polysaccharide and conjugate vaccines. Although these platforms have reduced invasive disease, their limitations, such as poor immunogenicity in infants, lack of durable protection, and restricted coverage, highlight the need to explore innovative preventive strategies. Next-generation vaccines that provide comprehensive protection, sustained immunity, and cost-effectiveness are urgently required. Live attenuated vaccines (LAVs) represent a promising frontier in this effort, with recent advances focused on overcoming developmental and safety challenges. This review highlights the evolving pneumococcal vaccine landscape, with emphasis on LAV strategies. We summarize the strengths and shortcomings of current vaccines, examine recent advances in LAV development, including key aspects of attenuation, immune-protective mechanisms, and delivery approaches. LAVs demonstrate potential to induce balanced mucosal, humoral, and cellular immunity, addressing critical gaps left by existing platforms. Key challenges related to genetic stability, safety, and translational applicability are also discussed. By synthesizing established knowledge and highlighting advancements, this review underscores the promise of LAVs as next-generation candidates that can provide broader, longer-lasting protection against pneumococcal disease.
DOI: https://doi.org/10.37349/ei.2026.1003236
This article belongs to the special issue Novel Vaccines development for Emerging, Acute, and Re-emerging Infectious Diseases

Pulsed radiofrequency (PRF) has emerged as a promising and versatile technology in pain management and immunological modulation. PRFʼs effects extend beyond pain modulation, demonstrating the ability to regulate inflammatory processes through cytokine modulation, reduction of microglial hyperactivity, and promotion of autophagy. These mechanisms position PRF as a potential therapeutic tool not only for neuropathic and musculoskeletal pain but also for conditions associated with neuroinflammation and immune dysfunction, including chronic inflammatory and degenerative diseases. Clinically, PRF has demonstrated potential in alleviating neuropathic pain in several clinical studies—including a limited number of small RCTs. However, most of the available evidence remains of low methodological quality, with many studies being observational, retrospective, or underpowered. Moreover, the lack of standardized protocols remains a barrier to its broader adoption. Establishing evidence-based guidelines and enhancing practitioner expertise are critical to ensuring consistent and optimal patient outcomes. Future research should focus on optimizing PRF technical parameters, elucidating molecular mechanisms, and expanding its clinical applications. Integrating PRF with emerging therapies, such as orthobiologics, biological drugs, and electrical stimulation, may further enhance its efficacy. Moreover, advancements in predictive biomarkers and device technologies hold promise for personalized treatments, improving the precision and effectiveness of PRF interventions. This narrative review explores the primary clinical applications, underlying biological mechanisms, and potential future directions of PRF, emphasizing its ability to address complex therapeutic challenges.
Pulsed radiofrequency (PRF) has emerged as a promising and versatile technology in pain management and immunological modulation. PRFʼs effects extend beyond pain modulation, demonstrating the ability to regulate inflammatory processes through cytokine modulation, reduction of microglial hyperactivity, and promotion of autophagy. These mechanisms position PRF as a potential therapeutic tool not only for neuropathic and musculoskeletal pain but also for conditions associated with neuroinflammation and immune dysfunction, including chronic inflammatory and degenerative diseases. Clinically, PRF has demonstrated potential in alleviating neuropathic pain in several clinical studies—including a limited number of small RCTs. However, most of the available evidence remains of low methodological quality, with many studies being observational, retrospective, or underpowered. Moreover, the lack of standardized protocols remains a barrier to its broader adoption. Establishing evidence-based guidelines and enhancing practitioner expertise are critical to ensuring consistent and optimal patient outcomes. Future research should focus on optimizing PRF technical parameters, elucidating molecular mechanisms, and expanding its clinical applications. Integrating PRF with emerging therapies, such as orthobiologics, biological drugs, and electrical stimulation, may further enhance its efficacy. Moreover, advancements in predictive biomarkers and device technologies hold promise for personalized treatments, improving the precision and effectiveness of PRF interventions. This narrative review explores the primary clinical applications, underlying biological mechanisms, and potential future directions of PRF, emphasizing its ability to address complex therapeutic challenges.
DOI: https://doi.org/10.37349/ei.2026.1003235

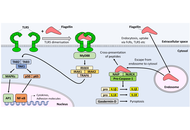
Vaccines can be highly safe and effective tools for disease prevention. However, improvements in the areas of cost, ease of manufacture, distribution, and administration are sought in the next generation of vaccine platforms. A promising candidate is the recombinant flagellin fusion protein platform, which comprises a protein antigen of interest genetically fused to the bacterial protein flagellin. As flagellin stimulates two distinct pattern recognition receptors of the human innate immune system (Toll-like receptor 5 and nucleotide-binding and oligomerization domain-like receptor family apoptosis inhibitory protein) and contains helper T-cell epitopes, it is capable of serving as both a carrier and an adjuvant for the target antigen. Studies in animal models and human clinical trials have shown that flagellin fusion proteins can induce diverse humoral (including various subtypes of IgG), mucosal (including secreted IgA), and cell-mediated (TH1 and TH2 CD4+ helper T-cell and CD8+ cytotoxic T-cell) responses to the covalently linked antigen. Such fusions are also capable of eliciting protective immunity in diverse experimental models of infection and cancer. They are effective via numerous routes of administration, including intranasal delivery, without the requirement for adjuvant or complex delivery vehicles. This review aims to cover recent progress in the investigation of flagellin fusion proteins for their potential to stimulate humoral and cellular immune responses to partner antigens, and their prospects for the prevention or treatment of viral infections and cancer.
Vaccines can be highly safe and effective tools for disease prevention. However, improvements in the areas of cost, ease of manufacture, distribution, and administration are sought in the next generation of vaccine platforms. A promising candidate is the recombinant flagellin fusion protein platform, which comprises a protein antigen of interest genetically fused to the bacterial protein flagellin. As flagellin stimulates two distinct pattern recognition receptors of the human innate immune system (Toll-like receptor 5 and nucleotide-binding and oligomerization domain-like receptor family apoptosis inhibitory protein) and contains helper T-cell epitopes, it is capable of serving as both a carrier and an adjuvant for the target antigen. Studies in animal models and human clinical trials have shown that flagellin fusion proteins can induce diverse humoral (including various subtypes of IgG), mucosal (including secreted IgA), and cell-mediated (TH1 and TH2 CD4+ helper T-cell and CD8+ cytotoxic T-cell) responses to the covalently linked antigen. Such fusions are also capable of eliciting protective immunity in diverse experimental models of infection and cancer. They are effective via numerous routes of administration, including intranasal delivery, without the requirement for adjuvant or complex delivery vehicles. This review aims to cover recent progress in the investigation of flagellin fusion proteins for their potential to stimulate humoral and cellular immune responses to partner antigens, and their prospects for the prevention or treatment of viral infections and cancer.
DOI: https://doi.org/10.37349/ei.2026.1003234
This article belongs to the special issue Novel Vaccines development for Emerging, Acute, and Re-emerging Infectious Diseases

Aim:
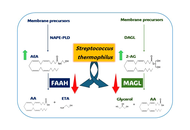
The benefit of topical application of probiotics on pain and itching associated with skin disorders has become an increasingly intriguing topic in recent years. These effects are mainly associated with the anti-inflammatory activity of probiotics. Given the crucial role of the endocannabinoid system (ECS) in skin pathophysiology, here, the ability of Streptococcus thermophilus was evaluated, in comparison with Lactobacillus acidophilus, to inhibit two enzymes involved in endocannabinoid (eCB) degradation: fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL).
Methods:
Bacterial lysates were obtained from both probiotics. FAAH and MAGL activities were assayed using fluorometric and colorimetric methods. The effect of probiotic lysates on FAAH and MAGL activities was also evaluated on human keratinocytes stimulated with lipopolysaccharide (LPS).
Results:
S. thermophilus inhibited both FAAH and MAGL, although to varying extents. In comparison, L. acidophilus had a minimal effect on FAAH and did not influence MAGL activity.
Conclusions:
Although preliminary, our findings suggest that S. thermophilus may exert both potential analgesic and anti-inflammatory effects by modulating the ECS and reducing the degradation of EC, known to play a key role in immune regulation and inflammation. Results presented confirm the selective actions of probiotics and propose a novel mechanism that may contribute to the beneficial effects of S. thermophilus in alleviating signs and symptoms associated with inflammatory skin conditions. Our evidence shows significant inhibitory activity of S. thermophilus on FAAH and MAGL activity, suggesting its ability to influence skin conditions by modulating ECS and preventing the eCB degradation.
Aim:
The benefit of topical application of probiotics on pain and itching associated with skin disorders has become an increasingly intriguing topic in recent years. These effects are mainly associated with the anti-inflammatory activity of probiotics. Given the crucial role of the endocannabinoid system (ECS) in skin pathophysiology, here, the ability of Streptococcus thermophilus was evaluated, in comparison with Lactobacillus acidophilus, to inhibit two enzymes involved in endocannabinoid (eCB) degradation: fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL).
Methods:
Bacterial lysates were obtained from both probiotics. FAAH and MAGL activities were assayed using fluorometric and colorimetric methods. The effect of probiotic lysates on FAAH and MAGL activities was also evaluated on human keratinocytes stimulated with lipopolysaccharide (LPS).
Results:
S. thermophilus inhibited both FAAH and MAGL, although to varying extents. In comparison, L. acidophilus had a minimal effect on FAAH and did not influence MAGL activity.
Conclusions:
Although preliminary, our findings suggest that S. thermophilus may exert both potential analgesic and anti-inflammatory effects by modulating the ECS and reducing the degradation of EC, known to play a key role in immune regulation and inflammation. Results presented confirm the selective actions of probiotics and propose a novel mechanism that may contribute to the beneficial effects of S. thermophilus in alleviating signs and symptoms associated with inflammatory skin conditions. Our evidence shows significant inhibitory activity of S. thermophilus on FAAH and MAGL activity, suggesting its ability to influence skin conditions by modulating ECS and preventing the eCB degradation.
DOI: https://doi.org/10.37349/ei.2025.1003233
This article belongs to the special issue Immunology and Pain

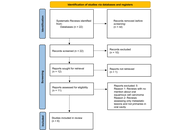
Inflammatory bowel disease (IBD), consisting of Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic inflammatory condition of the gastrointestinal tract with significant clinical impact, leading to debilitating symptoms, impaired quality of life, and an increased risk of complications such as colorectal cancer. This review provides a comprehensive overview of current and emerging therapeutic strategies for IBD. We conducted a narrative review to explore therapeutic advances in IBD treatment, focusing on mechanisms of action, clinical development, and current therapeutic challenges. We analyzed existing knowledge on clinical drug development for IBD, up to July 2025. Our search encompassed databases including PubMed, ClinicalTrials.gov, and Google Scholar, using keywords such as “Inflammatory bowel disease”, “Crohn’s disease”, “Ulcerative colitis”, “therapeutics”, and relevant drug names. We delve into key progress in approved drugs in recent years, including biologic and targeted small molecule therapies, which have advanced the treatment paradigms by offering more precise targeting of inflammatory pathways. This review also covers investigational drugs in clinical development, including biologics and small molecules against novel molecular targets, cell and gene therapies, precision medicine approaches, and microbiome-based interventions. Those novel therapies could potentially address unmet medical needs by achieving deeper and more durable responses, inducing remission, preventing disease progression, and ultimately improving long-term patient outcomes. This review summarizes the latest progress in IBD treatment, outlines the advantages, pitfalls, and research prospects of various drugs and therapies, aiming to provide a foundational understanding for both clinical decision-making and future IBD research.
Inflammatory bowel disease (IBD), consisting of Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic inflammatory condition of the gastrointestinal tract with significant clinical impact, leading to debilitating symptoms, impaired quality of life, and an increased risk of complications such as colorectal cancer. This review provides a comprehensive overview of current and emerging therapeutic strategies for IBD. We conducted a narrative review to explore therapeutic advances in IBD treatment, focusing on mechanisms of action, clinical development, and current therapeutic challenges. We analyzed existing knowledge on clinical drug development for IBD, up to July 2025. Our search encompassed databases including PubMed, ClinicalTrials.gov, and Google Scholar, using keywords such as “Inflammatory bowel disease”, “Crohn’s disease”, “Ulcerative colitis”, “therapeutics”, and relevant drug names. We delve into key progress in approved drugs in recent years, including biologic and targeted small molecule therapies, which have advanced the treatment paradigms by offering more precise targeting of inflammatory pathways. This review also covers investigational drugs in clinical development, including biologics and small molecules against novel molecular targets, cell and gene therapies, precision medicine approaches, and microbiome-based interventions. Those novel therapies could potentially address unmet medical needs by achieving deeper and more durable responses, inducing remission, preventing disease progression, and ultimately improving long-term patient outcomes. This review summarizes the latest progress in IBD treatment, outlines the advantages, pitfalls, and research prospects of various drugs and therapies, aiming to provide a foundational understanding for both clinical decision-making and future IBD research.
DOI: https://doi.org/10.37349/ei.2025.1003232
This article belongs to the special issue Advances in Cellular and Molecular Treatment of Autoimmune Diseases

Aim:
To determine whether PepTivator® melanoma-associated antigen-A3 (MAGE-A3) primes early T-cell activation and memory skewing in human peripheral blood mononuclear cells (PBMCs).
Methods:
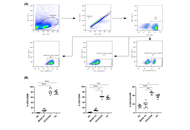
PBMCs from 10 donors were stimulated with MAGE-A3 (manufacturer-recommended dose), negative control (NC), or CD3/CD28 and CytoStim™ (PC: positive control). Activation [CD69, CD25, HLA-DR (human leukocyte antigen-DR isotype)], proliferation, cytokines [24 h; GM-CSF (granulocyte-macrophage colony-stimulating factor), IFN-γ (interferon-gamma), IL-2 (interleukin-2), TNF-α (tumor necrosis factor-alpha)], and memory phenotypes (CD45RO/CD27 at days 0/7/14) were quantified by flow cytometry and MACSPlex. Paired statistics used repeated-measures models with Šidák correction; cytokines were analyzed on log10 scale.
Results:
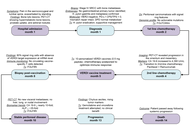
MAGE-A3 significantly increased early activation (CD69 ↑, CD25 ↑) and modestly increased proliferation, with selective IL-2/TNF-α rise and minimal IFN-γ and modest HLA-DR. Across two weeks, 6/10 donors showed increased central memory T cell (TCM)/effector memory T cell (TEM) with a corresponding decline in naïve cells relative to NC. Variability across donors was evident.
Conclusions:
MAGE-A3 primes partial activation and memory skewing of human T cells in vitro, suggesting utility as a component antigen that likely benefits from professional antigen-presenting cell (APC) presentation and/or costimulation. We discuss limitations (single dose, in vitro context, donor variability) and implications for future dose-response, HLA-stratified, and APC-supported studies.
Aim:
To determine whether PepTivator® melanoma-associated antigen-A3 (MAGE-A3) primes early T-cell activation and memory skewing in human peripheral blood mononuclear cells (PBMCs).
Methods:
PBMCs from 10 donors were stimulated with MAGE-A3 (manufacturer-recommended dose), negative control (NC), or CD3/CD28 and CytoStim™ (PC: positive control). Activation [CD69, CD25, HLA-DR (human leukocyte antigen-DR isotype)], proliferation, cytokines [24 h; GM-CSF (granulocyte-macrophage colony-stimulating factor), IFN-γ (interferon-gamma), IL-2 (interleukin-2), TNF-α (tumor necrosis factor-alpha)], and memory phenotypes (CD45RO/CD27 at days 0/7/14) were quantified by flow cytometry and MACSPlex. Paired statistics used repeated-measures models with Šidák correction; cytokines were analyzed on log10 scale.
Results:
MAGE-A3 significantly increased early activation (CD69 ↑, CD25 ↑) and modestly increased proliferation, with selective IL-2/TNF-α rise and minimal IFN-γ and modest HLA-DR. Across two weeks, 6/10 donors showed increased central memory T cell (TCM)/effector memory T cell (TEM) with a corresponding decline in naïve cells relative to NC. Variability across donors was evident.
Conclusions:
MAGE-A3 primes partial activation and memory skewing of human T cells in vitro, suggesting utility as a component antigen that likely benefits from professional antigen-presenting cell (APC) presentation and/or costimulation. We discuss limitations (single dose, in vitro context, donor variability) and implications for future dose-response, HLA-stratified, and APC-supported studies.
DOI: https://doi.org/10.37349/ei.2025.1003231

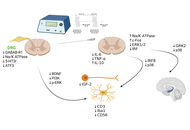
Aim:
Develop A/H1N1pdm09-based live attenuated influenza vaccines (LAIVs) presenting chimeric hemagglutinin (HA) fused to fragments of Streptococcus pneumoniae (PspA, Spr1875) or S. agalactiae (ScaAB) to elicit combined anti-influenza and anti-bacterial immunity.
Methods:
Recombinant LAIVs were generated by reverse genetics. Replicative fitness was measured in embryonated chicken eggs (CE; log10 EID50/0.1 mL, n = 5 per delution) and MDCK cells (log10 TCID50/mL, n = 5). BALB/c mice (n = 20 per group; serology n = 6 per group; lung titers n = 5 per group) received intranasal 106 EID50. Systemic IgG and mucosal IgA to influenza and to the recombinant pneumococcal peptide were quantified by ELISA (GMT ± SD). Early cytokine responses were profiled in THP-1 cells.
Results:
All recombinant strains replicated in CE at 33°C but were temperature-sensitive at 39°C. H1-ScaAB retained relatively high replication and exhibited a cold-adapted phenotype despite a large N-terminal insert. In MDCK cells, H1-PspA showed significantly reduced replication compared with the parental LAIV. In mouse lungs, replication on day 3 post-immunization was significantly lower for H1-ScaAB and H1-PspA compared with the parental LAIV strain (p < 0.05). The parental LAIV induced robust systemic anti-influenza IgG and, uniquely for H1-ScaAB, significant mucosal anti-influenza IgA (p < 0.05). H1-Spr generated stronger antibody responses to the inserted pneumococcal peptide (p < 0.05). THP-1 assays revealed construct-specific cytokine patterns (unmodified H1N1: highest IFN-α; H1-Spr: elevated IL-6; H1-ScaAB: greatest MCP-1).
Conclusions:
Multiple A/H1N1pdm09-based recombinant LAIVs with chimeric HA can replicate in eggs and murine respiratory tract and induce dual influenza/pneumococcal antigen responses. Expanded biophysical validation, functional antibody assays and challenge studies are needed to optimize insert design without compromising viral fitness.
Aim:
Develop A/H1N1pdm09-based live attenuated influenza vaccines (LAIVs) presenting chimeric hemagglutinin (HA) fused to fragments of Streptococcus pneumoniae (PspA, Spr1875) or S. agalactiae (ScaAB) to elicit combined anti-influenza and anti-bacterial immunity.
Methods:
Recombinant LAIVs were generated by reverse genetics. Replicative fitness was measured in embryonated chicken eggs (CE; log10 EID50/0.1 mL, n = 5 per delution) and MDCK cells (log10 TCID50/mL, n = 5). BALB/c mice (n = 20 per group; serology n = 6 per group; lung titers n = 5 per group) received intranasal 106 EID50. Systemic IgG and mucosal IgA to influenza and to the recombinant pneumococcal peptide were quantified by ELISA (GMT ± SD). Early cytokine responses were profiled in THP-1 cells.
Results:
All recombinant strains replicated in CE at 33°C but were temperature-sensitive at 39°C. H1-ScaAB retained relatively high replication and exhibited a cold-adapted phenotype despite a large N-terminal insert. In MDCK cells, H1-PspA showed significantly reduced replication compared with the parental LAIV. In mouse lungs, replication on day 3 post-immunization was significantly lower for H1-ScaAB and H1-PspA compared with the parental LAIV strain (p < 0.05). The parental LAIV induced robust systemic anti-influenza IgG and, uniquely for H1-ScaAB, significant mucosal anti-influenza IgA (p < 0.05). H1-Spr generated stronger antibody responses to the inserted pneumococcal peptide (p < 0.05). THP-1 assays revealed construct-specific cytokine patterns (unmodified H1N1: highest IFN-α; H1-Spr: elevated IL-6; H1-ScaAB: greatest MCP-1).
Conclusions:
Multiple A/H1N1pdm09-based recombinant LAIVs with chimeric HA can replicate in eggs and murine respiratory tract and induce dual influenza/pneumococcal antigen responses. Expanded biophysical validation, functional antibody assays and challenge studies are needed to optimize insert design without compromising viral fitness.
DOI: https://doi.org/10.37349/ei.2025.1003230
This article belongs to the special issue Old and New Paradigms in Viral Vaccinology

Killer immunoglobulin-like receptors (KIRs) and human leukocyte antigen (HLA) molecules play an essential role in regulating immune responses against hepatitis B virus (HBV) and hepatitis C virus (HCV) infections. HLA-KIRs interactions are crucial for activating and inhibiting the natural killer (NK) cell system through a modulation that shapes these cells to kill infected cells and release cytokines. Regulation underlies the anti-viral function of the NK cell and profoundly affects viral clearance, immune evasion, and the course of disease. Activating KIRs such as KIR2DS1 and KIR3DS1 cooperate with specific HLA ligands in boosting NK cell responses against the virus, thereby facilitating viral elimination. In contrast, inhibitory KIRs like KIR2DL1 and KIR3DL1 bind to HLA-C2 and HLA-Bw4, respectively, imposing a dampening influence on NK cell activation, which allows the virus to persist and progress to chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC). These variations in KIRs and HLA genes will also affect an individual’s susceptibility to infections, disease severity, and their response to antiviral therapies. Observation of the role of KIRs and their interaction with HLA at the immunogenetic level provides valuable insight into host-virus dynamics and opens up many therapeutic avenues. Targeting immunotherapies toward NK cell pathways and developing personalized medicine may boost antiviral immune responses and improve treatment outcomes in chronic viral hepatitis patients. This review recognizes HLA-KIRs interactions as potent biomarkers for disease progression and determining treatment strategies.
Killer immunoglobulin-like receptors (KIRs) and human leukocyte antigen (HLA) molecules play an essential role in regulating immune responses against hepatitis B virus (HBV) and hepatitis C virus (HCV) infections. HLA-KIRs interactions are crucial for activating and inhibiting the natural killer (NK) cell system through a modulation that shapes these cells to kill infected cells and release cytokines. Regulation underlies the anti-viral function of the NK cell and profoundly affects viral clearance, immune evasion, and the course of disease. Activating KIRs such as KIR2DS1 and KIR3DS1 cooperate with specific HLA ligands in boosting NK cell responses against the virus, thereby facilitating viral elimination. In contrast, inhibitory KIRs like KIR2DL1 and KIR3DL1 bind to HLA-C2 and HLA-Bw4, respectively, imposing a dampening influence on NK cell activation, which allows the virus to persist and progress to chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC). These variations in KIRs and HLA genes will also affect an individual’s susceptibility to infections, disease severity, and their response to antiviral therapies. Observation of the role of KIRs and their interaction with HLA at the immunogenetic level provides valuable insight into host-virus dynamics and opens up many therapeutic avenues. Targeting immunotherapies toward NK cell pathways and developing personalized medicine may boost antiviral immune responses and improve treatment outcomes in chronic viral hepatitis patients. This review recognizes HLA-KIRs interactions as potent biomarkers for disease progression and determining treatment strategies.
DOI: https://doi.org/10.37349/ei.2025.1003229
This article belongs to the special issue Immunogenetics of Chronic Illnesses

Tumor-infiltrating lymphocytes (TILs) play a critical role in the ability of the immune system to combat cancer, offering a foundation for personalized immunotherapies. However, the effectiveness of TILs is often reduced by problems like becoming less active, the tumor making the immune system weaker, and not lasting long in the tumor environment. Recent advancements in single-cell technologies, including single-cell RNA sequencing (scRNA-seq), single-cell T-cell receptor sequencing (scTCR-seq), and mass cytometry (CyTOF), have revolutionized our understanding of TIL heterogeneity and dynamics. These tools offer new perspectives on the diverse phenotypes, functional states, and spatial organization of TILs, enabling the identification of key exhaustion markers, regulatory pathways, and neoantigen-specific clones. Concurrently, genetic reprogramming strategies have emerged to address TIL limitations by reversing exhaustion, enhancing metabolic resilience, and improving persistence in vivo. This review explores the synergistic integration of single-cell technologies and genetic engineering in refining TIL-based therapies. We talk about how spatial transcriptomics can help us understand how TILs work in different areas of the body and how changing their epigenetics can help them become more effective at fighting cancer. Additionally, we highlight emerging approaches to overcome immunosuppressive barriers in the tumor microenvironment (TME), including targeting regulatory immune cells, neutralizing suppressive cytokines, and enhancing antigen presentation. Together, these strategies promise to unlock the full therapeutic potential of TILs, paving the way for more effective and durable cancer immunotherapy.
Tumor-infiltrating lymphocytes (TILs) play a critical role in the ability of the immune system to combat cancer, offering a foundation for personalized immunotherapies. However, the effectiveness of TILs is often reduced by problems like becoming less active, the tumor making the immune system weaker, and not lasting long in the tumor environment. Recent advancements in single-cell technologies, including single-cell RNA sequencing (scRNA-seq), single-cell T-cell receptor sequencing (scTCR-seq), and mass cytometry (CyTOF), have revolutionized our understanding of TIL heterogeneity and dynamics. These tools offer new perspectives on the diverse phenotypes, functional states, and spatial organization of TILs, enabling the identification of key exhaustion markers, regulatory pathways, and neoantigen-specific clones. Concurrently, genetic reprogramming strategies have emerged to address TIL limitations by reversing exhaustion, enhancing metabolic resilience, and improving persistence in vivo. This review explores the synergistic integration of single-cell technologies and genetic engineering in refining TIL-based therapies. We talk about how spatial transcriptomics can help us understand how TILs work in different areas of the body and how changing their epigenetics can help them become more effective at fighting cancer. Additionally, we highlight emerging approaches to overcome immunosuppressive barriers in the tumor microenvironment (TME), including targeting regulatory immune cells, neutralizing suppressive cytokines, and enhancing antigen presentation. Together, these strategies promise to unlock the full therapeutic potential of TILs, paving the way for more effective and durable cancer immunotherapy.
DOI: https://doi.org/10.37349/ei.2025.1003227
This article belongs to the special issue Advances and Novel Insights into Immunoinformatics

Innate lymphoid cells are lymphocytes that are neither T cells nor B cells. They are relatively rare in lymphoid tissues and peripheral blood and are distinguished by their absence of an adaptive antigen receptor. In the present study, we describe the mechanisms underlying the generation of the various cell populations and highlight the functional importance of their plasticity. These cells are indeed capable of transdifferentiating from one type to another. This adds complexity to their functional program, and this feature appears to be crucial for adapting and modulating immune responses under different conditions. These lymphoid cells are of great hematological interest due to their pathophysiological and therapeutic role in many onco-hematological pathologies such as acute myeloid leukemia, multiple myeloma, and several types of lymphomas. In hematological disorders, innate lymphoid cells may exert differential effects on the pathogenesis of hematologic malignancies. Furthermore, within the same disease, certain cell populations have been shown to play a protective role in antitumor immune responses, whereas others appear to suppress these responses. This review aims to provide an integrated description of innate lymphoid cells, their alterations in hematological malignancies, and potential preventive strategies, by proposing new specific targets for correcting anomalies. We also discuss the use of innate lymphoid cells as new therapies by applying chimeric antigen receptor-modified natural killer cells. We examine the current knowledge and outline future perspectives.
Innate lymphoid cells are lymphocytes that are neither T cells nor B cells. They are relatively rare in lymphoid tissues and peripheral blood and are distinguished by their absence of an adaptive antigen receptor. In the present study, we describe the mechanisms underlying the generation of the various cell populations and highlight the functional importance of their plasticity. These cells are indeed capable of transdifferentiating from one type to another. This adds complexity to their functional program, and this feature appears to be crucial for adapting and modulating immune responses under different conditions. These lymphoid cells are of great hematological interest due to their pathophysiological and therapeutic role in many onco-hematological pathologies such as acute myeloid leukemia, multiple myeloma, and several types of lymphomas. In hematological disorders, innate lymphoid cells may exert differential effects on the pathogenesis of hematologic malignancies. Furthermore, within the same disease, certain cell populations have been shown to play a protective role in antitumor immune responses, whereas others appear to suppress these responses. This review aims to provide an integrated description of innate lymphoid cells, their alterations in hematological malignancies, and potential preventive strategies, by proposing new specific targets for correcting anomalies. We also discuss the use of innate lymphoid cells as new therapies by applying chimeric antigen receptor-modified natural killer cells. We examine the current knowledge and outline future perspectives.
DOI: https://doi.org/10.37349/ei.2025.1003226

Background:
Oral squamous cell carcinoma (OSCC), a significant health burden in developing nations, is linked to risk factors such as tobacco use, alcohol consumption, betel nut chewing, HPV infection, and genetic susceptibility. A hallmark of OSCC is impaired T cell function, driven in part by the PD-L1/PD-1 immune checkpoint pathway, which enables tumor immune evasion and progression. Despite growing interest in immunotherapy, a focused synthesis of PD-L1 expression and its clinical implications in OSCC remains limited. This review aims to consolidate existing evidence on PD-L1 in OSCC, evaluating its expression patterns, correlation with disease progression, and therapeutic relevance.
Methods:
A systematic search was conducted across multiple databases to identify studies examining PD-L1 expression in OSCC and its relationship with clinicopathological parameters and immune response.
Results:
The findings revealed a higher PD-L1 positivity in female patients, non-smokers, and non-drinkers. Positive PD-L1 expression rate correlated with poor differentiation, lymph node metastasis, and advanced TNM stage. Although it didn’t significantly impact overall survival, higher PD-L1 expression was observed in HPV-positive patients and correlated with increased CD8+ TIL levels.
Discussion:
Understanding the role of PD-L1 in OSCC elucidates immune evasion mechanisms and offers insights into potential treatments, such as checkpoint inhibitors, for personalized therapies and innovative cancer treatments. This comprehensive synthesis provides valuable insights into the complex interplay between PD-L1 expression and OSCC progression, laying the groundwork for additional studies in this area.
Background:
Oral squamous cell carcinoma (OSCC), a significant health burden in developing nations, is linked to risk factors such as tobacco use, alcohol consumption, betel nut chewing, HPV infection, and genetic susceptibility. A hallmark of OSCC is impaired T cell function, driven in part by the PD-L1/PD-1 immune checkpoint pathway, which enables tumor immune evasion and progression. Despite growing interest in immunotherapy, a focused synthesis of PD-L1 expression and its clinical implications in OSCC remains limited. This review aims to consolidate existing evidence on PD-L1 in OSCC, evaluating its expression patterns, correlation with disease progression, and therapeutic relevance.
Methods:
A systematic search was conducted across multiple databases to identify studies examining PD-L1 expression in OSCC and its relationship with clinicopathological parameters and immune response.
Results:
The findings revealed a higher PD-L1 positivity in female patients, non-smokers, and non-drinkers. Positive PD-L1 expression rate correlated with poor differentiation, lymph node metastasis, and advanced TNM stage. Although it didn’t significantly impact overall survival, higher PD-L1 expression was observed in HPV-positive patients and correlated with increased CD8+ TIL levels.
Discussion:
Understanding the role of PD-L1 in OSCC elucidates immune evasion mechanisms and offers insights into potential treatments, such as checkpoint inhibitors, for personalized therapies and innovative cancer treatments. This comprehensive synthesis provides valuable insights into the complex interplay between PD-L1 expression and OSCC progression, laying the groundwork for additional studies in this area.
DOI: https://doi.org/10.37349/ei.2025.1003228
This article belongs to the special issue The Role of Immune Checkpoint Molecules in Cancer and Hematological Malignancies

DOI: https://doi.org/10.37349/ei.2025.1003225

Cancer treatment regimens are significantly more intricate than commonly perceived. Nonetheless, both immunotherapy and chemotherapy may produce adverse consequences. Immunotherapy represents a significant advancement in the battle against cancer; nonetheless, it is not devoid of challenges. This research elucidates the mechanisms underlying immunotherapy-induced cardiovascular damage, highlighting the significant role of immune regulators, such as soluble urokinase plasminogen activator receptor (suPAR), in inducing vascular leakage. We also examine the role of matrix metalloproteinase (MMP14/15) in this process, and antigens associated with cardiovascular illness and malignancies, including native proteins, mutated tumor antigens, and viral components. Besides, we studied predictive biomarkers, such as circulating T-cell populations associated with the probability of myocarditis. We discuss treatment approaches and strategies to mitigate these issues, particularly through the use of antihypertensive medications to reduce their impact. Losartan alters the tumor microenvironment (TME) to improve immunotherapy. It restores immune effector cells in triple-negative breast cancer (TNBC), overcomes resistance in unresponsive tumors, and modifies TMEs’ immune system suppression in ovarian cancer and melanoma. It is important to note that its effects differ by cancer kind. It may promote fibrosarcoma tumor growth and improve cholangiocarcinoma treatment. This shows that losartan’s dangers in cancer treatment should be carefully considered and that more research is needed. This suggests that there must be careful consideration of the potential risks associated with losartan use in cancer treatment and underscores the requirement for additional research on this topic. This study may enhance our comprehension and management of cardiovascular adverse effects in cancer immunotherapy by integrating novel insights on immunological predictors and vascular dysfunction. Experts assert that oncology programs must provide and promote continuous monitoring of cardiac health for breast cancer patients. To mitigate the risk of cardiovascular disease in this demographic, comprehensive patient care must be administered.
Cancer treatment regimens are significantly more intricate than commonly perceived. Nonetheless, both immunotherapy and chemotherapy may produce adverse consequences. Immunotherapy represents a significant advancement in the battle against cancer; nonetheless, it is not devoid of challenges. This research elucidates the mechanisms underlying immunotherapy-induced cardiovascular damage, highlighting the significant role of immune regulators, such as soluble urokinase plasminogen activator receptor (suPAR), in inducing vascular leakage. We also examine the role of matrix metalloproteinase (MMP14/15) in this process, and antigens associated with cardiovascular illness and malignancies, including native proteins, mutated tumor antigens, and viral components. Besides, we studied predictive biomarkers, such as circulating T-cell populations associated with the probability of myocarditis. We discuss treatment approaches and strategies to mitigate these issues, particularly through the use of antihypertensive medications to reduce their impact. Losartan alters the tumor microenvironment (TME) to improve immunotherapy. It restores immune effector cells in triple-negative breast cancer (TNBC), overcomes resistance in unresponsive tumors, and modifies TMEs’ immune system suppression in ovarian cancer and melanoma. It is important to note that its effects differ by cancer kind. It may promote fibrosarcoma tumor growth and improve cholangiocarcinoma treatment. This shows that losartan’s dangers in cancer treatment should be carefully considered and that more research is needed. This suggests that there must be careful consideration of the potential risks associated with losartan use in cancer treatment and underscores the requirement for additional research on this topic. This study may enhance our comprehension and management of cardiovascular adverse effects in cancer immunotherapy by integrating novel insights on immunological predictors and vascular dysfunction. Experts assert that oncology programs must provide and promote continuous monitoring of cardiac health for breast cancer patients. To mitigate the risk of cardiovascular disease in this demographic, comprehensive patient care must be administered.
DOI: https://doi.org/10.37349/ei.2025.1003224
This article belongs to the special issue The Role of Immune Checkpoint Molecules in Cancer and Hematological Malignancies

Cutaneous lupus erythematosus (CLE) is the most common organ manifestation in individuals diagnosed with systemic lupus erythematosus (SLE). CLE can occur either alone or in association with SLE; in the latter case, it substantially increases the occurrence of disease flares and can cause disfigurement. The clinical pathogenesis of CLE is well established, as exposure to ultraviolet (UV) light and/or other environmental triggers, such as smoking or drug use, can lead to keratinocyte death in genetically susceptible individuals. This in turn activates cytotoxic T cells, plasmacytoid dendritic cells (pDCs), and B cells, creating a continuous interaction between the innate and adaptive immune systems. This interaction plays a pivotal role in CLE development, driving the formation of skin lesions. However, the molecular mechanisms underlying these cutaneous manifestations are not yet fully understood. While significant advances have been made in SLE treatment over the past few decades, U.S. Food and Drug Administration (FDA)-approved therapies remain limited to hydroxychloroquine, glucocorticoids, belimumab, and anifrolumab. Although new therapies for CLE have emerged, given the highly heterogeneous nature of the condition, personalized medicine is essential to prevent disfigurement and systemic disease flares. Understanding the molecular pathogenesis of CLE is crucial for developing targeted therapies and improving patient outcomes. This review presents current insights into CLE pathogenesis, highlighting key mechanisms driving the disease and exploring recent advances in treatments that have shown promise in clinical practice.
Cutaneous lupus erythematosus (CLE) is the most common organ manifestation in individuals diagnosed with systemic lupus erythematosus (SLE). CLE can occur either alone or in association with SLE; in the latter case, it substantially increases the occurrence of disease flares and can cause disfigurement. The clinical pathogenesis of CLE is well established, as exposure to ultraviolet (UV) light and/or other environmental triggers, such as smoking or drug use, can lead to keratinocyte death in genetically susceptible individuals. This in turn activates cytotoxic T cells, plasmacytoid dendritic cells (pDCs), and B cells, creating a continuous interaction between the innate and adaptive immune systems. This interaction plays a pivotal role in CLE development, driving the formation of skin lesions. However, the molecular mechanisms underlying these cutaneous manifestations are not yet fully understood. While significant advances have been made in SLE treatment over the past few decades, U.S. Food and Drug Administration (FDA)-approved therapies remain limited to hydroxychloroquine, glucocorticoids, belimumab, and anifrolumab. Although new therapies for CLE have emerged, given the highly heterogeneous nature of the condition, personalized medicine is essential to prevent disfigurement and systemic disease flares. Understanding the molecular pathogenesis of CLE is crucial for developing targeted therapies and improving patient outcomes. This review presents current insights into CLE pathogenesis, highlighting key mechanisms driving the disease and exploring recent advances in treatments that have shown promise in clinical practice.
DOI: https://doi.org/10.37349/ei.2025.1003222

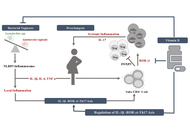
The human vaginal microbiome plays a pivotal role in maintaining female reproductive health through its Lactobacillus-dominated microbial ecology. These bacteria contribute to the acidic pH of the vagina by producing lactic acid, ultimately preventing the colonization of pathogens. Additionally, they produce bacteriocins and hydrogen peroxide, which are detrimental to other microorganisms. Human vaginal microbiota is subjected to alterations with advancement in age, hormonal status, puberty, menstruation cycle, pregnancy and gestation, vaginal tract diseases, exposure to antibiotics, etc. Diet, lifestyle factors, obesity, and gestational diabetes are also reported to cause a shift in vaginal microbiota. This review thoroughly illustrates the perpetually changing dynamics of vaginal microbiota throughout women’s lives, as well as focuses on the impact of dysbiosis in bacterial vaginosis. More emphasis is given on immunological changes observed during bacterial vaginosis, mainly IL-1β, and its involvement in the development of preeclampsia. Thereby, this review highlights a mechanistic link between lower genital tract disease, bacterial vaginosis, and a hypertensive disorder of pregnancy, preeclampsia, via IL-1β–ROR-γt–Th17 axis, which is regulated by vitamin D, with a suggestion on how shifts in vaginal microbial community may pose a risk for preeclampsia.
The human vaginal microbiome plays a pivotal role in maintaining female reproductive health through its Lactobacillus-dominated microbial ecology. These bacteria contribute to the acidic pH of the vagina by producing lactic acid, ultimately preventing the colonization of pathogens. Additionally, they produce bacteriocins and hydrogen peroxide, which are detrimental to other microorganisms. Human vaginal microbiota is subjected to alterations with advancement in age, hormonal status, puberty, menstruation cycle, pregnancy and gestation, vaginal tract diseases, exposure to antibiotics, etc. Diet, lifestyle factors, obesity, and gestational diabetes are also reported to cause a shift in vaginal microbiota. This review thoroughly illustrates the perpetually changing dynamics of vaginal microbiota throughout women’s lives, as well as focuses on the impact of dysbiosis in bacterial vaginosis. More emphasis is given on immunological changes observed during bacterial vaginosis, mainly IL-1β, and its involvement in the development of preeclampsia. Thereby, this review highlights a mechanistic link between lower genital tract disease, bacterial vaginosis, and a hypertensive disorder of pregnancy, preeclampsia, via IL-1β–ROR-γt–Th17 axis, which is regulated by vitamin D, with a suggestion on how shifts in vaginal microbial community may pose a risk for preeclampsia.
DOI: https://doi.org/10.37349/ei.2025.1003223

T cell-based immunotherapies increasingly include personalized neoantigen vaccines that target tumor-specific mutations. However, despite their promise, current neoantigen vaccines show limited and unpredictable clinical benefit, with T cell responses observed in only a subset of patients. To overcome these limitations, we developed the VERDI (Vaccine Epitopes Ranked by Digital Intelligence) System—a cloud-based computational platform that integrates a patient’s human leukocyte antigen (HLA) class I and II genotype with selected tumor-associated antigens (TAAs), including cancer-testis antigens (CTAs), to identify peptides with high predicted immunogenicity and low risk of immune-related adverse events (irAEs). Using the VERDI System, we designed ten personalized peptide vaccines for a patient with metastatic signet ring cell carcinoma (SRCC), a rare and aggressive gastric cancer with limited treatment options. All ten VERDI vaccines were well tolerated and consistently induced tumor-specific T cell responses following a single administration, without the need for checkpoint inhibitors. The patient survived for 15 months—substantially longer than the reported median survival of 5.6 months in metastatic SRCC—highlighting the potential of this individualized, predictive vaccine platform to improve outcomes in advanced cancer.
T cell-based immunotherapies increasingly include personalized neoantigen vaccines that target tumor-specific mutations. However, despite their promise, current neoantigen vaccines show limited and unpredictable clinical benefit, with T cell responses observed in only a subset of patients. To overcome these limitations, we developed the VERDI (Vaccine Epitopes Ranked by Digital Intelligence) System—a cloud-based computational platform that integrates a patient’s human leukocyte antigen (HLA) class I and II genotype with selected tumor-associated antigens (TAAs), including cancer-testis antigens (CTAs), to identify peptides with high predicted immunogenicity and low risk of immune-related adverse events (irAEs). Using the VERDI System, we designed ten personalized peptide vaccines for a patient with metastatic signet ring cell carcinoma (SRCC), a rare and aggressive gastric cancer with limited treatment options. All ten VERDI vaccines were well tolerated and consistently induced tumor-specific T cell responses following a single administration, without the need for checkpoint inhibitors. The patient survived for 15 months—substantially longer than the reported median survival of 5.6 months in metastatic SRCC—highlighting the potential of this individualized, predictive vaccine platform to improve outcomes in advanced cancer.
DOI: https://doi.org/10.37349/ei.2025.1003221

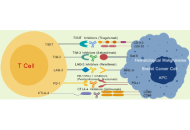
Cancer is a multifaceted and heterogeneous disease characterized by uncontrolled growth, evasion of immune surveillance, and resistance to conventional therapies. The immune system plays a crucial role in tumor surveillance. However, tumors exploit immune checkpoint pathways to inhibit T cell activation and evade immune destruction. Immune checkpoint inhibitors (ICIs) have markedly improved outcomes in certain cancers by restoring T cell function and enhancing anti-tumor immunity. Despite these advances, the presence of immune resistance mechanisms contributes to variability in responses and ongoing challenges in overcoming resistance. Triple-negative breast cancer (TNBC), compared to other breast cancer (BC) subtypes, exhibits higher immunogenicity, but its anti-tumor immunity is profoundly suppressed by immune checkpoint molecules, creating a paradoxical scenario of “high immunogenic potential yet restrained by inhibitory signals”. Consequently, TNBC has become a significant target for ICI therapy. However, response rates vary among BC subtypes, with hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-positive (HER2+) BC demonstrating lower immunogenicity. Hematological malignancies, including leukemia, lymphoma, and multiple myeloma, also exhibit distinct immune checkpoint dynamics, influencing their responsiveness to ICIs. This review comprehensively examines the mechanisms of immune checkpoint regulation, their role in cancer immune evasion, and the clinical applications of ICIs in both solid and hematological malignancies. It further discusses emerging strategies to counteract ICI resistance, such as dual checkpoint blockade, tumor microenvironment modulation, metabolic targeting, and epigenetic reprogramming. An enhanced understanding of immune checkpoint biology is essential for optimizing immunotherapy strategies and improving patient outcomes. The literature selection for this study was guided by relevance to the research topic, focusing on peer-reviewed articles, monographs, and conference proceedings published between 2010 and 2025, sourced from databases like PubMed and Google Scholar.
Cancer is a multifaceted and heterogeneous disease characterized by uncontrolled growth, evasion of immune surveillance, and resistance to conventional therapies. The immune system plays a crucial role in tumor surveillance. However, tumors exploit immune checkpoint pathways to inhibit T cell activation and evade immune destruction. Immune checkpoint inhibitors (ICIs) have markedly improved outcomes in certain cancers by restoring T cell function and enhancing anti-tumor immunity. Despite these advances, the presence of immune resistance mechanisms contributes to variability in responses and ongoing challenges in overcoming resistance. Triple-negative breast cancer (TNBC), compared to other breast cancer (BC) subtypes, exhibits higher immunogenicity, but its anti-tumor immunity is profoundly suppressed by immune checkpoint molecules, creating a paradoxical scenario of “high immunogenic potential yet restrained by inhibitory signals”. Consequently, TNBC has become a significant target for ICI therapy. However, response rates vary among BC subtypes, with hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-positive (HER2+) BC demonstrating lower immunogenicity. Hematological malignancies, including leukemia, lymphoma, and multiple myeloma, also exhibit distinct immune checkpoint dynamics, influencing their responsiveness to ICIs. This review comprehensively examines the mechanisms of immune checkpoint regulation, their role in cancer immune evasion, and the clinical applications of ICIs in both solid and hematological malignancies. It further discusses emerging strategies to counteract ICI resistance, such as dual checkpoint blockade, tumor microenvironment modulation, metabolic targeting, and epigenetic reprogramming. An enhanced understanding of immune checkpoint biology is essential for optimizing immunotherapy strategies and improving patient outcomes. The literature selection for this study was guided by relevance to the research topic, focusing on peer-reviewed articles, monographs, and conference proceedings published between 2010 and 2025, sourced from databases like PubMed and Google Scholar.
DOI: https://doi.org/10.37349/ei.2025.1003220
This article belongs to the special issue The Role of Immune Checkpoint Molecules in Cancer and Hematological Malignancies

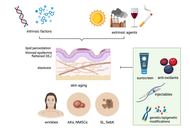
The skin covers the entire surface of the body and therefore is the largest organ in humans. The skin has various functions, primarily defence from infections and trauma. With aging, profound changes occur that compromise its key functions, leading to impaired barrier protection and immune responses. This is in part due to the increased low-grade systemic inflammation known as inflammaging, driven by senescent cells, and release of pro-inflammatory cytokines, to which the skin also significantly contributes. As a consequence of inflammaging, the skin’s function is compromized. The cellular and molecular components involved are summarized in this review.
The skin covers the entire surface of the body and therefore is the largest organ in humans. The skin has various functions, primarily defence from infections and trauma. With aging, profound changes occur that compromise its key functions, leading to impaired barrier protection and immune responses. This is in part due to the increased low-grade systemic inflammation known as inflammaging, driven by senescent cells, and release of pro-inflammatory cytokines, to which the skin also significantly contributes. As a consequence of inflammaging, the skin’s function is compromized. The cellular and molecular components involved are summarized in this review.
DOI: https://doi.org/10.37349/ei.2025.1003218
This article belongs to the special issue Immunosenescence: Mechanisms and Its Impact

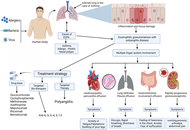
Eosinophilic granulomatosis with polyangiitis (EGPA) is an uncommon form of necrotizing vasculitis that affects the respiratory system and other organs, characterized by asthma, eosinophilia, and multiple organ involvement that complicates the diagnosis and treatment. There are no definitive open-label clinical data on the diagnosis or treatment of EGPA to guide clinicians. The diverse presentation of the disease, distinct from other eosinophilic disorders; the presence of competing conditions such as allergy and asthma, which also have potential biomarkers; lack of a definitive test to confirm diagnosis; difficulties in obtaining endoscopic biopsies for histologic confirmation, etc., are significant barriers to early detection. Even with recent advances in imaging, immunological approaches, and molecular testing to determine the disease’s identity and characteristics, clinicians still misdiagnose or delay treatment, sometimes leading to life-threatening and irreversible complications. Though EGPA is pharmacotherapeutically controlled with glucocorticoids, it has typically included the use of cytotoxic agents such as cyclophosphamide for induction in cases of severity. Recently, several clinical trials have examined targeted biologic treatments (such as mepolizumab, benralizumab, and omalizumab) and demonstrated that these medications can reduce exacerbations, decrease the need for glucocorticoids, and improve asthma control. New drugs such as dupilumab and new anti-IL-5/IL-5R monoclonal antibodies are being studied in phase II and phase III trials, and these drugs may provide additional avenues for refractory disease. Treatment will be organized based on individualization of treatment strategy, depending on disease severity, organ involvement, and biomarker profile. Vertical investment in multicenter longitudinal studies is necessary to formulate therapeutic algorithms and evaluate new targets.
Eosinophilic granulomatosis with polyangiitis (EGPA) is an uncommon form of necrotizing vasculitis that affects the respiratory system and other organs, characterized by asthma, eosinophilia, and multiple organ involvement that complicates the diagnosis and treatment. There are no definitive open-label clinical data on the diagnosis or treatment of EGPA to guide clinicians. The diverse presentation of the disease, distinct from other eosinophilic disorders; the presence of competing conditions such as allergy and asthma, which also have potential biomarkers; lack of a definitive test to confirm diagnosis; difficulties in obtaining endoscopic biopsies for histologic confirmation, etc., are significant barriers to early detection. Even with recent advances in imaging, immunological approaches, and molecular testing to determine the disease’s identity and characteristics, clinicians still misdiagnose or delay treatment, sometimes leading to life-threatening and irreversible complications. Though EGPA is pharmacotherapeutically controlled with glucocorticoids, it has typically included the use of cytotoxic agents such as cyclophosphamide for induction in cases of severity. Recently, several clinical trials have examined targeted biologic treatments (such as mepolizumab, benralizumab, and omalizumab) and demonstrated that these medications can reduce exacerbations, decrease the need for glucocorticoids, and improve asthma control. New drugs such as dupilumab and new anti-IL-5/IL-5R monoclonal antibodies are being studied in phase II and phase III trials, and these drugs may provide additional avenues for refractory disease. Treatment will be organized based on individualization of treatment strategy, depending on disease severity, organ involvement, and biomarker profile. Vertical investment in multicenter longitudinal studies is necessary to formulate therapeutic algorithms and evaluate new targets.
DOI: https://doi.org/10.37349/ei.2025.1003219
 Previous
Previous