Affiliation:
Biotechnology Department, College of Applied Science, University of Fallujah, Fallujah 31002, Iraq
Email: hanan.exas@uofallujah.edu.iq
ORCID: https://orcid.org/0009-0008-2955-1494
Explor Immunol. 2025;5:1003210 DOI: https://doi.org/10.37349/ei.2025.1003210
Received: March 18, 2025 Accepted: July 22, 2025 Published: August 17, 2025
Academic Editor: Giuseppe Murdaca, University of Genova, Italy
Aim: Vitiligo is an autoimmune skin disorder characterized by melanocyte destruction and progressive depigmentation. Cytokine imbalance plays a key role in its pathogenesis. This study aims to evaluate and compare serum levels of the anti-inflammatory cytokine interleukin-10 (IL-10) and the pro-inflammatory cytokine IL-17 in vitiligo patients and healthy individuals, to explore their potential as biomarkers of disease activity.
Methods: A total of 60 vitiligo patients and 40 age- and sex-matched healthy controls were recruited. Serum concentrations of IL-10 and IL-17 were measured using enzyme-linked immunosorbent assay (ELISA). Disease severity and duration were also assessed in relation to cytokine levels.
Results: Vitiligo patients showed significantly lower IL-10 levels (9.37 ± 0.17 pg/mL) compared to controls (11.38 ± 0.22 pg/mL, P < 0.01), and significantly higher IL-17 levels (326.48 ± 5.49 pg/mL) compared to controls (270.47 ± 8.48 pg/mL, P < 0.01).
Conclusions: These findings suggest an inflammatory cytokine imbalance in vitiligo, characterized by decreased IL-10 and elevated IL-17 levels. The significant correlation of IL-17 with disease progression supports its role as a potential biomarker of disease activity. Targeting cytokine pathways may offer new directions for immunomodulatory treatment strategies in vitiligo.
Vitiligo is defined as a chronic autoimmune skin disease that causes a progressive loss of melanocytes, resulting in skin patches devoid of pigmentation [1–3]. It affects approximately 0.5% to 2% of the global population, with no significant differences based on gender, race, or geographic region [4, 5]. Although vitiligo has long been regarded as an autoimmune disease, its etiopathogenesis is complex and involves a dynamic interaction between genetic predisposition, oxidative stress, and immune system dysregulation [6, 7]. Among the contributing factors, immune-mediated destruction of melanocytes is considered one of the most critical, with growing evidence indicating that cytokine imbalance is a major driver of disease progression [3, 8]. However, the exact roles of pro-inflammatory and anti-inflammatory cytokines in the pathogenesis of vitiligo remain incompletely understood.
The pathogenesis of vitiligo involves a complex interplay between oxidative stress and immune dysfunction [9]. Oxidative stress is recognized as an early triggering factor that leads to melanocyte damage, which subsequently activates immune responses [10–12]. The autoimmune hypothesis suggests that melanocyte destruction is driven by cytotoxic T cells and inflammatory cytokines [13, 14]. Notably, interleukin-17 (IL-17) has been found to be elevated in various autoimmune conditions, further supporting its potential role in vitiligo pathophysiology [15, 16]. In contrast, IL-10 deficiency may impair immune tolerance, thereby perpetuating immune responses against melanocytes [17, 18]. The influence of IL-10 downregulation and IL-17 upregulation on vitiligo severity and progression remains an unresolved question.
Cytokine dysregulation is a key factor in autoimmune disorders [19]. Therefore, in this study, we analyzed the serum levels of IL-10 and IL-17 in vitiligo patients and healthy controls using enzyme-linked immunosorbent assay (ELISA) [20]. The primary aim of this study is to determine and compare serum levels of IL-10 and IL-17 in vitiligo patients versus healthy controls, and to evaluate their association with disease severity and duration. Unlike previous studies, this research investigates both cytokines simultaneously in a well-defined patient population using stringent inclusion/exclusion criteria, providing clearer insight into their role as potential biomarkers for disease activity and progression. The findings may provide evidence for IL-17 as a biomarker of disease activity. Understanding these cytokine dynamics could offer insights into future diagnostic and therapeutic strategies focused on immune modulation in vitiligo patients.
The present study included a total of 100 participants, comprising 60 vitiligo patients and 40 age- and sex-matched healthy controls. Participants were recruited from the Dermatology Outpatient Clinic at the Hospital for Dermatology Diseases, Fallujah Teaching Hospital, Anbar, Iraq, between April 2023 and March 2024. Based on strict inclusion and exclusion criteria, individuals were selected to ensure a homogeneous study population. All patients were diagnosed with vitiligo through clinical examination by a qualified dermatologist, who assessed characteristic features of the lesions along with the patients’ clinical histories. To minimize potential bias, participants were selected using a consecutive sampling method, meaning all eligible patients who visited the clinic during the study period were invited to participate. Healthy control subjects were recruited from the same population and confirmed to have no history of dermatological, autoimmune, or systemic inflammatory disorders, ensuring a comparable demographic background.
The patient and control groups were balanced for age and sex, helping to reduce confounding related to these variables. Participants were aged between 15 and 40 years, as this age range represents a high prevalence of vitiligo while limiting variability due to immature or aging immune systems. To ensure diagnostic clarity, patients with mixed or unclassifiable forms of vitiligo were excluded. Disease severity was recorded, and patients were categorized into mild, moderate, or severe groups based on the extent of skin depigmentation. Strict exclusion criteria were applied to avoid potential confounding factors. Individuals diagnosed with other autoimmune diseases (e.g., psoriasis, rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, or autoimmune thyroid disorders) were excluded due to their independent effects on cytokine production. Additionally, patients receiving phototherapy, topical corticosteroids, or systemic treatments for vitiligo were excluded to ensure that cytokine levels reflected the untreated, natural state of the disease. Environmental and lifestyle factors, which may influence cytokine profiles, were considered during recruitment [21, 22]. While these were not directly analyzed in this study, their documentation allows for potential future research. Moreover, due to the known effects of hormonal changes on immune function, pregnant and lactating women were excluded to prevent hormonal fluctuations from influencing cytokine levels and disease activity.
Blood samples from all participants were collected using standardized procedures to ensure consistency and cytokine stability. Venous blood (5 mL) was drawn from each subject in the morning to account for circadian variations in cytokine expression. Samples were collected in serum separator tubes (SSTs), allowed to clot for 30 minutes at room temperature, and centrifuged at 3,000 rpm for 15 minutes. Serum was then separated and stored at –80°C until analysis using ELISA. The concentrations of IL-10 and IL-17 in serum samples were quantified using commercially available ELISA kits (e.g., Bioassay Technology Lab, IL-10: Cat# E0102Hu; Sunlong Medical, IL-17: Cat# SL0978Hu, Hangzhou City, Zhejiang Province, China), following the manufacturer’s instructions. These kits provided high sensitivity and specificity for human cytokine detection. To minimize pre-analytical variability, all samples were processed within one hour of collection, and only non-hemolyzed specimens were included. The use of stringent inclusion/exclusion criteria and standardized sample handling strengthens the reliability and accuracy of cytokine measurement. Recruiting vitiligo patients and healthy controls from the same geographic area and accounting for potential confounders further enhances the validity of the findings. These methodological considerations provide a solid foundation for advancing understanding of the roles of IL-10 and IL-17 in the pathogenesis of vitiligo.
Table 1 compares the demographic and clinical characteristics of vitiligo patients (n = 60) and healthy controls (n = 40). The median age in both groups was approximately 25 years, with no significant difference observed (P = 0.925), indicating that age did not influence cytokine levels. Since vitiligo is more common in young adults, the selected age range (15–40 years) helps reduce variability due to immune response differences in pediatric or elderly populations. Sex distribution was also balanced, with 55% males in the vitiligo group and 55% in the control group (P = 0.798). This sex matching helps eliminate bias related to sex-based variations in immune response, thereby strengthening the validity of cytokine comparisons between groups. Vitiligo patients were further categorized by disease duration: 20% had disease onset within the past year, 50% had vitiligo for 1–5 years, and 30% had the disease for more than five years. This stratification allows for the analysis of cytokine trends over time, as prolonged disease may be associated with chronic inflammation that could affect IL-17 and IL-10 levels. In terms of treatment status, 43.3% of patients were receiving treatment, while 56.7% were untreated (P = 0.547). Including both treated and untreated patients provides insight into cytokine expression in the context of disease progression rather than treatment effects. Importantly, patients on systemic immunomodulatory therapy were excluded to ensure that cytokine measurements reflected the natural disease state. Overall, Table 1 demonstrates the careful demographic and clinical matching between groups, minimizing potential confounders. This structured sample selection enhances the reliability of the study’s findings regarding IL-17 and IL-10 as potential biomarkers of vitiligo disease activity.
Demographic and clinical characteristics of the study participants
| Demographic and clinical characteristics | Control group (n = 40) | Vitiligo group (n = 60) | P-value |
|---|---|---|---|
| Age (years) | 25 (15–36) | 25 (16–38) | 0.925 |
| Sex | |||
| Male | 22 (55%) | 33 (55%) | 0.798 |
| Female | 18 (45%) | 27 (45%) | |
| Period of disease | |||
| < 1 year | 12 (20%) | NA | |
| 1–5 years | 30 (50%) | ||
| > 5 years | 18 (30%) | ||
| Treatment | |||
| Yes | 26 (43.3%) | 0.547 | |
| No | 34 (56.7%) | ||
Data presented as median (interquartile range) for age and frequency (percentage) for other parameters. P-values from Mann-Whitney U test for age and Fisher’s exact test for other parameters. NA: non-applicable
Table 2 presents a comparative analysis of IL-10 and IL-17 levels between vitiligo patients and healthy controls, revealing significant cytokine dysregulation that may contribute to disease pathogenesis and progression. IL-10, an anti-inflammatory cytokine crucial for immune regulation, was significantly reduced in vitiligo patients (9.37 ± 0.17 pg/mL) compared to controls (11.38 ± 0.22 pg/mL), with a statistically significant difference (P < 0.01). This decrease suggests a compromised immunosuppressive mechanism, resulting in an unchecked immune response against melanocytes. IL-10 downregulation reflects a diminished capacity to suppress inflammation, potentially facilitating sustained autoimmune-mediated melanocyte destruction. Given IL-10’s role in maintaining immune tolerance, its suppression may play a key role in disease persistence and progression.
Comparison between patients and control groups in IL-10 and IL-17
| Group | Means ± SE | |
|---|---|---|
| IL-10 (pg/mL) | IL-17 (pg/mL) | |
| Patients | 9.37 ± 0.17 | 326.48 ± 5.49 |
| Control | 11.38 ± 0.22 | 270.47 ± 8.48 |
| T-value | 7.23 | 5.54 |
| P-value | ** | ** |
The symbol “**” indicates highly significant IL-10 and IL-17 differences between vitiligo patients and controls (P < 0.01)
Conversely, IL-17—a pro-inflammatory cytokine predominantly secreted by Th17 cells—was significantly elevated in vitiligo patients (326.48 ± 5.49 pg/mL) relative to healthy controls (270.47 ± 8.48 pg/mL), with a highly significant difference (P < 0.01). IL-17 is known to amplify inflammation by inducing other inflammatory mediators such as IL-1, IL-6, and tumor necrosis factor-alpha (TNF-α). Its substantial upregulation in vitiligo suggests a heightened inflammatory state, likely contributing to progressive melanocyte destruction. Furthermore, the increased IL-17 levels observed in patients with more severe disease and longer disease duration underscore its potential utility as a biomarker for disease progression. The T-test results in Table 2 reinforce the statistical significance of these findings. For IL-10, a T-value of 7.23** reflects a statistically meaningful, though numerically smaller, difference between groups—still significant due to the low standard error (P < 0.01). In contrast, the T-value for IL-17 (5.54**) indicates a striking and highly significant difference, supporting its central role in the disease immunopathology.
Overall, these results highlight a clear imbalance between anti- and pro-inflammatory cytokines in vitiligo. The simultaneous reduction of IL-10 and elevation of IL-17 suggests a shift in immune homeostasis toward a pro-inflammatory state, perpetuating melanocyte loss. This cytokine dysregulation not only supports the autoimmune basis of vitiligo but also provides a rationale for exploring IL-17 as a diagnostic biomarker and therapeutic target. Targeted inhibition of IL-17 or strategies aimed at restoring IL-10 levels may offer promising avenues for immune modulation in vitiligo treatment. Future research should investigate longitudinal cytokine profiles and evaluate the clinical benefits of IL-17-targeted therapies in mitigating disease severity and progression. In conclusion, the findings in Table 2 provide robust evidence for the role of cytokine imbalance—particularly IL-10 downregulation and IL-17 upregulation—in the pathogenesis of vitiligo.
To enhance the clarity of cytokine comparisons, a histogram was generated to visually represent the serum levels of IL-10 and IL-17 in vitiligo patients and healthy controls in Figure 1. As shown, IL-10 levels were markedly reduced in patients compared to controls, underscoring a compromised anti-inflammatory response. In contrast, IL-17 levels were significantly elevated in the patient group, highlighting a pro-inflammatory shift in immune signaling. This graphical representation complements the tabulated statistical data and reinforces the conclusion that cytokine imbalance, characterized by diminished IL-10 and elevated IL-17, plays a central role in the immunopathogenesis of vitiligo.
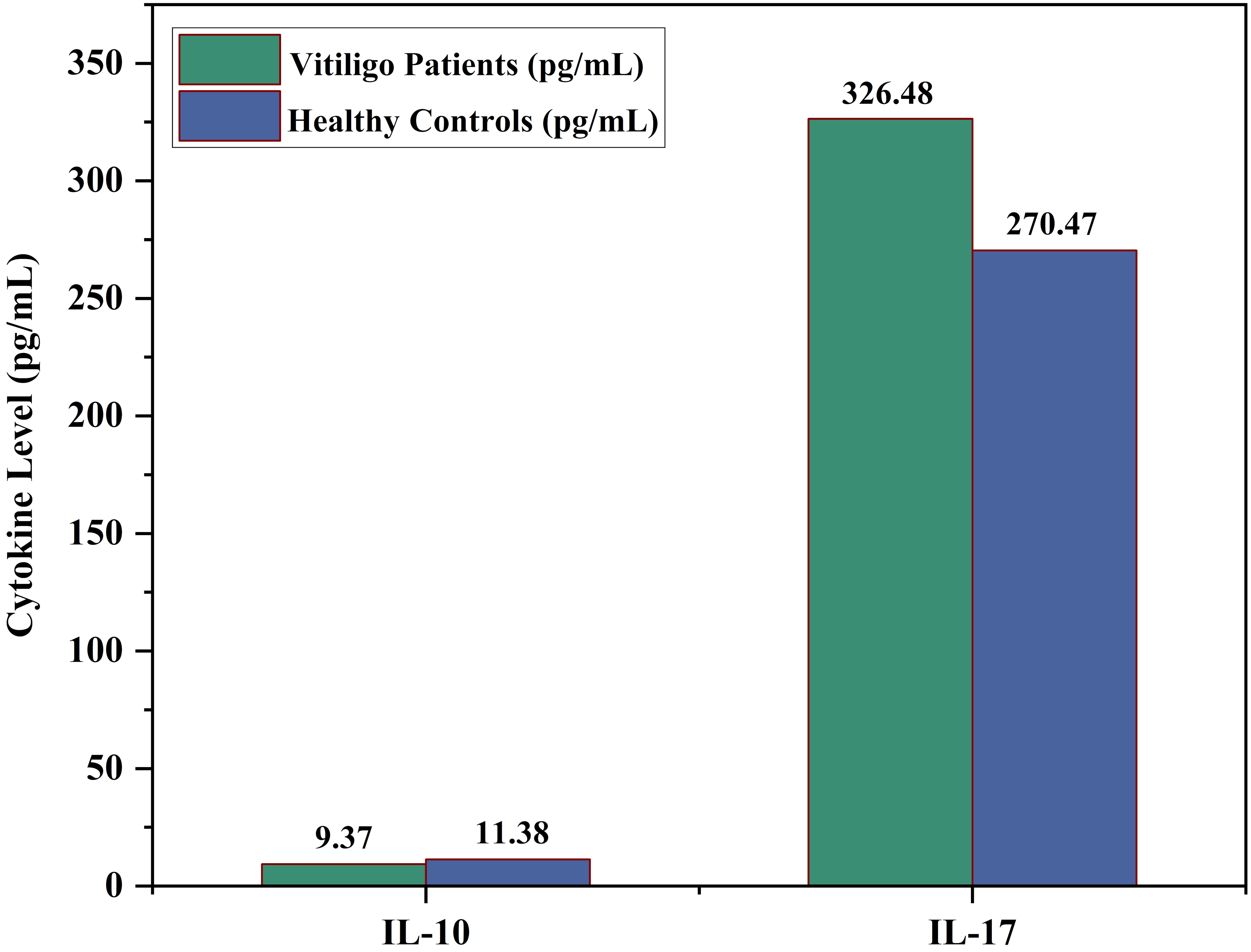
Comparison of serum cytokine levels (IL-10 and IL-17) between vitiligo patients and healthy controls
This study underscores the pivotal role of cytokine imbalance in the pathogenesis of vitiligo, particularly the dysregulation of IL-10 and IL-17. The observed reduction in IL-10 levels suggests a weakened anti-inflammatory response, potentially allowing persistent immune-mediated attacks on melanocytes. In contrast, elevated IL-17 levels indicate a heightened inflammatory state that may exacerbate melanocyte destruction.
The imbalance between IL-10 and IL-17 reflects a shift toward a pro-inflammatory immune environment, reinforcing the autoimmune nature of vitiligo. The insufficient IL-10 response fails to counteract IL-17-driven inflammation, a mechanism that parallels immune dysregulation seen in other autoimmune diseases. Clinically, these findings point toward promising therapeutic strategies—such as enhancing IL-10 activity or inhibiting IL-17 signaling. Biologic agents targeting IL-17 have demonstrated efficacy in other autoimmune conditions, such as psoriasis, and may offer similar therapeutic benefits in vitiligo. Additionally, interventions aimed at augmenting IL-10 function could help restore immune tolerance and suppress disease progression.
However, the cross-sectional design of this study limits causal inference regarding the relationship between cytokine levels and disease progression. Longitudinal studies are warranted to monitor cytokine fluctuations over time and determine their direct impact on vitiligo development and severity. Moreover, while serum cytokine levels provide valuable systemic insights, they may not fully capture local immune responses within vitiligo lesions. This study has several limitations. First, its cross-sectional design limits the ability to establish causal relationships between cytokine levels and disease progression. Second, only serum cytokine levels were measured, which may not fully represent local cytokine activity within skin lesions. Third, the sample size, though adequate, may limit generalizability to all populations. Lastly, while treatment status was documented, further stratification based on treatment history or response was not conducted. Future studies should address these limitations through longitudinal designs, tissue-based analyses, and larger multi-center cohorts.
In conclusion, the findings of this study highlight the critical role of cytokine imbalance in vitiligo pathogenesis, particularly the decreased levels of IL-10 and the elevated levels of IL-17. This dysregulation fosters a pro-inflammatory environment that contributes to melanocyte destruction and disease progression. The observed correlation between IL-17 levels and disease severity supports its potential utility as a biomarker for monitoring vitiligo activity. Concurrently, IL-10 downregulation may impair immune tolerance, thereby intensifying autoimmune responses. These insights lay a valuable foundation for future therapeutic strategies focused on cytokine modulation. Further research is warranted to investigate the clinical potential of IL-17 inhibition or IL-10 enhancement as targeted treatments, which may pave the way for improving management and outcomes in vitiligo patients.
ELISA: enzyme-linked immunosorbent assay
IL-17: interleukin-17
The author would like to express her sincere gratitude to the Deanship of the College of Applied Science at the University of Fallujah for their support of this project. Their guidance and resources were instrumental in the successful completion of this research. Additionally, the author extends her appreciation to colleagues and peers who provided valuable insights and feedback throughout the study.
HMS: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. The author read and approved the submitted version.
The author declares that she has no conflicts of interest.
According to our hospital policy, particularly at the Hospital for Dermatology Diseases, ethical committee approval is not required for case reports in this manuscript as long as written informed consent is obtained from the patients.
Informed consent to participate in the study was obtained from all participants, or their parents or legal guardians of the participants.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1691
Download: 25
Times Cited: 0
