Affiliation:
1Department of Anesthesia, Intensive Care and Pain Therapy, University of Verona, 37134 Verona, Italy
Email: marta.nizzero@gmail.com
ORCID: https://orcid.org/0000-0001-6175-0620
Affiliation:
1Department of Anesthesia, Intensive Care and Pain Therapy, University of Verona, 37134 Verona, Italy
ORCID: https://orcid.org/0000-0001-9833-4997
Affiliation:
1Department of Anesthesia, Intensive Care and Pain Therapy, University of Verona, 37134 Verona, Italy
ORCID: https://orcid.org/0000-0002-7280-5356
Affiliation:
1Department of Anesthesia, Intensive Care and Pain Therapy, University of Verona, 37134 Verona, Italy
ORCID: https://orcid.org/0000-0002-2504-5913
Affiliation:
2Fondazione Paolo Procacci, 00193 Roma, Italy
3College of Medicine, University of Baghdad, Baghdad 10064, Iraq
ORCID: https://orcid.org/0000-0002-3822-2923
Affiliation:
4Department of Diagnostics and Public Health, Section of Forensic Medicine, University of Verona, 37134 Verona, Italy
ORCID: https://orcid.org/0000-0002-9147-9373
Affiliation:
5Department of Maternal, Child and Adult Medical and Surgical Sciences, University of Verona, 37134 Verona, Italy
ORCID: https://orcid.org/0000-0001-7924-5577
Affiliation:
1Department of Anesthesia, Intensive Care and Pain Therapy, University of Verona, 37134 Verona, Italy
Affiliation:
6European League Against Pain, CH-8057 Zurich, Switzerland
ORCID: https://orcid.org/0009-0000-9053-2139
Affiliation:
1Department of Anesthesia, Intensive Care and Pain Therapy, University of Verona, 37134 Verona, Italy
ORCID: https://orcid.org/0000-0001-5628-184X
Explor Immunol. 2025;5:1003206 DOI: https://doi.org/10.37349/ei.2025.1003206
Received: December 10, 2024 Accepted: June 19, 2025 Published: August 06, 2025
Academic Editor: Giuseppe Bardi, Istituto Italiano di Tecnologia, Italy
The article belongs to the special issue Immunology and Pain
Fibromyalgia syndrome (FMS) is a chronic condition characterized by widespread musculoskeletal pain, fatigue, cognitive impairments, and sleep disturbances. Although traditionally considered psychogenic, recent research supports a multifactorial etiology involving central nervous system (CNS) dysregulation and significant immune involvement. This narrative review synthesizes current evidence regarding the role of immune mechanisms in FMS, with comparative insights into chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) and irritable bowel syndrome (IBS)—previously grouped under functional somatic syndromes (FSS). In FMS, immune dysregulation is evidenced by elevated levels of pro-inflammatory cytokines (e.g., IL-6, IL-8, TNF-α) and decreased anti-inflammatory mediators such as IL-10, contributing to symptomatology including pain amplification and fatigue. Neuroinflammation, as indicated by microglial activation in pain-processing CNS regions, further supports the role of immune signaling in central sensitization. Other contributing factors include oxidative stress, mitochondrial dysfunction, and immune cell alterations, particularly involving regulatory T cells and natural killer (NK) cells. Compared to FMS, CFS/ME exhibits greater systemic immune activation and more severe mitochondrial impairment, correlating with profound fatigue and cognitive decline. IBS, on the other hand, shows immune activation localized to the gastrointestinal tract, emphasizing the gut-brain axis. These findings highlight both shared and syndrome-specific immune features. To better reflect their systemic and immunological complexity, this review refers to these conditions collectively as chronic multisystem immune-related disorders (CMIRDs). The evidence supports the development of biomarker-based diagnostics and personalized immunomodulatory therapies. A multidisciplinary approach that integrates immunology and neurology is essential to improve outcomes for patients with FMS and related disorders.
Fibromyalgia syndrome (FMS) is a chronic syndrome characterized by widespread musculoskeletal pain, sleep disturbances, fatigue, and cognitive impairment, often referred to as “fibro fog” [1, 2]. Predominantly affecting women, FMS is a significant contributor to chronic pain, with an estimated prevalence of 2–8% in the general population [3, 4]. FMS was historically considered a psychogenic disorder linked to somatization. However, it is now increasingly recognized as a multifactorial condition involving central nervous system (CNS) dysfunction, dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, and, critically, immune system alterations [5, 6]. Immune involvement in FMS was initially evidenced by dysregulated cytokine profiles, with studies reporting elevated levels of pro-inflammatory cytokines (IL-6, IL-8, and TNF-α) and reduced anti-inflammatory cytokines such as IL-10 [7, 8]. In addition, neuroinflammation has emerged as a key mechanism in FMS pathophysiology. Activation of glial cells and the subsequent release of cytokines, including IL-1β, TNF-α, and IL-6, contribute to central sensitization [9, 10]. This has been supported by advanced imaging studies demonstrating microglial activation in brain regions involved in pain processing [11, 12].
FMS shares several clinical and biological features with other chronic multisystem immune-related disorders (CMIRDs), such as chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) and irritable bowel syndrome (IBS). CFS/ME is characterized by elevated systemic inflammation, mitochondrial dysfunction, and impaired natural killer (NK) cell activity, which contribute to symptoms such as profound fatigue and post-exertional malaise [13, 14]. In contrast, IBS shows localized immune activation within the gastrointestinal tract, highlighting the gut-brain axis as a key pathogenic mechanism [15, 16].
A comparative analysis of these syndromes is valuable, as they all involve interactions between the CNS and immune system, yet differ in the extent, localization, and nature of immune dysregulation [17]. These differences underscore the heterogeneity within CMIRDs and highlight the need for individualized, mechanism-based therapeutic strategies.
This review aims to synthesize current knowledge on immune system involvement in FMS and to compare it with that of CFS/ME and IBS, emphasizing both shared and syndrome-specific immunopathological features.
A narrative review methodology was employed to synthesize relevant literature on immune dysregulation in FMS and other CMIRDs. A comprehensive search was conducted across PubMed, Scopus, and Web of Science, using a combination of keywords and Boolean operators including ‘fibromyalgia’, ‘immune system’, ‘cytokines’, ‘neuroinflammation’, ‘FSS’, ‘CFS/ME’, and ‘IBS’. The review included peer-reviewed clinical and pre-clinical studies published in English from January 2000 to March 2024. Inclusion criteria comprised studies providing empirical evidence on immune dysregulation in FMS, CFS/ME, or IBS. Exclusion criteria included non-English publications, editorials, commentaries, and studies focusing solely on psychiatric or unrelated systemic disorders. Data extraction prioritized pathological features in terms of cytokine profiles, neuroinflammatory findings, mitochondrial dysfunction, and immune cell characterization using validated methods such as ELISA, PET imaging, and flow cytometry. When available, data on actual or potential clinical implications were also extracted.
The interplay between cytokine imbalances and neuroinflammation highlights the complexity of FMS pathogenesis. A hallmark of immune dysregulation in FMS is the imbalance between pro- and anti-inflammatory cytokines. Studies consistently report elevated levels of pro-inflammatory cytokines such as IL-6, IL-8, and TNF-α in FMS patients [8, 18]. These cytokines not only exacerbate peripheral inflammation but also act on the CNS, promoting microglial activation and contributing to central sensitization, causing pain amplification, fatigue, and depression-like symptoms [9, 19].
Conversely, in FMS, levels of anti-inflammatory cytokines, such as IL-10, are reduced, further perpetuating a pro-inflammatory state [20]. Table 1 highlights the imbalance between pro-inflammatory and anti-inflammatory cytokines in FMS patients.
Cytokine pattern, oxidative stress, and specific immune cells dysfunction in FMS patients
| Category | Biomarkers/Cells | Observed changes in FMS | Functional/Clinical implications |
|---|---|---|---|
| Pro-inflammatory cytokines | IL-6, IL-8, TNF-α | ↑ levels | Pain amplification, fatigue, mood disturbances |
| Anti-inflammatory cytokines | IL-10 | ↓ levels | Sustained pro-inflammatory state |
| Oxidative stress | Malondialdehyde, protein carbonyls | ↑ levels | Correlate with symptom severity |
| Antioxidant defense | Glutathione, superoxide dismutase | ↓ activity | Increased oxidative damage |
| Immune system cells | T-reg cells | ↓ cell count and function | Failure to suppress immune hyperactivation |
| Immune system cells | NK cells | ↓ activity | Compromised immune surveillance |
Most studies cited used blood or serum samples and cytokine assays, such as ELISA, to quantify cytokine concentrations in FMS patients. Biomarkers such as malondialdehyde were assessed in plasma using spectrophotometric assays. NK cell activity was commonly evaluated using flow cytometry-based cytotoxicity assays. T-reg cell counts were determined through immunophenotyping. FMS: fibromyalgia syndrome; T-reg cells: regulatory T cells; NK: natural killer; ↑: increased; ↓: decreased
Advancements in neuroimaging have provided compelling evidence for neuroinflammation in FMS. PET studies have demonstrated increased microglial activation in brain regions associated with pain processing, including the thalamus, prefrontal cortex, and insula [11, 12]. Microglia, the resident immune cells of the CNS, release pro-inflammatory mediators such as IL-1β, TNF-α, and reactive oxygen species (ROS), which contribute to the hyperexcitability of pain pathways [10].
Sustained microglial activation may arise from chronic peripheral immune signaling or stress-induced disruption of the blood-brain barrier (BBB). This dysregulation likely contributes to the sensory, cognitive, and affective disturbances observed in FMS [9].
Oxidative stress, defined as an imbalance between free radicals and antioxidant defenses, is prevalent in FMS and closely linked to immune dysfunction [21]. Elevated markers of oxidative stress, including malondialdehyde and protein carbonyls, have been detected in FMS patients, correlating with symptom severity [22]. Oxidative stress can activate the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, amplifying pro-inflammatory cytokine production.
Although mitochondrial dysfunction and increased ROS are present in FMS, evidence suggests these alterations are more pronounced in CFS/ME. Metabolomic studies in CFS/ME have identified significant alterations in lipid metabolism and energy production pathways, supporting a more profound mitochondrial impairment [23, 24]. Dysfunctional mitochondria may contribute to the metabolic and immunological dysregulation observed in FMS, exacerbating fatigue and pain [14].
Table 1 illustrates the presence of oxidative stress in FMS patients, evidenced by elevated levels of markers such as malondialdehyde and protein carbonyls. Concurrently, a reduction in the antioxidant defenses, including glutathione and superoxide dismutase, leads to increased oxidative damage, correlating with the severity of FMS symptoms.
Reduced numbers and impaired function of regulatory T cells (T-reg cells) have been observed in FMS patients, leading to a failure in suppressing immune hyperactivation [22]. NK cell activity is variably impaired in FM but shows a more consistent and pronounced reduction in CFS/ME, where decreased cytotoxicity is a well-documented immunological feature, likely to significantly compromise immune homeostasis [25, 26].
As illustrated in Table 1, a decrease in the number and function of the T-regs leads to inadequate suppression of immune responses, resulting in hyperactivation. Additionally, altered activity of NK cells compromises the body’s ability to surveil and respond to pathological changes, contributing to the persistence of FMS symptoms.
In summary, FMS is characterized by moderate systemic immune activation, with elevated proinflammatory cytokines, including IL-6, IL-8, and TNF-α, and reduced levels of the anti-inflammatory cytokine IL-10 [8, 18]. Neuroinflammation is a hallmark, as demonstrated by PET imaging showing microglial activation in CNS regions associated with pain processing, such as the thalamus, limbic system, and prefrontal cortex [11, 12]. Peripheral immune alterations include reduced T-reg cell counts and increased oxidative stress markers, which contribute to a chronic inflammatory state [21, 22].
CFS/ME exhibits the highest level of systemic immune activation among CMIRDs, with elevated IL-1β, TNF-α, and IFN-γ levels, alongside reduced NK cell cytotoxicity [13, 25]. This dysregulation correlates with profound fatigue and cognitive impairments. Neuroinflammation in CFS/ME is coupled with mitochondrial dysfunction and impaired energy metabolism, suggesting a distinct interplay between immune activation and cellular energetics [14].
In IBS, immune activation is primarily localized to the gastrointestinal tract, with elevated IL-6 and IL-8 levels in the gut mucosa but minimal systemic immune involvement [15, 16]. Mast cell activation in the intestinal wall contributes to localized inflammation and visceral hypersensitivity, emphasizing the gut-brain axis as a key mechanism.
As synthesized in Table 2, FMS demonstrates moderate systemic immune activation, reflected in elevated IL-6, IL-8, and TNF-α levels. These cytokines are associated with pain amplification, fatigue, and systemic symptoms [18]. In terms of neuroinflammation, PET imaging studies have revealed microglial activation in pain-related CNS regions such as the thalamus, limbic system, and prefrontal cortex, suggesting a link between immune activity and central sensitization [11].
A comparative analysis of systemic immune activation (SIA) in three CMIRDs
| Syndrome | SIA | Cytokines elevated | Neuroinflammation | Predominant cellular dysfunction |
|---|---|---|---|---|
| FMS | Moderate | IL-6, IL-8, TNF-α | Microglial activation | Reduced T-reg cells |
| CFS/ME | High | IL-1β, TNF-α, IFN-γ | Neuroinflammation and mitochondrial dysfunction | Reduced NK cell activity |
| IBS | Low | IL-6, IL-8 | Localized (gut-brain axis) | Mast cell activation (localized) |
CMIRDs: chronic multisystem immune-related disorders; FMS: fibromyalgia syndrome; CFS/ME: chronic fatigue syndrome/myalgic encephalomyelitis; IBS: irritable bowel syndrome; NK: natural killer
As for cellular dysfunction, reduced T-reg cells compromise immune homeostasis, while elevated oxidative stress markers suggest prolonged immune activation. These immune alterations correlate with clinical features including widespread pain, cognitive dysfunction, and fatigue [21]. CFS/ME presents a different immunopathological profile, exhibiting high systemic immune involvement. Pro-inflammatory cytokines such as IL-1β, TNF-α, and IFN-γ are elevated, and NK cell cytotoxicity is significantly reduced, with recent meta-analytic data confirming this reduction to nearly half the activity found in healthy individuals [26]. These alterations are linked to profound fatigue and post-exertional malaise [13, 25]. The combination of neuroinflammation and mitochondrial dysfunction highlights a unique interplay between immune activation and cellular energy deficits, exacerbating cognitive and physical fatigue [14]. Cellular dysfunction can be seen primarily in terms of reduced NK cell activity as a hallmark feature, compromising immune surveillance and further aggravating chronic immune dysregulation [25].
IBS demonstrates low systemic immune activation, with immune activity primarily localized to the gastrointestinal tract. Elevated IL-6 and IL-8 levels are observed in the gut mucosa. Localized neuroinflammation in the gut-brain axis drives visceral hypersensitivity. This process involves interactions between the enteric nervous system and the immune system [15, 16]. Mast cell activation within the intestinal mucosa is the key feature in terms of cellular dysfunction for this clinical entity, and it plays a pivotal role in localized inflammation, contributing to symptoms such as abdominal pain and bloating [15]. However, mast cell activation has also been reported in FMS and CFS/ME. Recent studies suggest that mast cells may contribute to pain and inflammation in FMS by releasing neuro-sensitizing mediators, while increased cutaneous mast cell counts have been observed in patients with CFS/ME [27].
Furthermore, as represented in the bar chart in Figure 1, comparing the levels of systemic immune activation among FMS, CFS/ME, and IBS, FMS exhibits moderate activation, while CFS/ME shows the highest systemic involvement, and IBS remains primarily localized in its immune response.
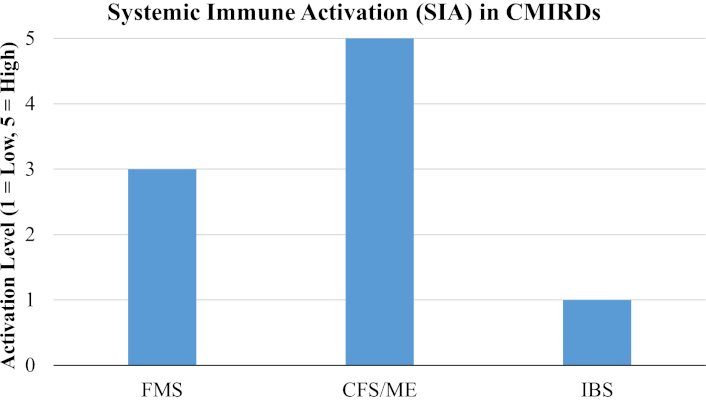
Bar chart showing the SIA levels in the three main CMIRDs. FMS: fibromyalgia syndrome; CFS/ME: chronic fatigue syndrome/myalgic encephalomyelitis; IBS: irritable bowel syndrome; CMIRDs: chronic multisystem immune-related disorders
It seems fair to assume that CMIRDs, including CFS/ME and IBS, share overlapping features with FMS, such as chronic pain and fatigue, and exhibit varying degrees of immune involvement. CFS/ME is characterized by elevated pro-inflammatory cytokines (e.g., IL-1β, TNF-α) and profound mitochondrial dysfunction, resulting in severe fatigue and post-exertional malaise. Reduced NK cell activity is more pronounced in CFS/ME compared to FMS [13, 14]. Immune activation in IBS is predominantly localized to the gut mucosa, with increased mast cell activity and elevated IL-6 and IL-8 levels. Unlike FMS and CFS/ME, systemic immune dysregulation is less prominent in IBS [15, 16, 28]. This comparative analysis underscores the heterogeneity in immune system involvement across CMIRDs, emphasizing the need for syndrome-specific biomarkers and tailored therapeutic strategies.
This review highlights the pivotal role of immune dysregulation in the pathogenesis of FMS, with substantial evidence implicating cytokine imbalance, neuroinflammation, oxidative stress, and immune cell dysfunction. These findings align with a growing body of literature suggesting that FMS is not merely a functional disorder but rather a systemic condition with significant neuroimmune involvement. Neuroinflammation, particularly the activation of microglia in pain-processing regions of the CNS, emerges as a key mechanism underlying the amplification of pain and associated symptoms such as fatigue and cognitive dysfunction. This process appears to be mediated by pro-inflammatory cytokines and sustained immune signaling, supporting the notion that immune-CNS interactions contribute directly to clinical symptomatology. While FMS shares many immunological mechanisms with CFS/ME, including elevated cytokines and NK cell abnormalities, the magnitude and consistency of these findings differ. In CFS/ME, immune dysfunction is more pronounced and often includes marked reductions in NK cell cytotoxicity and greater mitochondrial impairment. In contrast, IBS primarily involves localized immune activity within the gastrointestinal tract, with less systemic immune involvement.
A critical aspect in reviewing the immunological evidence in fibromyalgia and related syndromes is the degree of consistency across findings. Most studies report elevated IL-6 in FMS, though some failed to detect significant differences compared to controls, possibly due to heterogeneity in diagnostic criteria, disease duration, or sampling methods. Similarly, findings on NK cell dysfunction in FMS are inconsistent, with some studies reporting reduced activity while others found no significant changes. These discrepancies may stem from small sample sizes, methodological variability, or differences in immune profiling techniques. This variability emphasizes the need for standardized protocols and replication in larger, well-characterized cohorts.
The recognition of immune dysregulation in FMS has significant implications for diagnosis and treatment. Biomarkers such as IL-6, IL-8, and TNF-α could aid in differentiating FMS from other chronic pain syndromes. The recognition of neuroinflammation as a core component of FMS pathophysiology also opens venues for novel therapeutic interventions. Targeting glial cell activation, particularly microglia, represents a promising strategy. Both microglia inhibitors and cytokine antagonists hold promise for improving outcomes in FMS [29, 30]. Low-dose naltrexone (LDN), a toll-like receptor-4 (TLR-4) antagonist, has shown potential in modulating microglial activity and reducing pro-inflammatory cytokine release, resulting in decreased pain and fatigue in FMS patients [31, 32]. Other glial-modulating agents, such as minocycline (a broad-spectrum tetracycline antibiotic) and ibudilast (a phosphodiesterase inhibitor and TLR-4 modulator), are being explored for their anti-inflammatory and neuroprotective effects [33–37]. Additionally, interventions aimed at reducing systemic inflammation—such as omega-3 fatty acids, cytokine antagonists—may indirectly hinder neuroinflammatory signaling [35, 38]. These approaches emphasize the importance of targeting both central and peripheral immune pathways in the management of FMS.
Non-pharmacological interventions might also mitigate oxidative stress and improve immune function. A peculiar, novel non-pharmacological therapeutic option in the practice of whole-body cryotherapy (WBC), a fast-acting and effective procedure that has been proven to positively alter cytokine profiles in FMS patients, leading to significant clinical benefits in terms of reduced pain sensation and disease activity [39]. Tailored therapies addressing the unique immunological profile of FMS patients are essential for advancing treatment approaches.
In conclusion, FMS is a chronic pain syndrome underpinned by complex immune system alterations. Dysregulated cytokine production, neuroinflammation, oxidative stress, and cellular immune dysfunction interact to perpetuate the debilitating symptoms of FMS. While these mechanisms overlap with other CMIRDs, such as CFS/ME and IBS, FMS presents a unique profile of systemic immune activation and CNS involvement. Researchers should then focus on identifying biomarkers, such as cytokine profiles or neuroimaging markers, to enhance diagnostic accuracy and guide therapeutic interventions. Additionally, longitudinal studies tracking immune changes over the disease course could illuminate causal mechanisms and inform precision medicine approaches.
Future research should prioritize the identification of immune-specific biomarkers and investigate the efficacy of immune-modulating therapies. A multidisciplinary approach integrating immunological, neurological, and psychological perspectives is crucial for advancing FMS management and improving patient quality of life.
CFS/ME: chronic fatigue syndrome/myalgic encephalomyelitis
CMIRDs: chronic multisystem immune-related disorders
CNS: central nervous system
FMS: fibromyalgia syndrome
IBS: irritable bowel syndrome
NK: natural killer
ROS: reactive oxygen species
TLR-4: toll-like receptor-4
T-regs: regulatory T cells
The authors wish to express their gratitude to colleagues of the Rheumatology Department and Clinical Psychology of the University of Verona for their valuable insights, constructive feedback, and critical discussions that greatly enhanced the quality of this review. Their expertise and thoughtful suggestions contributed to the refinement of the manuscript. Any remaining errors or omissions are solely the responsibility of the authors.
MN: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. VS and GV: Conceptualization, Writing—review & editing. LG and EP: Validation, Writing—review & editing, Supervision. AM, SC, and GDB: Supervision, Writing—review & editing. LP: Investigation. ES: Writing—original draft. All authors read and approved the submitted version.
Giustino Varrassi, who is the Editorial Board Member and a Guest Editor of Exploration of Immunology, had no involvement in the decision-making or the review process of this manuscript. The other authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Rucha A. Kelkar ... Giustino Varrassi
Marco Cascella ... The TRIAL Group
Mariateresa Giglio ... Filomena Puntillo
Antonella Ciaramella, Giancarlo Carli
