Affiliation:
Perm Federal Research Center of the Ural Branch of the Russian Academy of Sciences, 614081 Perm, Russia
Email: olia15_77@mail.ru
ORCID: https://orcid.org/0000-0002-7580-6848
Affiliation:
Perm Federal Research Center of the Ural Branch of the Russian Academy of Sciences, 614081 Perm, Russia
ORCID: https://orcid.org/0000-0003-1195-8962
Affiliation:
Perm Federal Research Center of the Ural Branch of the Russian Academy of Sciences, 614081 Perm, Russia
ORCID: https://orcid.org/0000-0001-6050-8656
Affiliation:
Perm Federal Research Center of the Ural Branch of the Russian Academy of Sciences, 614081 Perm, Russia
ORCID: https://orcid.org/0000-0001-5766-7496
Explor Immunol. 2025;5:1003214 DOI: https://doi.org/10.37349/ei.2025.1003214
Received: February 06, 2025 Accepted: July 10, 2025 Published: August 26, 2025
Academic Editor: Jinming Han, Capital Medical University, China
Aim: Regulatory T (Treg) cells and interleukin-17-producing T helper (Th17) cells play a critical role in successful pregnancy. Treg and Th17 cells differentiate predominantly in the thymus. Despite steroid-induced pregnancy thymic involution, the peripheral blood Treg number increases, indicating peripheral expansion. Thymic atrophy is accompanied by a decrease in T-cell receptor diversity, but is compensated for by activation of RAG2 (recombination activating genes) in the periphery, which initiates extrathymic T-cell differentiation. In addition, naive Treg enhance their suppressive activity during pregnancy, which may play an important role in the development of maternal tolerance to fetal antigens. The changes in naive Th17 thymic output during pregnancy have not been studied. The aim of the study is to determine the percentages of peripheral blood Treg and Th17 and the expression of CD45RA, CD31, RAG2, and Tim-3 on these subsets during physiological pregnancy and in non-pregnant (NP) women.
Methods: Peripheral blood samples (n = 80) from healthy NP and pregnant women (1st, 2nd, and 3rd trimesters) were analyzed by flow cytometry to determine Treg (CD4+CD25+FOXP3+) and Th17 (CD4+RORγt+IL-17A+), and the expression of RAG2 and Tim-3 in these subsets. Treg and Th17 then subdivided into mature naive (MN, CD45RA+CD31–), recent thymic migrants (RTE, CD45RA+CD31+), CD31– memory, and CD31+ memory cells.
Results: An increase in the Treg percentage, a decrease in Th17, and a shift in the Treg/Th17 ratio shift towards Treg were revealed in pregnant women compared to NP. A Tim-3+ Treg increase in the 1st and 3rd trimesters and Tim-3+ Th17 in the 3rd trimester were found. There was a decrease in RTE-Treg and RTE-Th17, an increase in the MN-Treg percentage, but MN-Th17 did not change during pregnancy. The RAG2 expression was increased only in Treg.
Conclusions: The obtained data indicate that a healthy pregnancy is characterized by significant changes in the composition of naive Th17 and Tregs in peripheral blood.
Physiologic pregnancy is characterized by the immune tolerance formation towards semi-allogenic fetus [1]. Regulatory T (Treg) cells and interleukin (IL)-17-producing T helper (Th17) cells have a leading role in the establishment and maintenance of immune tolerance to semi-allogeneic fetus throughout pregnancy [2–5]. Treg are CD4+ cells characterized by high cell membrane expression of CD25 molecule—the α chains of the IL-2 receptor, intracellular expression of the X-linked Foxhead box P3 (FOXP3) transcription factor, production of immunosuppressive cytokines—transforming growth factor β (TGF-β), IL-10 [6, 7]. During pregnancy, Treg cells suppress the T-cell-mediated immune responses against fetal antigens, contributing to the predominance of humoral immune reactions, and also have an essential role in implantation and fetal growth [8, 9]. In pregnancy, the proportion of Treg increases in both peripheral blood and decidua [10]. A reduced number of Treg cells occurs in the peripheral blood and decidua of women with implantation failure or recurrent miscarriage [11–14].
In contrast, Th17 cells stimulate pro-inflammatory immune responses and play an essential role in host defense against extracellular pathogens and in autoimmune disorder development [15, 16]. Th17 cells are determined as CD4+ T-lymphocytes expressing the transcription factor RORγt (retinoic acid receptor-related orphan receptors) and producing the IL-17a [7]. An increase in the numbers and activity of Th17 cells is observed in various pregnancy pathologies [17]. Therefore, the Treg/Th17 balance appears to be involved in the tolerogenic mechanisms required for semi-allogenic fetus development during pregnancy.
According to origin, Treg and Th17 are divided into natural cells maturing in the thymus from single-positive CD4+ precursors and inducible cells formed in peripheral lymphoid organs from naive CD4+ T-cells after antigen recognition [18]. Treg and Th17 of peripheral blood are mainly represented by cells that have differentiated in the thymus [18, 19]. Only a small part of Treg and Th17 in peripheral blood are generated extrathymically in peripheral sites [18, 19]. Pregnancy is characterized by the steroid-induced thymus involution and inhibition of the productive function of the thymus [20]. However, an increased number of Tregs in peripheral blood during healthy pregnancy was found [21, 22]. The changes in natural Th17 output from the thymus during pregnancy have not been studied.
Naive CD4+ T-lymphocytes after being released from the thymus into peripheral blood have high expression of CD45RA and CD31 molecules [recent thymic migrants (RTE)], which correlates with intracellular T-cell receptor (TCR) excision circle (TREC) [23]. CD31 [platelet/endothelial cell adhesion molecule 1 (PECAM-1)] is required for transendothelial migration of naive T-lymphocytes to secondary lymphoid organs and could also be involved in modulating the TCR-driven extrathymic proliferation of thymic naive RTE T-cells [24]. Some of the RTE cells undergo peripheral extrathymic selection and transform into long-lived mature naive (MN) T-cells capable of homeostatic proliferation [24]. A decrease in CD31 expression on thymic MN lymphocytes correlates well with a decline in TREC [23, 24]. Therefore, CD31 combined with CD45RA expression can be used as markers to distinguish 2 subsets of naive CD4+ T-cells in the peripheral blood: RTE (TREC+) and MN (TREC–) lymphocytes capable of homeostatic proliferation [23]. After stimulation with appropriate antigens, both RTE-/MN-CD4+T-lymphocytes can differentiate directly into memory CD45RA–CD31+ (CD31–-memory) and CD45RA–CD31– (CD31+-memory) T cells [24, 25]. We proposed that Th17 and Treg composition, as determined by CD31 and CD45RA expression, reflects changes in thymic output during steroid atrophy during healthy pregnancy.
On the other hand, thymus involution during pregnancy activates the extrathymic differentiation of T-lymphocytes [26, 27]. Thus, an increase in the number of RAG1 (recombination activating genes)/RAG2+ T-lymphocytes with activated rearrangement of TCR genes was detected in the peripheral lymphoid organs of pregnant mice—mainly in lymph nodes draining the uterus [26, 27]. RAG1 and RAG2 are two essential DNA processing enzymes required for the rearrangement of the TCR genes [28]. Extrathymic rearrangement in T-cells was detected in different pregnancy variants—syngenic, allogeneic, and a combination of prone to abortion [26, 27]. Considering the appearance of fetal antigens in the mother’s body during pregnancy, extrathymic differentiation of Treg and Th17 involves not just compensation for atrophic processes occurring in the thymus but is also a fundamental mechanism for local correction of the antigen-recognizing repertoire of TCR Treg-lymphocytes. However, the possibility of extrathymic differentiation of Th17 and Treg during healthy pregnancy has not been investigated yet.
The study of the effect of Tim-3 on maternal and fetal immunity is only at an early stage. However, a growing number of studies suggest that Tim-3 is an important factor in regulating the immune system during pregnancy. A recent study showed that Tim-3 was expressed on Th17 cells, Treg cells, dendritic cells, monocytes, and NK cells [29–32]. The Tim-3 pathway is observed to inhibit the differentiation and proliferation of Th17 cells and promote the expansion of Treg [33–35]. Treg express galectin 9 (Gal-9), which is a ligand for Tim-3 [36]. The interaction of Tim-3 with Gal-9 can induce Th1 and Th17 depletion or apoptosis [37]. Thus, it can be assumed that during pregnancy, Tim-3 molecules regulate the balance of Th1/Th2 and Treg/Th17 and promote immunological tolerance [33, 38]. We hypothesize that during physiological pregnancy, the shift in Treg/Th17 peripheral blood composition is not only due to the changes in T-cells thymic output, but also in homeostatic proliferation and extrathymic differentiation of T-cells in peripheral blood. Therefore, we examined the peripheral blood Treg and Th17 compositions according to CD45RA and CD31 expression as well as RAG2 and Tim-3 expression in healthy non-pregnant (NP) and healthy pregnant women in the 1st, 2nd, and 3rd trimesters.
The peripheral blood of healthy women with physiological pregnancy (in the 1st trimester, 2nd trimester, and 3rd trimester) and healthy NP women (in the follicular phase of the menstrual cycle; control group) was studied. Women with a history of recent infections, endocrine, genetic, autoimmune diseases, neoplasia, or abnormal pregnancies in the past were excluded. The control group included women with one or more successful pregnancies sampled at least one and а half years after their last delivery and no breastfeeding for at least two months before the study inclusion. The individuals taking hormonal drugs were excluded from participation. All pregnant women participating in the experiment successfully delivered babies and gave birth to healthy children. The inclusion criteria were voluntary informed consent for examination and absence of hormonal drugs. In women with physiological pregnancy, pregnancy complications such as gestational diabetes, gestational hypertension, multiple pregnancies, and others were excluded. The subjects of the study were peripheral blood mononuclear cells (PBMCs). Characteristics of pregnant (1st trimester, 2nd trimester, and 3rd trimester) and NP women were shown in Table 1. All participants were aged between 21 and 32 years old. The study groups were matched by weight. The body mass index (BMI) in the control group (NP women) averaged 21.7 (18.5–24.9). Weight gain during the 1st trimester of pregnancy averaged 1.25 (0.5–2.0) kg, weight gain during the 2nd trimester of pregnancy averaged 8.8 (7.7–10.0) kg, and weight gain during the 3rd trimester of pregnancy averaged 13.6 (11.5–15.9) kg.
Clinical and demographic characteristics of the study participants
| Study groups | n | Age (years) | Gestation period (weeks) |
|---|---|---|---|
| NP women | 20 | 26 (21−29) | - |
| Pregnant women, 1st trimester | 20 | 26 (22−32) | 12 (10−12) |
| Pregnant women, 2nd trimester | 20 | 30 (28−31) | 20 (13−26) |
| Pregnant women, 3rd trimester | 20 | 30 (28−31) | 30 (28−31) |
Data are presented as median and interquartile range, Me (Q1; Q3). NP: non-pregnant
Fasting venous blood was collected from the ulnar vein (2 mL, in the morning) into vacuum tubes with EDTA. PBMCs were isolated by sedimentation in a Ficoll-Urografin density gradient (1.077 g/cm3) using a standard technique. PBMCs were washed twice in phosphate salt buffer (PBS) containing 2 mM EDTA and 0.1% bovine serum albumin (BSA) and stained with monoclonal antibodies (Table 2).
Characteristics of monoclonal antibodies used for flow cytometry
| Cellular markers | Fluorochrome | Clone | Isotype | Manufactured |
|---|---|---|---|---|
| Panel 1 | ||||
| CD4 | FITC | OKT-4 | Mouse IgGb, κ | eBioscience |
| CD25 (IL-2Rα) | Brilliant Violet 605 | BC96 | Mouse IgG1, κ | BioLegend |
| FOXP3 | PerCP-Cyanine5.5 | PCH101 | Rat IgG2a, κ | eBioscience |
| RORγt | APC | AFKJS-9 | Rat IgG2a, κ | eBioscience |
| IL-17A | PerCP-Cyanine5.5 | eBio64DEC17 | Mouse IgG1, κ | eBioscience |
| CD45RA | Alexa Fluor 700 | HI100 | Mouse IgG2b, κ | BioLegend |
| CD31 | Pacific Blue | WM59 | Mouse IgG1, κ | BioLegend |
| RAG2 | - | 3G4A26 | Mouse IgG2b, κ | BioLegend |
| Anti-mouse IgG2b | PE | RMG2b-1 | Rat IgG2a, κ | BioLegend |
| Panel 2 | ||||
| CD4 | PE | T4 | Mouse IgG2b, κ | BioLegend |
| CD25 (IL-2Rα) | Brilliant Violet 605 | BC96 | Mouse IgG1, κ | BioLegend |
| FOXP3 | PerCP-Cyanine5.5 | PCH101 | Rat IgG2a, κ | eBioscience |
| RORγt | PerCP | NR1F3 | Mouse IgG1, κ | R&D Systems |
| IL-17A | FITC | BL168 | Mouse IgG1, κ | BioLegend |
| Tim-3 (CD366) | APC | F38-2E2 | Mouse IgG1, κ | BioLegend |
FOXP3: X-linked Foxhead box P3
Cells were analyzed by flow cytometry (CytoFLEX S, Beckman Coulter, USA) using the CytExpert 2.0 software (Beckman Coulter, USA). At least 100,000 events per sample were analyzed. The discrimination of adherent cells (doublets) was performed using the forward scatter area/height (FSC-A/FSC-H) parameters. Live and dead cells were divided using Zombie UV fluorescent dye (Zombie UV™ Fixable Viability Kit, BioLegend). The lymphocyte gating was performed using the FSC-A and side scatter height (SSC-H) parameters. The gating strategy included the identification of CD4+, CD4+CD25+, Treg (FOXP3+ in CD4+CD25+), and Th17 (RORγt+IL-17A+ in CD4+) (Figure 1A). Subdivision of investigated subsets into MN (CD45RA+CD31–), RTE (CD45RA+CD31+), CD31–-memory, and CD31+-memory was carried out based on the expression of CD45RA and CD31 (Figure 1B). In addition, RAG2 expression was investigated in all studied subsets (Figure 1C). The second stage of the study (Figure 1D) included an assessment of Tim-3 expression on CD4+ T-cells, Treg, and Th17 (Figure 1E). True-nuclear buffers (BioLegend, USA) and fluorescence minus one (FMO) controls were used to measure intracellular cytokines and transcription factors.
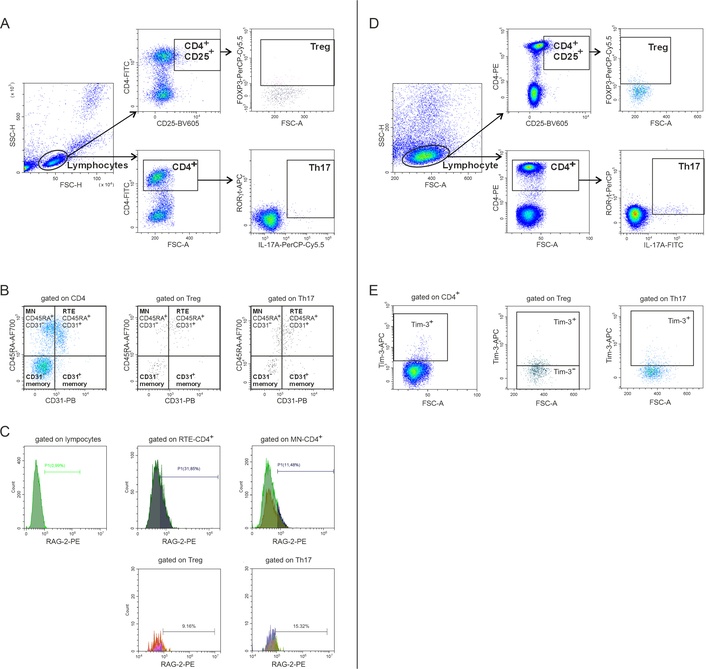
Evaluation of cell subsets by flow cytometry. (A and D) Gating strategy for CD4+, CD4+CD25+, Treg, and Th17 selection; (B) assessment of the studied cell distribution into MN, RTE, CD31–-memory and CD31+-memory subsets; (C) evaluation of RAG2; and (E) Tim-3 expression. SSC-H: side scatter height; FSC-H: forward scatter height; FOXP3: X-linked Foxhead box P3; Treg: regulatory T; Th17: interleukin-17-producing T helper; MN: mature naive; RTE: recent thymic migrants
The statistical analysis of the data was conducted through GraphPad Prism software version 8.01 (StatSoft, USA). The normality of distribution was assessed using the Kolmogorov-Smirnov test. The data were presented as median and lower and upper quartiles, Me (Q0.25–Q0.75). For multiple comparisons between unmatched groups (NP women, 1st trimester, 2nd trimester, 3rd trimester), the Kruskal-Wallis with Dunn’s post hoc test for non-normal variables was used. Correlations were assessed by calculating Spearman’s r correlation coefficient (r). Statistical significance was established at P < 0.05 for all analyses.
Analysis of peripheral blood was performed during the following periods: 1st trimester, 2nd trimester, 3rd trimester, and in NP women. CD4+ T-cell subsets were determined in PBMC using flow cytometry. As shown in Figure 2A, the percentage of CD4+ T-cells remained relatively stable throughout pregnancy in comparison with NP women. The percentage of activated CD4+CD25+ T-cells was decreased compared to NP women in all trimesters (Figure 2A). The percentage of RTE-CD4+ T-cells decreased proportionally to the increase of MN-CD4+ T-cells in all trimesters compared to NP women (Figure 2A). It should be noted that a significant negative correlation was revealed (r = –0.39; P = 0.01) between a decrease in the number of RTE cells and an increase in MN-CD4+ T-lymphocytes, which may indicate increased transformation of RTE cells to MN during pregnancy (Figure 2A).
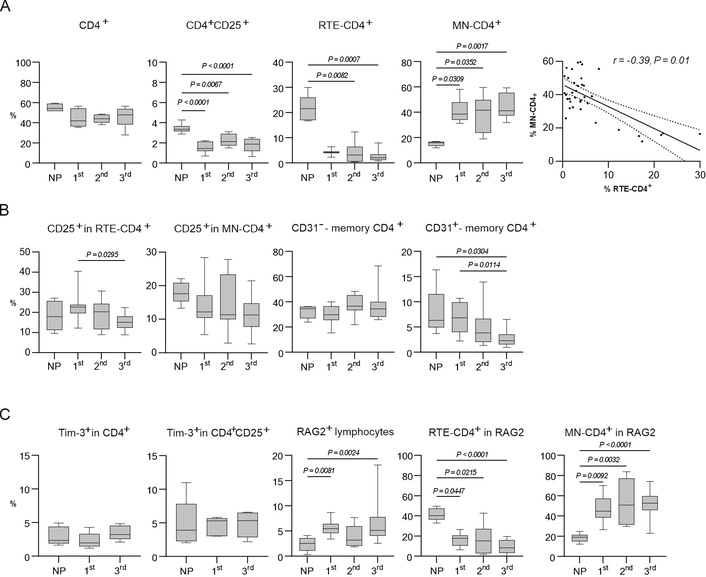
Analysis of cell subsets during pregnancy and in NP women. (A) Percentages of: CD4+; activated CD4+CD25+ T-lymphocytes; RTE-CD4+ T-cells; MN-CD4+ T-cells in peripheral blood in pregnant and NP women; statistically significant correlation between MN-CD4+ and RTE-CD4+ T-cells. (B) Percentages of: CD25+ in RTE-CD4+ T-lymphocytes; CD25+ in MN-CD4+ T-lymphocytes; changes in the percentage of CD31–-memory CD4+ T-cells; and CD31+-memory CD4+ T-cells in peripheral blood in pregnant and NP women. (C) Percentages of: Tim-3+ in CD4+ T-lymphocytes in the 1st and 3rd trimesters of pregnancy (data for the 2nd trimester are not presented); Tim-3+ in CD4+CD25+ T-lymphocytes in the 1st and 3rd trimesters of pregnancy (data for the 2nd trimester are not presented); RAG2+ in CD4+ T-lymphocytes; RTE-CD4+ T-lymphocytes in RAG2; MN-CD4+ T-lymphocytes in RAG2 in peripheral blood in pregnant and NP women. In A–С, all data are presented as medians (horizontal lines), interquartile ranges (boxes), and min–max (whiskers). P values were established between unmatched groups (NP women, 1st trimester, 2nd trimester, 3rd trimester) according to the Kruskal-Wallis with Dunn’s post hoc test for non-normal variables. RTE: recent thymic migrants; MN: mature naive; NP: non-pregnant
We observed a decrease in the percentage of CD25+ in RTE-CD4+ T-cells in the 3rd trimester of pregnancy, but the percentage of CD25+ in MN-CD4+ T-cells did not change during pregnancy (Figure 2B). It was found that the percentage of CD31–-memory СD4+ T-cells did not change regardless of the trimesters, but the percentage of CD31+-memory СD4+ T-cells decreased in the 3rd trimester of pregnancy compared to NP women and the 1st trimester of pregnancy (Figure 2B).
The expression of Tim-3+ did not change significantly in CD4+ T-cells and CD4+CD25+ T-cells in different trimesters of pregnancy compared to NP women (Figure 2C). The percentage of RAG2+ lymphocytes increased in the 1st and the 3rd trimesters of pregnancy compared to NP women (Figure 2C). In the gate of RAG2+ lymphocytes, the percentage of RTE-CD4+ T-cells was decreased proportionally to the increase of MN-CD4+ T-cells throughout pregnancy (Figure 2С).
To detect the percentage of Treg in PBMC, we gated FOXP3+ cells in the CD4+CD25+ lymphocyte gate (Figure 1A and D) [39]. The significant increase in the percentage of Treg cells was observed during the 1st, 2nd, and 3rd trimesters compared to NP women (Figure 3A). The expression of the Tim-3+ molecule on Treg increased regardless of the trimester of pregnancy (Figure 3A). The number of Tim-3– Treg did not change during pregnancy compared to NP women (Figure 3A). The percentage of RTE cells in Treg significantly increased in the 1st trimester compared to NP women, but in the 2nd and 3rd trimesters did not differ from NP women (Figure 3A). In contrast, the MN cells in Treg were significantly increased throughout pregnancy (Figure 3A).
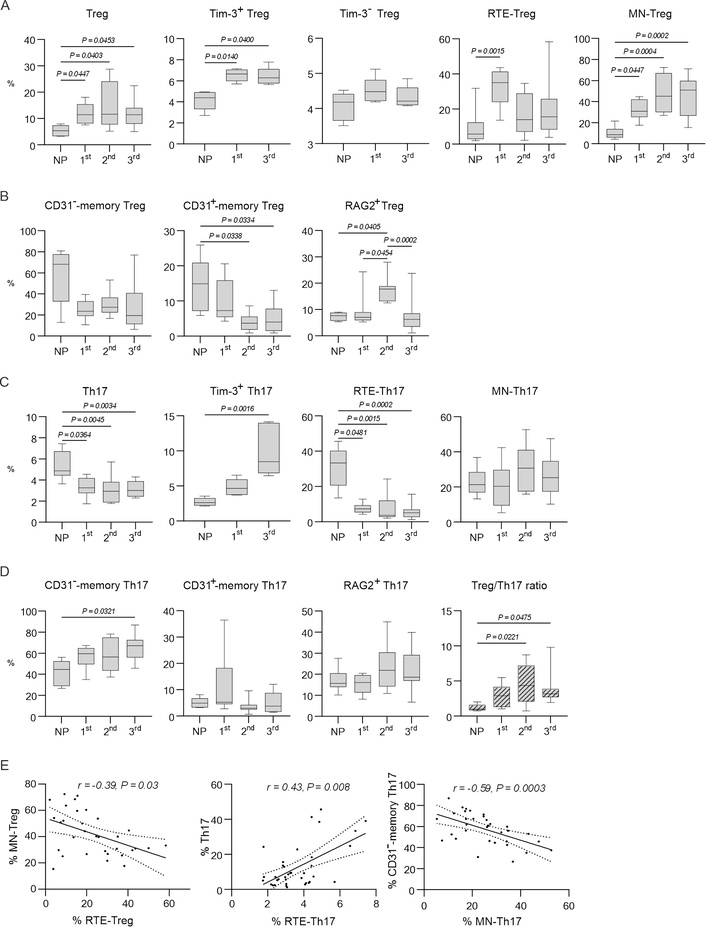
Measurement of Treg and Th17 levels during pregnancy and in NP women. (A) Percentages of: Treg; Tim-3+ Treg in the 1st and 3rd trimesters of pregnancy (data for the 2nd trimester are not presented); Tim-3– Treg in the 1st and 3rd trimesters of pregnancy (data for the 2nd trimester are not presented); RTE-Treg; MN-Treg in peripheral blood in pregnant and NP women. (B) Percentages of: CD31–-memory Treg; CD31+-memory Treg; RAG2+ Treg in peripheral blood in pregnant and NP women. (C) Percentages of: Th17; Tim-3+ Th17 in the 1st and 3rd trimesters (data for the 2nd trimester are not presented); RTE-Th17; MN-Th17 in peripheral blood in pregnant and NP women. (D) Percentages of: CD31–-memory Th17; CD31+-memory Th17; RAG2+ Th17 in peripheral blood in pregnant and NP women; Treg/Th17 ratio during pregnancy and in NP women. (E) Statistically significant correlations. In A–D, data are presented as medians (horizontal lines), interquartile ranges (boxes), and min–max (whiskers). P values were established between unmatched groups (NP women, 1st trimester, 2nd trimester, 3rd trimester) according to the Kruskal-Wallis with Dunn’s post hoc test for non-normal variables. Treg: regulatory T; NP: non-pregnant; RTE: recent thymic migrants; MN: mature naive; Th17: interleukin-17-producing T helper
It was found that the percentage of CD31–-memory Treg did not change regardless of the trimesters, but the percentage of CD31+-memory Treg decreased in the 2nd and 3rd trimesters of pregnancy compared to NP women (Figure 3B).
The percentage of RAG2+ in Treg was increased in the 2nd trimester of pregnancy compared to NP women and the 3rd trimester of pregnancy. But, in the 3rd trimester, the percentage of RAG2+ Treg was lower than in the 2nd trimester of pregnancy and did not differ from NP women (Figure 3B).
To determine the Th17 percentages, we gated RORγt+IL-17A+ cells in CD4+ lymphocytes (Figure 1A and D). The percentage of Th17 cells was decreased during the 1st, 2nd, and 3rd trimesters of pregnancy compared to NP women (Figure 3C). The expression of Tim-3+ on Th17 cells in the 3rd trimester was increased compared to NP women (Figure 3C). The percentage of RTE-Th17 cells was decreased significantly in the 1st, 2nd, and 3rd trimesters of pregnancy compared to NP women (Figure 3C). The percentage of MN-Th17 cells did not change during pregnancy (Figure 3C). The percentage of CD31+-memory Th17 cells did not change, but CD31–-memory Th17 increased in the 3rd trimester (Figure 3D). The percentage of RAG2+ cells in Th17 cells remained unchanged during pregnancy compared to NP women (Figure 3D).
Analysis of the Treg/Th17 ratio revealed a significant increase in the number of Treg, a decrease in the level of Th17, and a shift in the Treg/Th17 ratio towards Treg throughout pregnancy (Figure 3D).
During pregnancy, the maternal immune system changes in order to tolerate a semi-allogeneic fetus. The development of a tolerogenic immune profile is influenced by placenta-produced hormones and closely associated with thymus involution, changes in T-cell numbers, and subsets to decrease the cytotoxicity against fetal antigens [40]. We observed that the percentage of CD4+ T-cells remained relatively stable throughout pregnancy (Figure 2A). Similar dynamics of peripheral blood CD4+ T-lymphocytes in physiological pregnancy are also shown by other authors [41, 42]. Consistent with the thymic involution, we found a decrease in RTE-CD4+ cells throughout pregnancy (Figure 2A). Also, the decrease in RTE-CD4+ cells can be explained by their transformation into MN-CD4+ cells [24], which is confirmed by the proportional increase in the number of MN-CD4+ cells throughout pregnancy (Figure 2A) and the identified reverse correlation (Figure 2A). Both subsets of RTE and MN-CD4+ cells undergo homeostatic proliferation in vivo to maintain the naive T-cell pool [43]. We observed the decrease in the percentage of CD25+ RTE cells only in the 3rd trimester of pregnancy, but in the 1st and 2nd trimesters, the percentage of RTE-/MN-CD4+CD25+ cells did not change during pregnancy (Figure 2B). It is known that the expression of CD25 on peripheral blood CD4+ T-cells is a marker of activation associated with the proliferation of naive CD4+ T-cells [44]. Thus, it can be assumed that homeostatic proliferation of RTE and MN cells maintains the number of naive CD4+ T-lymphocytes in the peripheral blood during pregnancy to compensate for thymic involution. In addition, in the 1st trimester of pregnancy, RTE cells actively migrate to secondary lymphoid organs and the uterus [39]. Similar to conventional T cells, both RTE and MN can differentiate directly into memory (CD45RA–) T cells after stimulation with appropriate antigens [25]. We found that the percentage of CD31–-memory CD4+ T-cells did not change regardless of the trimesters, but the percentage of CD31+-memory CD4+ T-cells decreased in the 3rd trimester of pregnancy compared to NP women and the 1st trimester. The obtained results may strengthen our hypothesis that there is an increased proliferation of naive CD4+T-cells during the normal course of pregnancy.
The balance between Treg and Th17 plays a critical role in successful pregnancy development. In our study, we observed a significant increase in the Treg/Th17 ratio and a shift towards Treg throughout pregnancy (Figure 3D). The obtained results are in agreement with a healthy pregnancy because the decrease in Treg/Th17 ratio leads to several adverse obstetric outcomes like preterm birth [45], preeclampsia [46, 47], or recurrent pregnancy loss [3, 48, 49]. According to the literature and our previous investigations, the hormones produced by the placenta during pregnancy effectively shift the Treg/Th17 ratio towards Treg throughout healthy pregnancy due to regulation of their migration activity, plasticity, and apoptosis [50–55]. Understanding the mechanisms of regulation of Treg/Th17 balance is of great practical importance, since Treg/Th17 imbalance plays a critical role in the pathogenesis of autoimmune diseases [56, 57] and tumor growth [58].
The percentage of Treg was elevated in the peripheral blood of pregnant women regardless of the trimesters compared to NP women, which is completely consistent with the literature data [21, 22, 39]. We also observed the increase in the number of MN-Treg regardless of the trimester in comparison with NP women. Since the percentage of recently emigrated RTE-Treg from the thymus was increased only in the 1st trimester, and in the 2nd and the 3rd trimesters of pregnancy did not differ from NP women. The negative correlation between the percentages of RTE-Treg and MN-Treg during healthy pregnancy (r = –0.39, P = 0.03) was revealed (Figure 3E). But the proliferation of RTE-Treg did not exclude especially in the 1st trimester of pregnancy. The increased percentages of Ki67+-cells within RTE-Treg and relatively stable percentage of Ki67+ cells within MN-Treg during healthy pregnancy confirmed their differentiation capacities [25]. The percentage of CD31–-memory Treg does not change during pregnancy, but the percentage of CD31+-memory Treg decreases in the 2nd and the 3rd trimesters of pregnancy compared to NP women (Figure 3B). Thus, the composition of peripheral blood Treg is changed during healthy pregnancy. The peripheral Treg of NP women consists mainly of CD31− and CD31+-memory cells (Figure 4). During a healthy pregnancy, the proportion of naive Treg appears to increase significantly due to the proliferation of RTE-Treg and MN-Treg in the 1st trimester and MN-Treg in the second half of pregnancy (Figure 4). The memory Treg pool is stable and is maintained by differentiation of CD31+-memory Treg in CD31−-memory Treg (Figure 4) [25]. According to the data mentioned above, an increase in the total Treg pool of peripheral blood during pregnancy probably occurs due to the proliferation of MN-Treg.
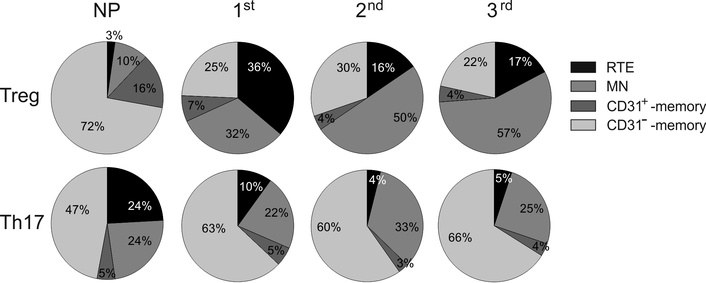
Proportions of RTE-Treg and RTE-Th17 during pregnancy and in NP women. Treg: regulatory T; NP: non-pregnant; Th17: interleukin-17-producing T helper; RTE: recent thymic migrants; MN: mature naive
We observed a decrease in the percentage of Th17 regardless of the trimesters of pregnancy in comparison with NP women, in agreement with the works of other authors [39, 41, 49] (Figure 3C). The maintenance of decreased Th17 number during healthy pregnancy is important to prevent pregnancy loss [59]. The change in the percentage of Th17 is directly correlated to the change in RTE-Th17 (r = 0.43; P = 0.008), which indicates the decrease in thymic output (Figure 3E). The composition of the total Th17 pool changed such that the percentage of RTE-Th17 in it decreased significantly, while the percentage of MN-Th17 and CD31+-memory Th17 changed insignificantly, and the percentage of CD31− memory Th17 complementarily increased (Figure 4).
We can propose that the decrease in thymic Th17 output leads to the differentiation of MN-Th17 into CD31–-Th17 memory cells, and the last subset becomes dominant in Th17 cells in peripheral blood during healthy pregnancy. This is confirmed by a significant negative correlation (r = –0.59; P = 0.0003) between a decrease in the number of MN-Th17 cells and an increase in CD31−-memory Th17 cells (Figure 3E). There are no investigations about the composition of the naive Th17 pool in peripheral blood during healthy pregnancy. The maintenance of the Th17 number in peripheral blood is important for protective properties against infectious agents.
It is known that during pregnancy, under the influence of estrogens and cytokines, the processes of rearrangement of antigenic receptor chains are repeatedly induced in mature peripheral T-lymphocytes, accompanied by the formation of TCR with a new specificity [26, 60, 61]. The RAGs encode two subunits, RAG1 and RAG2, that form the RAG complex to generate DNA double-stranded breaks between the receptor gene segments and to generate diverse antigen receptors [62]. To evaluate the processes of antigenic receptor chain rearrangements, we determine the RAG2+ cells. According to Gan et al. [62], human RAG1 exhibits an extremely low recombination activity in the absence of RAG2. In contrast, RAG2 stimulates RAG1 to initiate recombination between the receptor gene segments in lymphocytes [62]. We observed the increase in the percentage of RAG2+ cells in the lymphocyte gate in the 1st trimester and the 3rd trimesters. Earlier we reported that the combination of progesterone or estradiol with oncostatin M at concentrations typical for the 1st and 3rd trimesters of pregnancy effectively stimulated the expression of the TCR gene rearrangement markers in human peripheral blood lymphocytes in vitro [61]. In pregnant mice, the appearance of TCR gene rearrangement markers in lymphocytes was recorded from mid-pregnancy [60]. We also observed the increase in RAG2+ cells among MN-CD4+ lymphocytes during pregnancy. In contrast, the percentage of RAG2+ cells among RTE-CD4+ cells was decreased regardless of the trimesters of pregnancy. We may propose that MN-CD4+ lymphocytes are more susceptible to TCR rearrangement compared to naive RTE-CD4+ thymic cells.
The percentage of RAG2+ cells in Treg increased in the 2nd trimester of pregnancy, but in the Th17 population, it did not change significantly. In previous works, we showed the expression of the TCR rearrangement marker in human Treg under the influence of pregnancy hormones, but not for Th17 cells [61]. The functional activity of Treg is determined by the ability to recognize antigenic structures of the major histocompatibility complex (MHC) [8], and the possibility of TCR revision in these cells may enhance their suppressive functions with respect to fetal antigenic structures. A change in the antigen-recognition ability of these cells may serve as a condition for the appearance of fundamentally new mechanisms of immunological tolerance to fetal antigens during physiological pregnancy, in particular, the mechanism of formation of the pool of lymphocytes that recognize fetal molecules as autoantigens.
It was recently demonstrated that naive CD45RA+Treg in peripheral blood have high suppressive activity in pregnant women in comparison with NP women, which represents a key event in the induction of maternal tolerance to the fetal antigens [25]. The inhibitory molecule Tim-3 plays a critical role in regulating Treg suppressive function and associates with a shift of the Treg/Th17 ratio towards Treg [63, 64]. We found a significant increase in Tim-3+ Treg percentage in the 1st and the 3rd trimesters of pregnancy (Figure 3A) and the increase in the expression of the Tim-3+ on Th17 in the 3rd trimester of pregnancy, compared to NP women (Figure 3C). The expression of Tim-3+ in CD4+ T-cells and CD4+CD25+ T-cells in different trimesters of pregnancy did not significantly change compared to NP women (Figure 2C). According to the literature, Tim-3+ Treg exhibit enhanced suppressive function and proliferative activity compared with Tim-3– Treg [34, 65]. Tregs produce Gal-9, which interacts with Tim-3 on effector T cells (Th1 and Th17), and through the Tim-3/Gal-9 pathway, Tregs inhibit the proliferation and cytokine production by Th1 and Th17 or even promote apoptosis of these cells [36, 37]. Moorman and co-authors [35] found that the expression of Tim-3 on Treg positively correlates with the expression of the Ki67 proliferation marker in Treg but is inversely related to the proliferation of Th1 and Th17. It is important to note that the Gal-9/Tim-3 signaling pathway is triggered in Treg and Th17 сells and activates different second messenger cascades, leading to opposing effects [33]. Thus, increased expression of Tim-3 on Th1 and Th17 leads to their depletion and, as a result, a decrease in effector functions of these cells. On the contrary, an increase in Tim-3 expression on Treg leads to activation of Treg regulatory properties, leading to the depletion of cytotoxic T-cells [33]. The dysregulation of Tim-3 expression can cause excessive or suppressed inflammatory reactions, which can eventually lead to pregnancy complications [33]. A decrease in the suppressive ability of Treg leads to miscarriage due to the lack of control over activated T lymphocytes [66]. According to the literature, progesterone increases Tim-3 expression on Treg and Th17 during pregnancy [41]. Thus, the increased Tim-3 expression in Treg and Th17 during healthy pregnancy plays an important role in controlling the Treg/Th17 ratio and functions.
In conclusion, it is interesting to note that a healthy pregnancy is characterized by significant changes in the composition and functional activity of naive Th17 and Treg. In general, during a healthy pregnancy, there is an increase in the total number of Treg, a decrease in Th17, and a shift in the Treg/Th17 ratio towards Treg. Atrophic processes in the thymus during pregnancy are accompanied by the decrease in the percentage of RTE cells among CD4+, Treg, and Th17 cells. Compensation for the atrophic processes in the thymus during pregnancy occurs due to the increase of MN-CD4+ T-lymphocytes capable of homeostatic proliferation. RTE-Treg matures apparently in MN-Treg to maintain the Treg total pool in the peripheral blood. The decrease in Th17 number during healthy pregnancy occurs due to the decrease in RTE-Th17, but the proportion of MN-Th17 is stable, and CD31–- memory Th17 cell subset becomes dominant in Th17 cells in peripheral blood during healthy pregnancy. The increase in Tim-3 expression in Treg and Th17 cells in healthy pregnancy reflects one of the possible mechanisms of Treg suppressive properties realization and controlling the Treg/Th17 ratio. The increase in RAG2+ cells among MN-CD4+/Treg during healthy pregnancy testifies to the possibility of TCR revision in these cells in respect to fetal antigenic structures. Our results provide insight into the composition and functional features of peripheral blood Treg and Th17 during healthy pregnancy. The obtained results may explain the mechanisms of immune tolerance to fetal antigens and protective properties against infectious during healthy pregnancy.
The obtained results can be useful in the clinical settings. We determined the values of the Treg/Th17 ratio during different trimesters of healthy pregnancy. All pregnant women participating in our investigations successfully delivered babies and gave birth to healthy children. Monitoring of Treg, Th17 numbers, and Treg/Th17 ratio may serve as an early biomarker of high risk of pregnancy loss, preeclampsia, and help identify those patients who would benefit from therapy. According to literature, Th17 numbers are inversely correlated with Treg in recurrent spontaneous abortion patients [67], preeclampsia [68]. Treg/Th17 ratio is reduced in recurrent spontaneous abortion patients [67]. The occurrences of recurrent spontaneous abortion are related to Treg/Th17 imbalance [67]. Understanding the mechanisms of maintaining the Treg/Th17 balance in healthy pregnancy directs the development of new pharmacological approaches to pregnancy loss prevention and to treatment of autoimmune and tumor diseases.
The study included women with singleton pregnancies aged 21 to 32 years, which limits the applicability of the study results to pregnant women with multiple pregnancies and pregnant women of other age groups.
FOXP3: X-linked Foxhead box P3 transcription factor
FSC-A: forward scatter area
Gal-9: galectin 9
IL: interleukin
MN: mature naive
NP: non-pregnant
PBMCs: peripheral blood mononuclear cells
PECAM-1: platelet/endothelial cell adhesion molecule 1
RAG1/RAG2: recombination activating genes
RTE: recent thymic migrants
TCR: T-cell receptor
Th17: interleukin-17-producing T helper
TREC: T-cell receptor excision circle
Treg: regulatory T cells
We are grateful to Dr. Nina V. Karimova and all participants for their contribution in this study.
OG: Conceptualization, Methodology, Data curation, Investigation, Writing—original draft, Writing—review & editing. EO: Conceptualization, Investigation, Resources, Writing—review & editing, Supervision. OL: Conceptualization, Methodology, Validation, Data curation, Investigation. SS: Conceptualization, Methodology, Writing—original draft, Writing—review & editing, Supervision. All authors have read and agreed to the published version of the manuscript.
The authors claim that there are no conflicts of interest.
The experimental procedure was done in accordance with the regulations of the Ethical Review Committee of the Institute of Ecology and Genetics of Microorganisms, Ural Branch of the Russian Academy of Sciences (Minutes No. 16 of June 5, 2022). This study complies with the 2013 version of the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
All relevant data are available from the corresponding author on request [olia15_77@mail.ru].
This study was carried out within the framework of the state task, the state topic registration number: N 124020500027-7. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
