Affiliation:
Department of Zoology, School of Sciences, Maulana Azad National Urdu University, Gachibowli 500032, Telangana, India
ORCID: https://orcid.org/0000-0003-1911-2857
Affiliation:
Department of Zoology, School of Sciences, Maulana Azad National Urdu University, Gachibowli 500032, Telangana, India
Email: rashmibhuwalka04@gmail.com
ORCID: https://orcid.org/0009-0000-5220-0643
Affiliation:
Department of Zoology, School of Sciences, Maulana Azad National Urdu University, Gachibowli 500032, Telangana, India
ORCID: https://orcid.org/0000-0003-3203-1816
Explor Immunol. 2025;5:1003209 DOI: https://doi.org/10.37349/ei.2025.1003209
Received: November 08, 2024 Accepted: July 08, 2025 Published: August 17, 2025
Academic Editor: Calogero Caruso, University of Palermo, Italy
Aim: Recurrent pregnancy loss (RPL) is defined as the loss of two or more clinical pregnancies before the 20th week of gestation. Globally, RPL affects 1–5% of couples, with approximately 50% of cases remaining idiopathic. This study aimed to assess the circulating levels of interleukin-6 (IL-6) and IL-10 cytokines in pregnant women with and without a history of RPL.
Methods: A total of 170 pregnant women in their second trimester with and without a history of RPL were enrolled from Niloufer Hospital, South India. Serum samples isolated from blood were analyzed using a sandwich-enzyme linked immunosorbent assay (ELISA) to estimate IL-6 and IL-10 levels.
Results: The median age was significantly higher in the RPL group (25 years) compared to the non-RPL (NRPL) group (22 years) (p = 0.0001). Similarly, body mass index (BMI) was significantly elevated in the RPL group (25.64 kg/m2) vs. the NRPL group (22.51 kg/m2) (p = 0.0001). The analysis revealed significantly elevated IL-6 and reduced IL-10 levels in the RPL group compared to the NRPL group (p = 0.0001). Additionally, the IL-6/IL-10 ratio differed significantly between the two groups. Receiver operating characteristic (ROC) curve analysis indicated that IL-6 was a better marker for RPL than IL-6/IL-10 ratio and IL-10. IL-10 levels were found to be a reliable marker in relation to the extent of pregnancy loss history.
Conclusions: The study highlights the presence of a pro-inflammatory systemic milieu in mid-gestation among women with a history of RPL, potentially reflecting the immunological environment at the feto-placental interface. Further research to establish a distinct cytokine signature between RPL and NRPL groups may facilitate the development of targeted preventive and therapeutic strategies. However, the current findings are limited by a modest sample size and a homogenous ethnic population, which may affect generalizability. Larger, multi-ethnic studies are warranted to validate these observations and enhance clinical applicability.
Recurrent pregnancy loss (RPL) is a significant obstetric complication defined as the occurrence of two or more consecutive miscarriages before 20 weeks of gestation. It affects approximately 15–20% of clinically confirmed pregnancies [1]. Definitions of RPL vary among international organizations, with the American Society for Reproductive Medicine specifying it as two or more clinical pregnancy losses [2]. RPL impacts roughly 1 in every 300 pregnancies, and epidemiological data indicate that 1–2% of women experience recurrent losses [3]. The risk of RPL increases to 30% after two consecutive losses and rises further to 35% following a third loss [4]. While established causes of RPL include parental chromosomal abnormalities, endocrine disorders, uterine structural defects, and antiphospholipid antibody syndrome, nearly 50% of cases remain unexplained (idiopathic), presenting a major challenge for both researchers and clinicians worldwide.
Successful pregnancy depends heavily on immune tolerance toward semi-allogeneic fetal antigens, a process largely governed by regulatory T (Treg) cells. These Tregs, identified as CD4+CD25+FOXP3+ T lymphocytes, are essential for modulating immune responses and fostering an immunologically tolerant environment at the maternal-fetal interface [5]. Cytokines, which are small signaling molecules present both locally at the maternal-fetal interface and systemically, contribute to immune regulation by helping the embryo avoid rejection and supporting pregnancy progression. Extensive research highlights the critical involvement of cytokines in pregnancy [6, 7].
Cytokines serve as immune modulators, maintaining a balance between pro-inflammatory and anti-inflammatory signals to address specific physiological demands such as pregnancy or infection. At the maternal-fetal interface, intricate cytokine communication facilitates immune tolerance and promotes embryo survival [5]. The dynamic interplay of pro- and anti-inflammatory cytokines governs the three distinct phases of pregnancy [8, 9]. The initial phase is characterized by a predominance of pro-inflammatory cytokines that support embryo implantation. This is followed by a shift toward anti-inflammatory cytokines during the second phase, which establishes a symbiotic relationship between mother and fetus, ensuring healthy fetal development [10–12]. In the final phase, pro-inflammatory cytokines again become predominant, creating an inflammatory environment necessary for parturition [9]. Any imbalance or disruption in the levels of these cytokines across the pregnancy stages can contribute to pregnancy complications [8].
Interleukin-6 (IL-6) is a multifunctional cytokine produced by various cell types, including monocytes/macrophages, B and T lymphocytes, mast cells, endothelial cells, fibroblasts, and hepatocytes, which plays a central role in regulating inflammatory responses. IL-6 is critically involved in directing the differentiation of CD4+ T cells into Treg cells and T helper 17 (Th17) cells. In particular, in the presence of transforming growth factor-beta (TGF-β), IL-6 promotes the differentiation of Th17 cells [13–15], while simultaneously inhibiting the development of Treg cells [16–18]. The balance between these two cell populations is essential for the immune regulation necessary for a successful pregnancy [19–22].
IL-6 drives inflammation by stimulating the production of acute-phase proteins, enhancing neutrophil recruitment, and facilitating Th17 cell differentiation through trans-signalling mechanisms. At the same time, it suppresses Treg cell proliferation via the JAK/STAT3 signaling pathway, a process vital for embryo implantation and placental development [5, 23]. Conversely, IL-6’s classical signalling pathway mediates anti-inflammatory effects by inducing the production of IL-1 receptor antagonist (IL-1Ra) and IL-10, while inhibiting pro-inflammatory cytokines such as TNF-α and IL-1β, thereby contributing to the resolution of inflammation and tissue repair [24]. Dysregulated IL-6 activity is implicated in the pathogenesis of autoimmune diseases, where it perpetuates immune activation and favors Th17 cell dominance. It is also involved in cancer progression by sustaining chronic inflammation, promoting tumor growth, angiogenesis, and resistance to apoptosis through continuous JAK/STAT3 pathway activation, thus creating a pro-tumorigenic environment [25].
IL-10 is an immunomodulatory cytokine produced by a diverse range of immune and non-immune cells, including decidual cells involved in the immune regulation of pregnancy. IL-10 exerts its anti-inflammatory effects by activating the JAK/STAT and PI3K/Akt signaling pathways while inhibiting the NF-κB pathway. It reduces the production of pro-inflammatory cytokines such as IL-1, IL-6, IL-12, and TNF-α, and suppresses antigen presentation by downregulating major histocompatibility complex (MHC) class II expression [26–28]. In human pregnancy, decreased serum levels of IL-10 have been linked to complications including preeclampsia (PE) and RPL, highlighting the crucial role of IL-10 in maintaining a healthy pregnancy [29–33].
Interestingly, Calleja-Agius et al. (2008) [34] reported increased IL-10 levels in women experiencing RPL, which may reflect a compensatory anti-inflammatory response. In contrast, Vilotić et al. (2022) [35] found decreased IL-10 levels alongside elevated IL-6, indicating a shift towards a pro-inflammatory state.
Based on the existing literature, it can be inferred that the balance between pro- and anti-inflammatory cytokines, rather than their individual levels alone, plays a critical role in shaping the inflammatory environment during pregnancy, which may influence the risk of RPL. With this premise, our study aimed to examine the circulating levels of key pro-inflammatory and anti-inflammatory cytokines IL-6 and IL-10 and their relative concentrations in pregnant women with and without a history of RPL during mid-gestation. Additionally, we evaluated how these cytokine profiles correlate with the severity of previous pregnancy losses. To the best of our knowledge, this is the first study to explore this relationship in such a focused manner.
This case-control study included 170 pregnant women in their second trimester, equally divided into two groups: those with a history of RPL (RPL group; n = 85) and those without (NRPL group; n = 85). Participants were recruited from the Department of Gynaecology and Obstetrics at Niloufer Hospital, South India. The RPL group was further subdivided into women with two previous pregnancy losses (2-loss group; n = 53) and those with more than two (> 2-loss group; n = 32) losses. The demographic and clinical data were collected at the time of sample collection.
The sample size (n = 85 per group) was determined based on a prior power analysis using G*Power software (version 3.1.9.7). This was calculated to detect a medium effect size (Cohen’s d = 0.5) in cytokine levels (IL-6 and IL-10) between RPL and NRPL groups, with a two-tailed α = 0.05 and power (1−β) = 0.80, resulting in a minimum required sample size of 64 participants per group. To increase the robustness of the study and account for possible dropouts or data loss, we enrolled 85 participants in each group.
The RPL group included women in their second trimester with a history of 2 or more unexplained pregnancy losses. The NRPL group consisted of women in their second trimester with no history of pregnancy loss and at least two prior successful pregnancies.
Women with known causes of RPL, such as chromosomal abnormalities, anatomical defects, endocrine or immunological disorders [including thrombophilia and antiphospholipid syndrome (APS)], and TORCH infections were excluded. Although karyotyping was not performed due to resource limitations, detailed clinical histories and prior diagnostic records were reviewed to rule out known anatomical, endocrine, autoimmune, and chromosomal abnormalities. APS exclusion was based on documented negative lupus anticoagulant and anticardiolipin antibody tests.
A 5 mL blood sample was collected from each participant into EDTA tubes. Serum was then separated by centrifugation at 2,000–3,000 rpm for 20 min at 4°C and stored at −20°C in a deep freezer until further analysis.
Serum levels of IL-6 and IL-10 were measured using pre-coated anti-human IL-6 and IL-10 enzyme linked immunosorbent assay (ELISA) kits (BT LAB, Biosystems, China), following the manufacturer’s instructions. In the assay, cytokines present in the samples bound to specific antibodies immobilized on the microplate wells. A biotinylated detection antibody for each cytokine was then added, which subsequently bound to streptavidin-HRP. After a 60-minute incubation, unbound streptavidin-HRP was removed through five wash cycles using a programmable automatic microplate washer (Analytical Technologies Limited, AT2960R). The addition of substrate solution produced a colorimetric reaction proportional to the cytokine concentration. The reaction was stopped with an acidic solution, and optical density (OD) was read at 450 nm using an ELISA reader. Cytokine concentrations were determined from standard curves ranging from 2 to 600 ng/L for IL-6 and 5 to 1,500 pg/mL for IL-10. The assay detection limits were 1.03 ng/L for IL-6 and 2.59 pg/mL for IL-10.
Statistical analysis was performed using GraphPad Prism and MedCalc software, with a p-value of < 0.05 considered statistically significant. All results are presented as medians and percentiles. As the data did not pass the normality test (Shapiro-Wilk test), the non-parametric Mann-Whitney U test was used to compare differences between the NRPL and RPL groups, as well as within the RPL group (2 vs. > 2 previous losses). Receiver operating characteristic (ROC) curve analysis was conducted to assess the diagnostic accuracy of cytokine markers and determine optimal cut-off values.
Table 1 presents a comparative analysis of the demographic and clinical characteristics between women with no history of RPL (NRPL group, n = 85) and those with RPL (RPL group, n = 85). Additionally, within the RPL group, characteristics were compared between women with exactly 2 pregnancy losses (n = 53) and those with > 2 losses (n = 32). All comparisons were made using the Mann-Whitney U test, and values are reported as medians with interquartile ranges (25th–75th percentile).
Comparative demographic and clinical characteristics of RPL and NRPL groups and the RPL group with 2 and > 2 pregnancy losses
| Sample characteristic | NRPL group (n = 85); median (25th–75th percentile) | RPL group (n = 85); median (25th–75th percentile) | ||||
|---|---|---|---|---|---|---|
| Median (25th–75th percentile) | p-value | 2 losses (n = 53) | > 2 losses (n = 32) | p-value | ||
| Age (years) | 22 (21–25) | 25 (23–27) | 0.0001 | 25 (23–27) | 26 (23–28) | 0.29 |
| BMI (kg/m2) | 22.51 (19.39–24.45) | 25.64 (22.89–28.01) | 0.0001 | 25.54 (22.89–27.48) | 25.74 (23.19–28.93) | 0.54 |
| Age at menarche (years) | 12 (12–13) | 13 (12–13) | 0.34 | 13 (12–13) | 13 (12–14) | 0.11 |
| Age at first conception (years) | 20 (19–22) | 21 (19–23) | 0.21 | 21 (19–23) | 20 (19–21) | 0.10 |
| Gestational age (trimesters) | 2 (2–2) | 2 (2–2) | 0.68 | 2 (2–2) | 2 (2–2) | 0.82 |
| Number of abortions | 0 | 2 (2–3) | 0.0001 | 2 (2–2) | 3 (3–4.75) | 0.0001 |
RPL: recurrent pregnancy loss; NRPL: non-recurrent pregnancy loss; BMI: body mass index; p-value ≤ 0.05: statistically significant. Reprinted with permission from [5]. © 2023 Society for Biology of Reproduction & the Institute of Animal Reproduction and Food Research of Polish Academy of Sciences in Olsztyn
The median age was significantly higher in the RPL group (25 years) compared to the NRPL group (22 years) (p = 0.0001). A similar trend was observed for body mass index (BMI), with a significantly higher median BMI in the RPL group (25.64 kg/m2) compared to the NRPL group (22.51 kg/m2) (p = 0.0001). However, age at menarche, age at first conception, and gestational age did not differ significantly between the groups [5].
When stratifying the RPL group by the number of pregnancy losses, there was no statistically significant difference in age, BMI, age at menarche, age at first conception, or gestational age between women with 2 losses and those with > 2 losses. However, as expected, the number of abortions was significantly higher in the > 2 losses group (median = 3) compared to the 2 losses group (median = 2) (p = 0.0001).
Significantly elevated levels of IL-6 were seen in the RPL group compared to the NRPL group (Table 2; Figure 1). No significant differences (ns = p > 0.05) for IL-6 levels were observed between 2 losses vs. > 2 losses groups (Table 2; Figure 2). ROC curve analysis for IL-6 exhibited excellent (AUC = 0.992, p = 0.0001) ROC characteristics (Figure 3).
Comparison of median and percentile of cytokines between RPL and NRPL groups and the RPL group with 2 and > 2 previous pregnancy losses
| Parameters | NRPL group (n = 85); median (25th–75th percentile) | RPL group (n = 85); median (25th–75th percentile) | ||||
|---|---|---|---|---|---|---|
| Median (25th–75th percentile) | p-value | 2 losses (n = 53) | > 2 losses (n = 32) | p-value | ||
| IL-6 | 3.137 (3.131–3.149) | 6.478 (6.351–15.29) | 0.0001 | 6.477 (6.351–15.31) | 6.510 (6.334–15.27) | 0.869 |
| IL-10 | 14.17 (5.706–23.17) | 6.870 (5.108–8.671) | 0.0001 | 6.444 (5.090–8.216) | 7.320 (5.641–9.584) | 0.172 |
| IL-6/IL-10 | 0.222 (0.135–0.553) | 1.230 (0.892–1.814) | 0.0001 | 1.226 (0.916–2.162) | 1.244 (0.809–1.596) | 0.706 |
RPL: recurrent pregnancy loss; NRPL: non-recurrent pregnancy loss; IL-6: interleukin-6; 2: two; > 2: more than two; p < 0.05: statistically significant. Unit for IL-6, IL-10: pg/mL
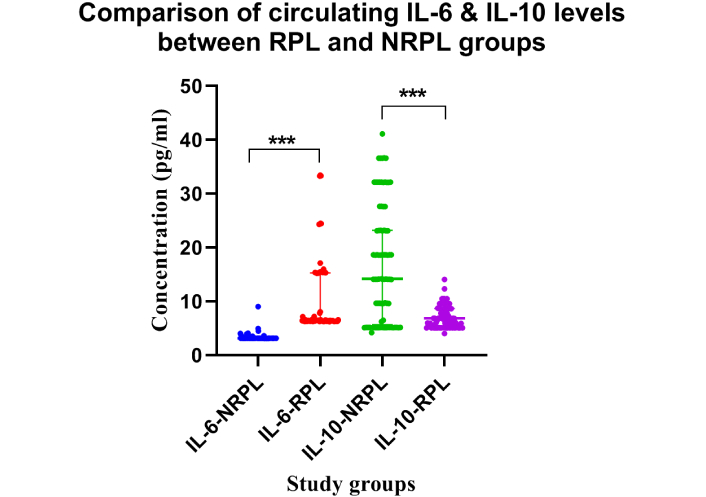
Comparison of circulating IL-6 and IL-10 concentrations between RPL and NRPL groups. Dot plots show the concentrations (pg/mL) of IL-6 and IL-10 in women with RPL (n = 85) and NRPL (n = 85). IL-6 was significantly elevated and IL-10 significantly reduced in the RPL group compared to the NRPL group (***p < 0.001). IL-6: interleukin-6; RPL: recurrent pregnancy loss; NRPL: non-recurrent pregnancy loss
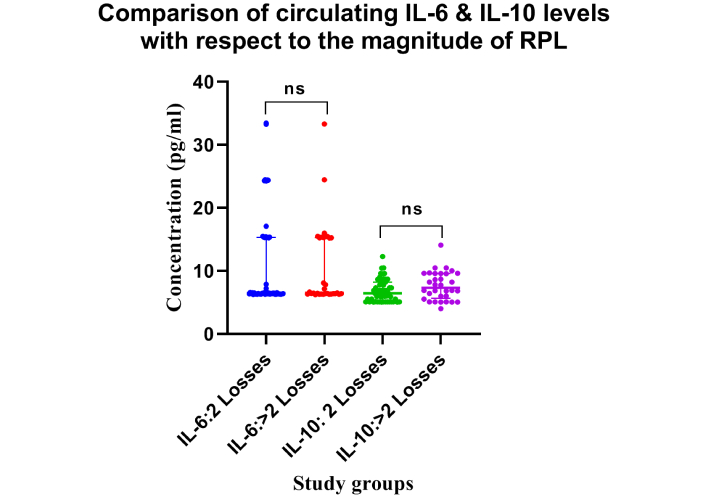
Comparison of circulating IL-6 and IL-10 levels with respect to the magnitude of RPL. Dot plot represents the concentrations (pg/mL) of IL-6 and IL-10 in women with 2 losses and > 2 losses. No statistically significant differences (ns = p > 0.05) were observed between the groups. Number of participants per group: IL-6 (2 losses, n = 53; > 2 losses, n = 32), IL-10 (2 losses, n = 53; > 2 losses, n = 32). IL-6: interleukin-6; RPL: recurrent pregnancy loss
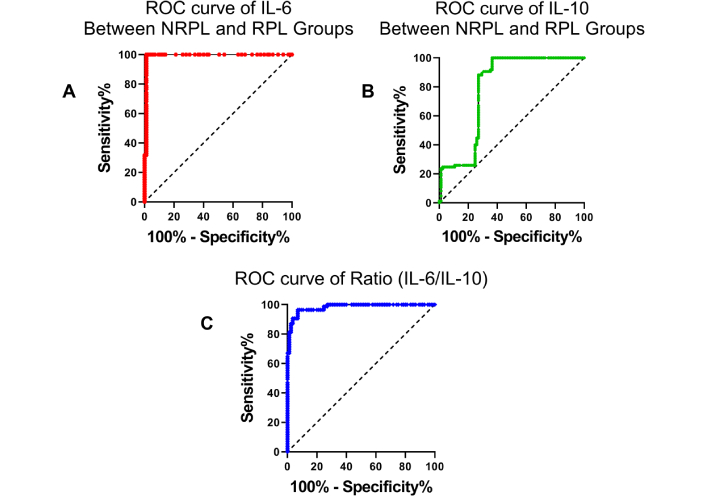
Receiver operating characteristic (ROC) curves of cytokine markers between NRPL and RPL groups. ROC curve for (A) IL-6 levels showing high sensitivity and specificity in distinguishing RPL cases from NRPL controls. (B) IL-10 levels have moderate diagnostic performance. (C) IL-6/IL-10 ratio, indicating improved classification ability compared to individual cytokines. IL-6: interleukin-6; RPL: recurrent pregnancy loss; NRPL: non-recurrent pregnancy loss
Decreased levels of IL-10 were seen in the RPL group compared to the NRPL group (Table 2; Figure 1). No variation (ns = p > 0.05) was observed for IL-10 concentrations between 2 and > 2 losses groups (Table 2; Figure 2). Receiver operating characteristics for IL-10 exhibited moderate (AUC = 0.789, p = 0.0001) characteristics for ROC (Figure 3).
Data analysis revealed a significantly elevated IL-6/IL-10 ratio in the RPL group compared to the NRPL group (Table 2; Figure 4). No difference was observed for IL-6/IL-10 ratio between 2 and > 2 losses groups (Table 2; Figure 5; p > 0.05). Receiver operating characteristics for IL-6/IL-10 exhibited excellent (AUC = 0.982, p = 0.0001) characteristics for ROC (Figure 3).
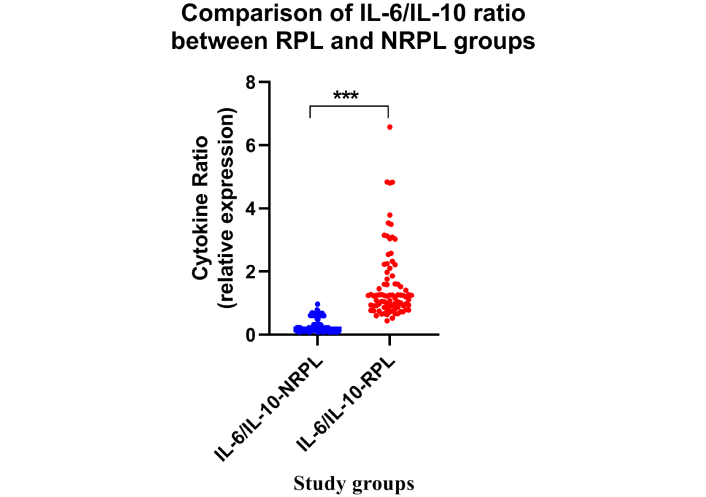
Comparison of IL-6/IL-10 ratio between the RPL and NRPL groups. Dot plots represent the IL-6/IL-10 ratio in RPL (n = 85) and NRPL (n = 85) women. A significantly higher IL-6/IL-10 ratio was observed in RPL cases (***p < 0.001). IL-6: interleukin-6; RPL: recurrent pregnancy loss; NRPL: non-recurrent pregnancy loss
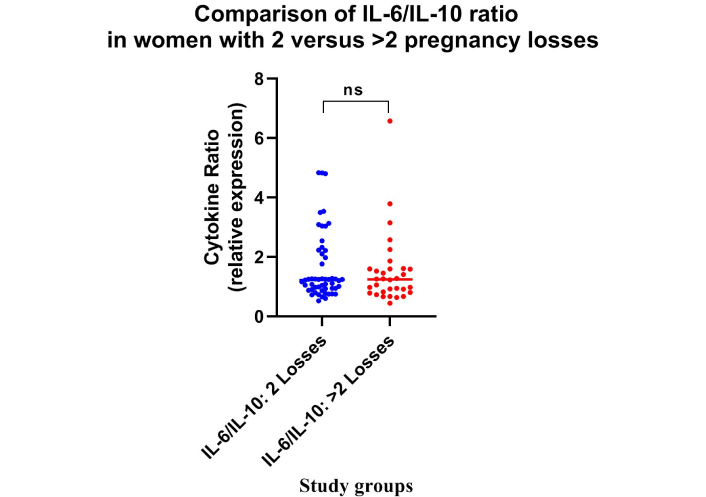
Comparison of IL-6/IL-10 ratio with respect to the magnitude of RPL. Dot plot represents the IL-6/IL-10 ratio in women with 2 losses and > 2 losses. No statistically significant differences (ns = p > 0.05) were observed between the groups. Number of participants per group: IL-6 (2 losses, n = 53; > 2 losses, n = 32), IL-10 (2 losses, n = 53; > 2 losses, n = 32). IL-6: interleukin-6; RPL: recurrent pregnancy loss
ROC curve analysis was performed to evaluate the diagnostic performance of IL-6, IL-10, and the IL-6/IL-10 ratio in differentiating between the NRPL and RPL groups, as well as between women with 2 vs. > 2 pregnancy losses (Table 3). As shown in Figure 3A–C, IL-6 and the IL-6/IL-10 ratio demonstrated high sensitivity and specificity in distinguishing NRPL from RPL cases, with the IL-6 curve approaching near-perfect discrimination. In contrast, IL-10 showed moderate discriminative ability. However, in the subgroup analysis (Figure 6), the ROC curves for IL-6, IL-10, and the IL-6/IL-10 ratio showed more limited predictive value when comparing 2 vs. > 2 losses, with AUCs closer to the reference line, indicating reduced classification accuracy in this subgroup.
ROC curve analysis of IL-6, IL-10, and IL-6/IL-10 ratio for differentiating NRPL vs. RPL and 2 vs. > 2 pregnancy losses
| Parameters | NRPL vs. RPL | 2 losses vs. > 2 losses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Cut-off | Sn% | Sp.% | p-value | AUC | Cut-off | Sn% | Sp.% | p-value | |
| IL-6 | 0.992 | > 5.59 | 100 | 98.82 | 0.0001 | 0.502 | > 6.64 | 46.88 | 66.04 | 0.974 |
| IL-10 | 0.789 | < 14.08 | 100 | 63.53 | 0.0001 | 0.589 | > 9.58 | 28.13 | 92.45 | 0.170 |
| IL-6/IL-10 | 0.982 | > 0.62 | 96.47 | 92.94 | 0.0001 | 0.524 | < 0.97 | 43.75 | 69.81 | 0.703 |
IL-6: interleukin-6; RPL: recurrent pregnancy loss; NRPL: non-recurrent pregnancy loss; 2: two; > 2: more than two; AUC: area under the curve; Sn%: sensitivity; Sp.%: specificity; p < 0.05: statistically significant. Unit for IL-6, IL-10: pg/mL
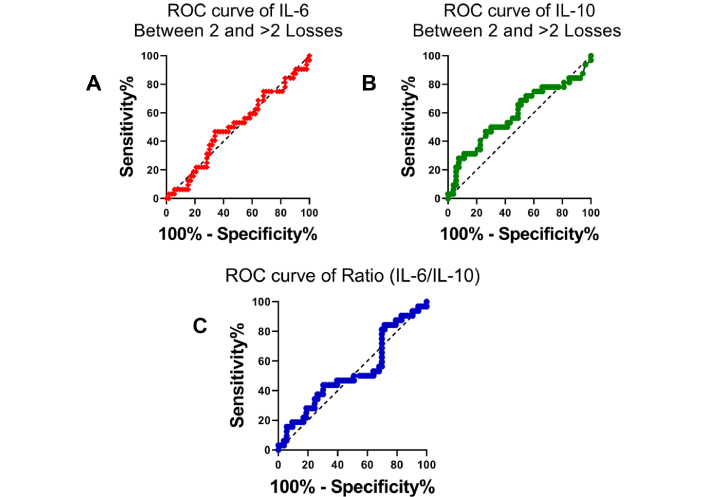
Receiver operating characteristic (ROC) curves comparing cytokine markers between women with two pregnancy losses and those with more than two losses. ROC curve for (A) IL-6 levels shows poor discriminatory ability between the two subgroups of RPL, (B) IL-10 levels, similarly, does not demonstrate strong diagnostic performance, (C) IL-6/IL-10 ratio also indicates limited potential in differentiating between women with 2 and > 2 pregnancy losses. IL-6: interleukin-6; RPL: recurrent pregnancy loss; NRPL: non-recurrent pregnancy loss
Cytokines are immune regulators with a fundamental role in human reproduction that mediate gametogenesis, implantation, and foetal development [5], and an imbalance in quantity or location of expression influences trophoblast-endometrial (feto-maternal) interaction, consequences in pregnancy impediments, and RPL is one such complication. For a steady maintenance of pregnancy, the appropriate homeostatic stability between Th1 and Th2 cytokines is obligatory. Favourable pregnancy outcome is accompanied by an overall higher expression of Th2 cytokines, whereas unfavourable ones generally go with Th1 cytokine dominance [30]. Table 4 depicts Previous Studies showing the effect of IL-6 and IL-10 in RPL.
Previous studies showing the effect of various cytokines including IL-6, IL-10, and the ratio (IL-6/IL-10) in RPL
| Author and year/reference | Title | Results | Ethnicity | IL-6 | IL-10 | IL-6/IL-10 |
|---|---|---|---|---|---|---|
| Bates et al., 2002 [44] | Aberrant cytokine production by peripheral blood mononuclear cells in recurrent pregnancy loss? | This study showed increased IL-10 levels in pregnant women compared with non-pregnant controls, and further increased in RPL patients (p = 0.026). | Europe | ND | ↑ | ND |
| Daher et al., 2004 [10] | Cytokines in recurrent pregnancy loss | This study comprised 29 women in the RPL group (at least three consecutive spontaneous abortions) and 27 women in the control group (with a history of successful pregnancies and no miscarriage). No significant difference was detected between RPL and control groups in relation to IL-6. | South America | No difference seen | ND | ND |
| Calleja-Agius et al., 2008 [34] | Recurrent miscarriages: What is the role of cytokines? | In this study, lower levels of IL-6 and IL-10 were found in RPL cases than in normal pregnant women. | Europe | ↓ | ↓ | ND |
| Abdullah et al., 2013 [45] | The role of cytokines among women with spontaneous miscarriage | This study enrolled 46 women as the patient group with first-trimester miscarriage and 38 women as the control group with first-trimester successful pregnancy with no history of miscarriage. There were significantly higher levels of IL-10 in the aborted women as compared to the controls, and no significant difference was detected between women with miscarriage and control groups in relation to IL-6. | Asia | No difference seen | ↑ | ND |
| Pandey et al., 2017 [40] | Interplay of cytokines in preterm birth | This study highlights the association of cytokine gene polymorphisms and their levels with preterm birth. Increased maternal levels of IL-6 and low levels of IL-10 have been found to be associated with preterm birth. | Asia | ↑ | ↓ | ND |
| Tyagi et al., 2020 [41] | Evaluation of Pro-inflammatory Cytokine Level in Cases of Idiopathic Recurrent Spontaneous Miscarriage in Saudi Arabia | This study comprised Group I—pregnant women with a history of recurrent spontaneous miscarriage (RSM, n = 50) and Group II—healthy pregnant controls (n = 50). IL-6 was found to be decreased in Group I patients (p < 0.001) as compared with the normal control Group II. | Saudi Arabia | ↓ | ND | ND |
| Vilotić et al., 2022 [35] | IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies | According to the reviewed literature, IL-6 appears to contribute to the establishment and maintenance of pregnancy by mediating uterine receptivity, trophoblast function at the implantation site and parturition, the immune-endocrine interactions at the feto-maternal interface, and other processes. Increased IL-6 expression in decidual tissues in RPL, compared to normal pregnancy, was found. | Europe | ↑ | ND | ND |
| Our findings—2025 | Association of circulating IL-6 and IL-10 levels during mid-gestation with recurrent pregnancy loss history and severity: a South Indianstudy | This study comprised a total of 170 pregnant women with (RPL, n = 85) and without (NRPL, n = 85) a history of RPL during the second trimester of pregnancy. The RPL group was further divided into women with 2 and > 2 previous pregnancy losses. Increased levels of IL-6 and IL-6/IL-10 ratio and decreased levels of IL-10 were found in the RPL group compared to the NRPL group. | Asia | ↑ | ↓ | ↑ |
IL-6: interleukin-6; RPL: recurrent pregnancy loss; NRPL: non-recurrent pregnancy loss; ↑: increased in RPL; ↓: decreased in RPL; ND: not defined
BMI, maternal age, age at first conception, age at menarche, gestational age, and number of abortions are key factors influencing pregnancy outcomes. Extremes in BMI (underweight or obesity) can lead to complications like gestational diabetes, PE, and preterm birth. Advanced maternal age (over 35) increases risks of chromosomal abnormalities, miscarriage, and labor complications. The age at first conception and menarche can also affect reproductive health, with earlier or later ages linked to varying risks of pregnancy complications. Gestational age, or the duration of pregnancy, is crucial, with preterm (before 37 weeks) and post-term (after 42 weeks) pregnancies carrying higher risks for both mother and baby. Together, these factors impact maternal and fetal health and pregnancy outcomes.
A significant difference was observed between the RPL and NRPL groups in terms of mean age (22 vs. 25 years), BMI (22.5 vs. 25.6 kg/m2), and the number of abortions (p = 0.0001). According to Magnus et al. (2019) [36], increasing maternal age may serve as an independent risk factor for miscarriage. Their study noted that the risk of miscarriage was lowest among women aged 25–29 (10%) but rose sharply after the age of 30, reaching as high as 53% in women aged 45 [24]. Furthermore, meta-analyses by Eapen et al. (2021) [37] and Ng et al. (2021) [38] indicated that women with a BMI above 25 kg/m2 are at significantly higher risk of RPL. Similarly, Cavalcante et al. (2019) [39] reported that a BMI greater than 25 kg/m2 further increases the likelihood of miscarriage, highlighting elevated BMI as a contributing factor to pregnancy loss.
Our study showed elevated circulating levels of IL-6 in the RPL group compared to NRPL. These results are consistent with earlier reports where elevated IL-6 is frequently evident in the altered cytokine profiles characteristic of unexplained infertility, recurrent miscarriage, indicating altered systemic IL-6 trans-signalling in women prone to recurrent miscarriage, with excessive IL-6 bioavailability potentially inhibiting the generation of CD4+ Treg cells required for pregnancy tolerance [5, 23]. Vilotić et al. (2022) [35] and Pandey et al. (2017) [40] reported increased IL-6 levels in RPL cases and preterm birth, respectively. However, Calleja-Agius et al. (2008) [34] and Tyagi et al. (2020) [41] reported reduced IL-6 levels in pregnancy complications. Further, similar levels of IL-6 were found within the RPL group between 2 and > 2 previous pregnancy losses (Table 2). We have not come across any literature pertaining to this aspect, warranting large studies in this angle.
It is well established that a successful pregnancy is associated with a balanced pro-inflammation in the first and final trimester, which corresponds to implantation and parturition, and the second trimester with anti-inflammatory dominance for fetal growth [42, 43]. The observed elevated levels of IL-6 in our study during the second trimester pregnancy in women with a history of RPL may indicate prevailing pro-inflammation, though not enough to cause an adverse pregnancy outcome, as it may require additional factors for an unfavourable outcome.
We noted decreased circulating levels of IL-10 in the mid-trimester of ongoing pregnancy of women with a history of RPL compared to the NRPL group, and a good characteristic of ROC analysis for IL-10. Further, with respect to the magnitude (2 vs. > 2) of pregnancy losses, no variation was observed in IL-10 levels between 2 and > 2 previous pregnancy losses. Calleja-Agius et al. (2009) [34] reported diminished and Bates et al. (2002) [44] elevated IL-10 production by stimulated peripheral blood mononuclear cells (PBMCs) from pregnant women with the previous idiopathic RPL at the time of delivery and during early pregnancy. Abdullah et al. (2013) [45] showed higher levels of IL-10 in first-trimester pregnancy losses in Iraqi women. Further, association of certain IL-10 gene polymorphism are also reported in RPL cases, Bahadori et al. (2014) [46] and Parveen et al. [47] (2013). Literature suggests that the inhibitory effects of IL-10 are primarily through decreasing pro-inflammatory cytokines (IL-6, IL-1β, TNF-α, etc.) via heme-oxygenase-1, impeding recruitment and expansion of Th1 cells like Th17, blocking antigen presentation via MHC class II expression [42]. These events control and curtail excessive inflammation through a feedback loop. Promotion of decidual natural killer cells (dNK) cell-mediated immune tolerance during pregnancy may be achieved by the synergistic action of IFN-γ, IL-10, and IL-1Ra that regulate the inhibition of Th17 cells [42, 43, 48].
Conclusive results pertaining to different physiological stages of pregnancy cannot be drawn from the existing studies (as this study deals with only second-trimester cases) except that the circulating levels of IL-10 are disturbed in mid-gestation, which may be a reflection of the feto-maternal milieu in the pregnancy of RPL cases. Multi-centred large single systematic perspective studies considering different gestational stages of RPL and NRPL cases with favourable and unfavourable pregnancy outcomes are needed to understand the pleotropic role of IL-10 and to exploit it in the cytokine therapy for RPL cases.
Our observation of significantly elevated IL-6 levels and reduced IL-10 levels individually may not serve as entirely reliable indicators of the overall inflammatory status. Therefore, the IL-6/IL-10 ratio was calculated to assess the relative systemic concentrations of these pro- and anti-inflammatory cytokines within the same individual, providing a more comprehensive reflection of the inflammatory state. Increased serum levels of the IL-6/IL-10 ratio in the RPL group compared to the NRPL group (cut-off > 0.626 pg/mL, p-value = 0.0001) were noted in the present study. The results are indicative of increased pro-inflammatory cytokines in relation to inhibitory cytokines in the RPL group. Makhseed et al. (2001) [49] support our observations reporting elevated pro/anti-inflammatory cytokines in pregnancy-related complications. It is assumed that the RPL group exhibits an underlying immune dysregulation characterized by heightened sterile inflammation compared to the NRPL group [50, 51]. In such a state, even a minor trigger, whether intrinsic or extrinsic, could potentially escalate inflammation to a critical threshold, increasing the risk of adverse pregnancy outcomes in the RPL group. However, this hypothesis requires further investigation, including studies involving cases with and without unfavourable outcomes.
To determine the cytokine scores, the sensitivity and specificity of each cytokine and the ratio were plotted in the RPL vs. NRPL groups. The ROC analysis revealed that both IL-6 and the IL-6/IL-10 ratio were strong predictors of RPL when comparing NRPL and RPL groups, demonstrating high sensitivity and specificity. In contrast, IL-10 alone showed moderate diagnostic potential, suggesting that the recurring rise in the number of miscarriages may be related to increasing inflammation. However, this predictive ability was notably diminished when stratifying the RPL group by the number of losses (2 vs. > 2). The closer alignment of the ROC curves to the diagonal reference line in this subgroup indicates limited discriminative capacity of these markers in predicting the magnitude of pregnancy loss, suggesting the need for additional biomarkers.
RPL is defined as the loss of two or more clinical pregnancies before the 20th week of gestation. Most studies have primarily focused on first-trimester losses, with limited research addressing second-trimester pregnancies. In our study, we identified increased maternal age and elevated BMI as significant risk factors for RPL, underlining the importance of early recognition and management of these modifiable factors to improve pregnancy outcomes. We also assessed the circulating levels of pro-inflammatory cytokine IL-6 and anti-inflammatory cytokine IL-10 in women during the second trimester of an ongoing pregnancy, all of whom had a history of two or more pregnancy losses followed by subsequent favourable outcomes. Our findings indicate a predominant pro-inflammatory state, evidenced by elevated IL-6 levels in women with RPL compared to those without a history of RPL. This suggests that women with RPL may possess an inherently heightened pro-inflammatory environment, which could be further influenced by internal or external factors with a lower threshold, potentially leading to either favourable or unfavourable pregnancy outcomes. Longitudinal studies involving a broader spectrum of cytokines, encompassing both favourable and unfavourable outcomes, are essential to better characterize the immunological profile of women in the second trimester and to identify potential biomarkers for the diagnosis and management of RPL.
APS: antiphospholipid syndrome
ELISA: enzyme linked immunosorbent assay
IL-1Ra: IL-1 receptor antagonist
IL-6: interleukin-6
MHC: major histocompatibility complex
NRPL: non-recurrent pregnancy loss
ROC: receiver operating characteristic
RPL: recurrent pregnancy loss
Th17: T helper 17
Treg: regulatory T
We are grateful to Dr. Rajeshwari Bonu, Gynaecologist, Niloufer Government Maternity Hospital, Hyderabad, India, for giving us the opportunity to carry forward our research by providing blood samples, and we express gratitude to the blood donors for their cooperation in sharing their clinical data.
SJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing. RB: Data curation, Investigation. PJ: Conceptualization, Project administration, Supervision, Funding acquisition, Resources, Formal analysis, Writing—review & editing.
The authors declare that they have no conflicts of interest.
The present study has approval from the Ethical Committee (Reg. No. ECR/300/Inst/AP/2013/RR-16), Osmania Medical College, Hyderabad, Telangana state, India, and informed consent from the study subjects. This study complies with the 2013 version of the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The preprint for this study is available on Authorea. The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
The authors have received research funding from the Indian Council of Medical Research, New Delhi, India [ICMR, Id No.: 2019–1022]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1872
Download: 22
Times Cited: 0
