Affiliation:
1Department of Basic Medicine, School of Health and Wellness Sciences, Guangzhou Kangda Vocational Technical College, Guangzhou 510555, Guangdong, China
2State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, Guangzhou 510060, Guangdong, China
†These authors contributed equally to this work and share the first authorship.
ORCID: https://orcid.org/0009-0000-0936-4474
Affiliation:
3Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou 510080, Guangdong, China
†These authors contributed equally to this work and share the first authorship.
Affiliation:
2State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, Guangzhou 510060, Guangdong, China
Affiliation:
4Department of Molecular Pharmacology & Physiology, Morsani College of Medicine, University of South Florida, Tampa, FL 33612, USA
Affiliation:
2State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, Guangzhou 510060, Guangdong, China
Email: tanghl@sysucc.org.cn
ORCID: https://orcid.org/0000-0002-3206-782X
Explor Immunol. 2025;5:1003220 DOI: https://doi.org/10.37349/ei.2025.1003220
Received: April 14, 2025 Accepted: August 15, 2025 Published: September 29, 2025
Academic Editor: Cunte Chen, South China University of Technology, China; Yangqiu Li, Jinan University, China
The article belongs to the special issue The Role of Immune Checkpoint Molecules in Cancer and Hematological Malignancies
Cancer is a multifaceted and heterogeneous disease characterized by uncontrolled growth, evasion of immune surveillance, and resistance to conventional therapies. The immune system plays a crucial role in tumor surveillance. However, tumors exploit immune checkpoint pathways to inhibit T cell activation and evade immune destruction. Immune checkpoint inhibitors (ICIs) have markedly improved outcomes in certain cancers by restoring T cell function and enhancing anti-tumor immunity. Despite these advances, the presence of immune resistance mechanisms contributes to variability in responses and ongoing challenges in overcoming resistance. Triple-negative breast cancer (TNBC), compared to other breast cancer (BC) subtypes, exhibits higher immunogenicity, but its anti-tumor immunity is profoundly suppressed by immune checkpoint molecules, creating a paradoxical scenario of “high immunogenic potential yet restrained by inhibitory signals”. Consequently, TNBC has become a significant target for ICI therapy. However, response rates vary among BC subtypes, with hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-positive (HER2+) BC demonstrating lower immunogenicity. Hematological malignancies, including leukemia, lymphoma, and multiple myeloma, also exhibit distinct immune checkpoint dynamics, influencing their responsiveness to ICIs. This review comprehensively examines the mechanisms of immune checkpoint regulation, their role in cancer immune evasion, and the clinical applications of ICIs in both solid and hematological malignancies. It further discusses emerging strategies to counteract ICI resistance, such as dual checkpoint blockade, tumor microenvironment modulation, metabolic targeting, and epigenetic reprogramming. An enhanced understanding of immune checkpoint biology is essential for optimizing immunotherapy strategies and improving patient outcomes. The literature selection for this study was guided by relevance to the research topic, focusing on peer-reviewed articles, monographs, and conference proceedings published between 2010 and 2025, sourced from databases like PubMed and Google Scholar.
Cancer is a complex and heterogeneous disease characterized by uncontrolled cellular proliferation, immune evasion, and resistance to conventional therapies. An expanding body of research indicates that the immune system plays a pivotal role in tumor surveillance by detecting and eliminating malignant cells via cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells [1]. However, tumors have evolved intricate strategies to circumvent immune detection, notably through the manipulation of immune checkpoint molecules (ICMs) [2].
Immune checkpoints are regulatory molecules that modulate immune activation and maintain self-tolerance, thereby preventing hyperactive immune responses that could result in autoimmunity [3]. In the context of the tumor microenvironment (TME), however, these checkpoints are often co-opted by cancer cells to inhibit T cell activity and facilitate immune escape [4]. Prominent immune checkpoints include programmed death-1 (PD-1), CTL-associated protein 4 (CTLA-4), lymphocyte activation gene-3 (LAG-3), T cell immunoglobulin and mucin-domain containing protein 3 (TIM-3), and T cell immunoreceptor with Ig and ITIM domains (TIGIT). The blockade of these checkpoints through immune checkpoint inhibitors (ICIs) has revolutionized cancer therapy by restoring T cell function and enhancing anti-tumor immune responses, as summarized in Table 1 [5].
Key immune checkpoint molecules in cancer.
| Immune checkpoint | Expressing cells | Primary ligand | Function | Representative inhibitors |
|---|---|---|---|---|
| PD-1 | T cells, B cells, NK cells | PD-L1, PD-L2 | Suppresses T cell activation | Pembrolizumab, nivolumab |
| CTLA-4 | T cells | B7-1, B7-2 | Inhibits early T cell activation | Ipilimumab |
| LAG-3 | CD4+, CD8+ T cells | MHC-II | Promotes Tex | Relatlimab |
| TIM-3 | CD4+ T cells, macrophages | Galectin-9, CEACAM1 | Regulates Tex | Sabatolimab |
| TIGIT | T cells, NK cells | CD155 | Suppresses T cell and NK cell activity | Tiragolumab |
PD-1: programmed death-1; NK: natural killer; PD-L1: programmed cell death ligand 1; CTLA-4: cytotoxic T lymphocyte-associated protein 4; LAG-3: lymphocyte activation gene-3; MHC: major histocompatibility complex; Tex: T cell exhaustion; TIM-3: T cell immunoglobulin and mucin-domain containing protein 3; CEACAM1: carcino-embryonic antigen related cellular adhesion molecule 1; TIGIT: T cell immunoreceptor with Ig and ITIM domains.
Table 1 encapsulates critical ICMs implicated in cancer, delineating their functions, representative inhibitors, resistance pathways, and co-expression of multiple checkpoints on exhausted T cells. Immune checkpoints serve to regulate immune responses and prevent overactivation. Nevertheless, tumors manipulate these mechanisms to evade immune surveillance.
Mechanisms of immune evasion:
Checkpoint overexpression: Numerous tumors increase the expression of programmed cell death ligand 1 (PD-L1), TIM-3, and LAG-3, which suppresses T cell activation.
Immunosuppressive TME: The recruitment of regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) intensifies immune suppression.
Metabolic adaptations: Tumors alter glucose and amino acid metabolism, restricting nutrient availability for immune cells.
Epigenetic modifications: Changes in DNA methylation and histone configurations lead to the downregulation of antigen presentation pathways.
These mechanisms collectively undermine the efficacy of ICIs, underscoring the necessity for integrative combination therapies.
This review meticulously examines the role of ICMs in breast cancer (BC) and hematological malignancies, distinguishing these from other cancer types. This focus is driven by their unique biological characteristics, immune microenvironments, and clinical outcomes following treatment with ICIs. BC, as a prototypical solid tumor, demonstrates substantial subtype-specific diversity in terms of immune checkpoint expression and immune cell infiltration—features that are notably less prevalent in other solid tumors. Triple-negative BC (TNBC), in particular, is marked by elevated PD-L1 expression and significant immune infiltration, making it the most responsive BC subtype to ICIs [6], as evidenced by pivotal studies such as KEYNOTE-522 and IMpassion130 [7]. In contrast, hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-positive (HER2+) BCs exhibit minimal ICI responses, attributed to sparse immune infiltration. This creates a distinct spectrum of ICM-mediated immune evasion that necessitates thorough examination. The variability in ICM dynamics and therapeutic responses across subtypes highlights the imperative for targeted research into the immunological underpinnings of BC [8]. Hematological malignancies, which originate from immune cells, engage in a complex and distinctive interaction with ICMs, setting them apart from solid tumors and other cancer types. Disorders such as leukemias, lymphomas, and multiple myeloma (MM) feature disruptions in immune checkpoint pathways that are essential for maintaining immune homeostasis. For instance, acute myeloid leukemia (AML) exhibits upregulation of PD-L1 and TIM-3, which contributes to T cell exhaustion (Tex) [9], while diffuse large B cell lymphoma (DLBCL) presents a high prevalence of PD-1+ T cells associated with NF-κB activation [10], and MM leverages PD-L1-positive stromal cells within the bone marrow niche to bolster immune suppression [11]. This intricate interaction between malignant immune cells and ICMs forms a unique landscape of immune evasion, distinct from the TME-driven mechanisms observed in most solid tumors. Additionally, investigations into dual checkpoint blockade to surmount resistance in these malignancies underscore their distinct dependence on ICMs, warranting specialized research [12]. Collectively, the subtype-specific ICM profiles in BC and the intrinsic ICM dysregulation in hematological malignancies, coupled with their variable responses to ICIs, render these tumor groups pivotal for enhancing our comprehension of immune checkpoints in cancer. This justifies the concentrated focus of this review.
The primary objectives of this review are to elucidate the role of ICMs in tumor immune evasion, summarize the current application and challenges of ICIs across diverse tumor types, including both solid tumors like BC and hematological malignancies, and to emphasize the necessity for developing innovative combination strategies to tackle ICI resistance. This review aims to furnish insights that will refine future approaches to cancer immunotherapy.
The immune system plays an integral role in regulating tumor growth and progression through its ability to identify and eliminate cancerous cells. However, tumors have evolved sophisticated mechanisms to evade immune surveillance, primarily by augmenting the expression of ICMs that suppress T cell activation and function. Immune checkpoints such as PD-1, CTLA-4, TIM-3, LAG-3, and TIGIT are pivotal in modulating immune responses and maintaining immunological homeostasis [5]. In oncological contexts, these checkpoints are frequently exploited by tumor cells to circumvent immune destruction, leading to immune exhaustion and facilitating tumor progression. This section delineates the fundamental mechanisms of critical immune checkpoints, elucidates their role in Tex, and explores their involvement in tumor immune evasion.
The immune system meticulously regulates its responses to pathogens and aberrant cells via a network of immune checkpoints, which serve as inhibitory signals to curtail excessive immune activation. Tumors exploit these regulatory mechanisms to avoid immune detection, culminating in immune suppression and disease progression [13]. The PD-1/PD-L1 pathway is a prominent example of such an immune checkpoint. Expressed on T cells, B cells, and NK cells, PD-1 engages with its ligands, PD-L1 and PD-L2, which are often upregulated on tumor cells, dendritic cells, and macrophages [14]. This interaction inhibits signaling through the T cell receptor (TCR), diminishes cytokine production [e.g., IL-2 and interferon-gamma (IFN-γ)], and attenuates the effector functions of cytotoxic T cells. Consequently, this enables tumors such as TNBC, non-small cell lung cancer (NSCLC), and Hodgkin lymphoma (HL) to escape destruction by the immune system [15].
CTLA-4, another pivotal immune checkpoint, operates during the initial activation phase of T cells. It competes with CD28 to bind B7-1 (CD80) and B7-2 (CD86) on antigen-presenting cells (APCs), thereby inhibiting the co-stimulatory signals essential for complete T cell activation. Contrary to PD-1, which predominantly functions in peripheral tissues, CTLA-4 primarily regulates T cell activity within lymphoid organs to curb overactive immune responses [16]. Tumors exploit this regulatory pathway by enhancing the prevalence of Tregs that express CTLA-4 at high levels, consequently suppressing the anti-tumor immune response. This exploitation allows tumors to foster an immunosuppressive microenvironment, thereby diminishing the efficacy of natural immune surveillance and enhancing tumor persistence [17].
Moreover, beyond PD-1 and CTLA-4, emerging immune checkpoints such as LAG-3, TIM-3, and TIGIT also contribute to the immune suppression mediated by tumors. LAG-3, which binds to major histocompatibility complex (MHC) class II molecules, suppresses T cell proliferation and often acts synergistically with PD-1 to induce Tex [18]. TIM-3 is present on CD4+ T cells and macrophages and engages with galectin-9 and carcino-embryonic antigen related cellular adhesion molecule 1 (CEACAM1) to induce apoptosis in effector T cells and confer resistance to PD-1 blockade [19]. TIGIT, expressed on T cells and NK cells, competes with CD226 for binding to CD155, thereby reducing NK cell cytotoxicity and attenuating T cell activation [20]. The simultaneous expression of these molecules on exhausted T cells within the TME highlights the potential of dual or multi-checkpoint blockade strategies to amplify the effectiveness of immunotherapies and to circumvent therapeutic resistance.
Tex is a state of dysfunction that develops from chronic antigen exposure, resulting in a gradual decline in T cell effector function. Tex cells exhibit reduced cytokine production, including IFN-γ, TNF-α, and IL-2, impaired proliferation, and diminished cytotoxic capabilities. Moreover, they express elevated levels of several inhibitory receptors, notably PD-1, CTLA-4, TIM-3, LAG-3, and TIGIT, as summarized in Table 2 [21].
The relationship between immune checkpoints and Tex.
| Immune checkpoint | Central role | Association with the Tex phenotype | Clinical strategies |
|---|---|---|---|
| PD-1 | Binding to ligands PD-L1/PD-L2, it inhibits pathways such as PI3K/Akt and MAPK, thereby blocking the activation signals of T cells | A characteristic molecule of Tex. PD-1 positive T cells are often accompanied by functional defects (cell toxicity ↓, secretion of IFN-γ/IL-2 ↓) | Anti-PD-1 monoclonal antibodies (pembrolizumab, nivolumab)Anti-PD-L1 antibodies (atezolizumab) |
| CTLA-4 | Competitive binding to the surface of APC CD80/CD86, blocking the CD28-mediated co-stimulatory signal, inhibiting the early activation of T cells | Initiation process of participation depletion | Anti-CTLA-4 antibodies (ipilimumab) |
| LAG-3 | Combined with MHC-II molecules and ligand FGL1, it inhibits TCR signal transduction | Tex’s “depth depletion” indicator | Anti-LAG-3 antibodies (relatlimab) |
| TIM-3 | Combined with ligands such as Gal-9 and CEACAM1, it inhibits the NF-κB pathway and induces T cell apoptosis | TIM-3 positive cells lose their ability to secrete cytokines and have a poor response to single-agent ICIs | Anti-TIM-3 antibody (MBG453)Combination with other checkpoint inhibitors (PD-1/LAG-3) |
| TIGIT | Competes with CD226 for the binding ligand CD155, blocks the co-stimulatory signal, and inhibits T cell activationHighly expressed in Tex, co-localizes with PD-1 | The levels of granzyme B and perforin in TIGIT positive Tex cells were reduced | Anti-TIGIT antibody (tiragolumab) |
Tex: T cell exhaustion; PD-1: programmed death-1; PD-L1: programmed cell death ligand 1; IFN-γ: interferon-gamma; CTLA-4: cytotoxic T lymphocyte-associated protein 4; APC: antigen-presenting cell; LAG-3: lymphocyte activation gene-3; MHC: major histocompatibility complex; FGL1: fibrinogen like protein 1; TCR: T cell receptor; TIM-3: T cell immunoglobulin and mucin-domain containing protein 3; Gal-9: galectin-9; CEACAM1: carcino-embryonic antigen related cellular adhesion molecule 1; ICIs: immune checkpoint inhibitors; TIGIT: T cell immunoreceptor with Ig and ITIM domains.
Chronic antigen stimulation perpetuates the Tex phenotypes, while epigenetic alterations, such as chromatin remodeling, solidify the expression of genes associated with exhaustion. Additionally, cytokine signaling within the TME, notably the increased presence of TGF-β and IL-10, reinforces Tex states. PD-1 is pivotal in Tex by attenuating TCR signaling and facilitating the recruitment of src homology 2 domain-containing protein tyrosine phosphatase (SHP-2) phosphatases, which then dephosphorylate crucial signaling molecules essential for T cell activation. Furthermore, LAG-3, TIM-3, and TIGIT intensify exhaustion by activating inhibitory pathways that inhibit effector T cell functionality [22].
ICIs that target PD-1/PD-L1 and CTLA-4 have proven effective in revitalizing exhausted T cells. The phase II clinical trial (NCT03147040) investigates the therapeutic efficacy of combining the PD-L1 inhibitor atezolizumab with carboplatin in metastatic lobular BC. Results indicate that carboplatin promotes the release of tumor antigens and diminishes immunosuppressive factors, whereas atezolizumab exerts its effect by blocking PD-L1, thereby reversing Tex [23]. Additionally, findings from another phase II clinical trial (NCT02395627) revealed that in the combined use of the histone deacetylase (HDAC) inhibitor vorinostat, endocrine agent tamoxifen, and pembrolizumab for treating patients with metastatic ER+ BC, vorinostat epigenetically primes tumors to achieve a state permissive for immune activation, while pembrolizumab is capable of reversing Tex [24]. However, tumors develop resistance by compensatorily upregulating alternative checkpoints such as TIM-3 and TIGIT, necessitating the adoption of combination therapeutic strategies [25].
ICIs have transformed the landscape of cancer therapy by reinvigorating T cell-mediated immunity to combat tumor cells. By targeting inhibitory pathways such as PD-1/PD-L1 and CTLA-4, ICIs have substantially improved clinical outcomes in a variety of cancers, including melanoma, NSCLC, TNBC, and hematological malignancies [26, 27]. However, challenges such as resistance to therapy, immune-related adverse events (irAEs), and inter-patient variability necessitate a deeper comprehension of their mechanisms of action, clinical applications, and resistance pathways.
ICIs operate by inhibiting negative regulatory pathways that curtail T cell activation, thereby augmenting anti-tumor immunity. The primary targets in cancer immunotherapy are the PD-1/PD-L1 axis and the CTLA-4 pathway [28].
Figure 1 delineates the signaling pathways of PD-1/PD-L1 and CTLA-4, illustrating their roles in the suppression of T cell activity, and the mechanisms through which FDA-approved ICIs, such as pembrolizumab, nivolumab, atezolizumab, and ipilimumab, function. By inhibiting these pathways, ICIs reactivate T cell functions and enhance the immune response against tumors [29].
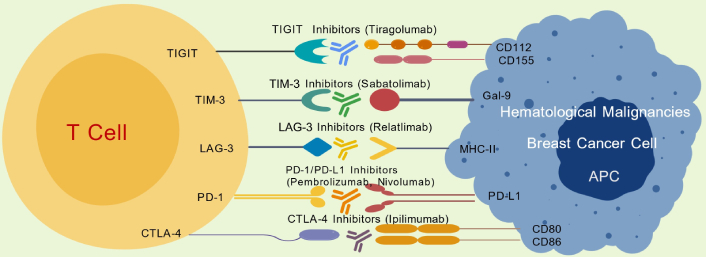
Mechanisms of action of immune checkpoint inhibitors. TIGIT: T cell immunoreceptor with Ig and ITIM domains; TIM-3: T cell immunoglobulin and mucin-domain containing protein 3; Gal-9: galectin-9; LAG-3: lymphocyte activation gene-3; MHC: major histocompatibility complex; PD-1: programmed death-1; PD-L1: programmed cell death ligand 1; CTLA-4: cytotoxic T lymphocyte-associated protein 4; APC: antigen-presenting cell.
PD-1 is an inhibitory receptor expressed on activated T cells, B cells, and NK cells. It engages with its ligands, PD-L1 and PD-L2, which are frequently upregulated on tumor cells and APCs. This interaction disrupts TCR signaling, diminishes cytokine production (e.g., IL-2, IFN-γ), and hampers the proliferation and cytotoxic function of T cells [30]. Therapeutic antibodies targeting PD-1 or PD-L1, such as pembrolizumab, nivolumab, and atezolizumab, counteract this inhibition by blocking the engagement of PD-1, thereby rejuvenating exhausted T cells and promoting the destruction of tumor cells [31]. In a landmark 2022 study, the KEYNOTE-355 trial revealed that the integration of pembrolizumab with chemotherapy significantly improved overall survival in patients with advanced TNBC exhibiting high PD-L1 expression [combined positive score (CPS) ≥ 10]. After a median follow-up period of 44 months, the median overall survival extended to 23.0 months for the combination therapy group, as opposed to 16.1 months for those receiving chemotherapy alone [32]. A subsequent meta-analysis in 2023 further corroborated that combinations of PD-1/PD-L1 inhibitors with chemotherapy markedly extend progression-free survival (PFS) in metastatic TNBC, with pronounced benefits in both the intention-to-treat population and, more significantly, in PD-L1-positive subgroups. Nevertheless, these regimens also showed an increased incidence of irAEs [33]. In the realm of hematological malignancies, nivolumab and pembrolizumab have been approved for the treatment of relapsed or refractory (R/R) classic HL (CHL). This approval is underpinned by the frequent occurrence of 9p24.1 genetic alterations in CHL, which lead to the overexpression of PD-1 ligands and render these tumors particularly susceptible to PD-1 blockade [34]. Anti-PD-1/PD-L1 antibodies, including pembrolizumab, nivolumab, and atezolizumab, re-establish T cell functionality by preventing PD-1 engagement, revitalizing exhausted T cells, and facilitating the elimination of tumor cells. While these agents have demonstrated effectiveness in both BC and hematological malignancies, ongoing challenges include the identification of reliable biomarkers for optimizing patient selection and managing the immune-related toxicities associated with these therapies.
CTLA-4 serves as a pivotal immune checkpoint that competes with CD28 for binding to B7 ligands (CD80/CD86) on APCs. In contrast to PD-1, which primarily operates in peripheral tissues, CTLA-4 is active in lymphoid organs and plays a crucial role in regulating early T cell activation [35]. Inhibition of CTLA-4 through ICIs such as ipilimumab enhances T cell priming and expansion, thereby intensifying immune responses against tumors.
In hematological malignancies, although the blockade of CTLA-4 has been extensively studied in melanoma, its significance in hematological neoplasms is increasingly acknowledged. Specifically, in CHL, there is a growing focus on integrating CTLA-4 inhibitors with other immunotherapies. This approach is propelled by the disease’s unique immune microenvironment and the integral role of immune checkpoints in its progression [36].
Beyond PD-1 and CTLA-4, additional immune checkpoints such as LAG-3, TIM-3, and TIGIT contribute to immune suppression. The strategy of dual checkpoint blockade, exemplified by combinations like PD-1 + LAG-3 (relatlimab) or PD-1 + TIM-3 (sabatolimab), has shown promise in enhancing anti-tumor immunity and overcoming resistance [37]. For instance, a phase I dose-escalation and cohort expansion clinical trial demonstrated that tebotelimab, a bispecific PD-1 × LAG-3 dual-affinity re-targeting (DART) molecule, exhibited safety and anti-tumor activity in patients with either solid tumors or hematological malignancies [38]. Another approach involves reprogramming the TME using targeted strategies, such as employing colony-stimulating factor-1 receptor (CSF1R) inhibitors to mitigate TAM-mediated suppression. The phase I clinical trial involving the CSF1R monoclonal antibody LY3022855 in patients with breast and prostate cancers indicated stable disease post-treatment and activation of immune-related genes in tumor biopsies [39]. Additionally, epigenetic modulation through the use of DNA methyltransferases (DNMTs) and HDAC inhibitors has been shown to enhance tumor antigen presentation, thereby improving immune recognition and response [40]. These inhibitors are also being explored as chemo-sensitizers in various BC trials, for instance, NCT00748553 and NCT00368875 [41].
Expanding beyond dual checkpoint blockade, combination therapeutic strategies hold significant potential in oncology. A noteworthy example includes the integration of ICIs with radiotherapy, which has undergone extensive investigation. Radiotherapy has the capability to modulate the TME, potentially enhancing the efficacy of ICIs. Studies suggest that while combining ICIs with radiotherapy for the treatment of solid tumors may lead to respiratory adverse events, radiotherapy could also synergize with ICIs through mechanisms such as the abscopal effect. This phenomenon involves radiation-induced cell death in the irradiated region, triggering an immune response that targets tumors in non-irradiated areas [42]. An additional innovative combinatorial approach includes pairing ICIs with vaccines. Specifically, in the context of MM, research exploring the use of a dendritic cell-based vaccine in conjunction with ICIs has shown promising results. The study revealed that this combination notably enhanced anti-myeloma immune responses. The vaccine was designed to present myeloma-specific antigens to the immune system, while ICIs worked to alleviate immune suppression, thereby resulting in increased T cell activation and improved disease control [43].
ICIs have markedly improved survival rates in various cancers, including melanoma, NSCLC, TNBC, and HL [44]. Nevertheless, their effectiveness is somewhat constrained in certain hematological malignancies, attributable to alternative mechanisms of immune evasion. Understanding the interactions between immune checkpoints and the TME is vital for advancing immunotherapeutic strategies.
ICIs have received approval for treating various cancer types, with ongoing clinical trials seeking to broaden their indications (Table 3) [45].
FDA-approved ICIs and their indications.
| Checkpoint target | ICI drug | Approval year | Notable trial | Key biomarkers | Approved indications |
|---|---|---|---|---|---|
| PD-1 | Pembrolizumab | 2014 | KEYNOTE-158 | PD-L1, MSI-H/dMMR | Melanoma, NSCLC, TNBC, Hodgkin lymphoma, gastric cancer |
| Nivolumab | 2014 | CheckMate 227, CheckMate 142 | PD-L1, MSI-H/dMMR, TMB | NSCLC, renal cell carcinoma, bladder cancer, colorectal cancer | |
| PD-L1 | Atezolizumab | 2016 | IMpassion130, IMpassion150, IMpassion133 | PD-L1 | TNBC, urothelial carcinoma, NSCLC |
| Durvalumab | 2018 | PACIFIC, ADRIATIC, NCT03732677 | PD-L1, TILs | Small cell lung cancer, NSCLC | |
| CTLA-4 | Ipilimumab | 2011 | NCT04008030, NCT01445379, NCT03408587 | CTLA-4, TILs, CTCs | Melanoma, NSCLC (in combination with nivolumab) |
| LAG-3 | Relatlimab | 2022 | RELATIVITY-047, NICHE-3 | dMMR, MLH1, MSH2, PD-L1 | Advanced melanoma (in combination with nivolumab) |
| TIGIT | Tiragolumab | 2021 | NCT04958811, SKYSCRAPER-11 | PD-L1, TAMs, Tregs, CD8+ effector T cells, serum myeloid protein markers | Investigational in NSCLC, TNBC |
ICIs: immune checkpoint inhibitors; PD-1: programmed death-1; PD-L1: programmed cell death ligand 1; MSI-H/dMMR: microsatellite instability high/deficient mismatch repair; NSCLC: non-small cell lung cancer; TNBC: triple-negative breast cancer; TMB: tumor mutational burdens; TILs: tumor-infiltrating lymphocytes; CTLA-4: cytotoxic T lymphocyte-associated protein 4; CTCs: circulating tumor cells; LAG-3: lymphocyte activation gene-3; MLH1: mutL homolog 1; MSH2: mutS homolog 2; TIGIT: T cell immunoreceptor with Ig and ITIM domains; TAMs: tumor-associated macrophages; Tregs: regulatory T cells.
The following content details the application of ICIs in four types of malignancies: For metastatic melanoma, the combination of nivolumab and ipilimumab yields clinical benefits, with over 50% of patients receiving this regimen as first-line therapy achieving 10-year survival free of melanoma-related death. In NSCLC, anti-PD-(L)1 agents serve as key therapeutic options, with treatment strategies customized based on PD-L1 expression levels. TNBC exhibits responsiveness to the combination of atezolizumab and nab-paclitaxel, yet the development of therapeutic resistance remains a major clinical challenge. HL shows favorable responses to PD-1 blockade, whereas AML and MM display limited responsiveness to ICIs.
Melanoma: In metastatic melanoma, ICIs have significantly improved survival rates. The combination of nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4) has demonstrated superior efficacy compared to monotherapy, resulting in sustained responses [46]. A recent 10-year follow-up analysis of the pivotal CheckMate 067 trial further highlighted the long-term benefits of the nivolumab-ipilimumab combination in managing metastatic melanoma. This trial, which involved patients with advanced melanoma, revealed that those treated with the combination therapy had a median survival of nearly six years. Remarkably, among those who received this combination as first-line therapy, over 50% were alive without melanoma-related death at the 10-year milestone. These long-term findings not only corroborate the sustained efficacy of this treatment strategy but also provide crucial insights into the long-term management of patients with metastatic melanoma [47].
NSCLC: Inhibitors that target PD-1 and PD-L1 form the cornerstone of treatment for NSCLC, employed either as monotherapies or in combination with chemotherapy. Pembrolizumab is designated as a first-line therapy for patients with high PD-L1 expression in NSCLC, while combinations of nivolumab and ipilimumab have received approval for tumors with low PD-L1 expression [48]. In 2024, Chu et al. [49] undertook a comprehensive network meta-analysis, integrating data from several phase II/III randomized controlled trials that assessed therapies based on anti-PD-(L)1 for stage IV NSCLC. This sophisticated statistical method facilitated a concurrent evaluation of various treatment options. The findings of the analysis indicated that for patients with PD-L1 expression levels below 1%, combinations of an anti-PD-(L)1 agent with chemotherapy and anti-vascular endothelial growth factor (VEGF) treatments, as well as a triple combination of anti-PD-(L)1, anti-CTLA4, and chemotherapy, proved to be relatively more beneficial. Conversely, for patients with PD-L1 expression of 50% or more, the combination of anti-PD-(L)1 and chemotherapy was shown to be an effective treatment strategy [49]. These groundbreaking results strongly support the idea that customizing treatment approaches based on specific PD-L1 levels can significantly enhance the efficacy of NSCLC treatment, thereby offering crucial insights for personalized medicine within this field.
TNBC: TNBC is characterized by high PD-L1 expression, which renders it susceptible to therapies combining atezolizumab with nab-paclitaxel, as well as to pembrolizumab-based treatments [50]. The IMpassion130 trial, an international, multicenter, randomized, double-blind, placebo-controlled study that involved 902 patients with unresectable locally advanced or metastatic TNBC (who had not received prior chemotherapy for metastatic disease), confirmed the effectiveness of atezolizumab in combination with nab-paclitaxel. In patients with PD-L1-positive tumors, the median PFS was 7.4 months in the atezolizumab plus nab-paclitaxel group, compared to 4.8 months in the placebo plus nab-paclitaxel group. The stratified hazard ratio for PFS was 0.60 [95% confidence interval (CI): 0.48–0.77; p < 0.0001], indicating a significant advantage for the atezolizumab-containing regimen. Additionally, the objective response rate (ORR) in patients with confirmed responses was 53% in the atezolizumab arm, compared to 33% in the placebo arm [50]. However, the development of resistance poses a significant challenge, necessitating the exploration of combinatorial approaches that target additional immune checkpoints.
Hematological malignancies: HL is notably responsive to PD-1 blockade, primarily due to genetic amplifications of PD-L1 [51]. Recent research has significantly advanced our understanding of these hematologic malignancies. In CHL, a phase II clinical trial assessed a sequential treatment regimen consisting of pembrolizumab followed by AVD chemotherapy in newly diagnosed patients. This study focused on genomic copy number alterations at chromosome 9p24.1, abnormalities that are known to promote elevated expression of PD-L1 and PD-L2. The findings revealed that a considerable proportion of patients exhibited positive responses to the initial pembrolizumab monotherapy, with some achieving near-complete metabolic responses. These results suggest that the integration of early PD-1 blockade with conventional chemotherapy could potentially enhance therapeutic outcomes for HL [52]. In contrast, AML and MM demonstrate limited responses, likely attributable to the highly suppressive nature of the bone marrow microenvironment [53]. Current clinical trials are exploring the efficacy of dual blockade strategies (e.g., PD-1 + TIM-3) and the combination of ICIs with epigenetic modulators.
Despite the clinical success of ICIs, the emergence of both primary and acquired resistance poses significant challenges, which are influenced by a variety of mechanisms.
The TME plays a critical role in influencing the response to ICIs. Tumors actively recruit immunosuppressive cells, such as Tregs, MDSCs, and TAMs, to hinder T cell activity. TAMs, in particular, secrete TGF-β and IL-10, which enhance PD-L1 expression and strengthen immune suppression. Current research efforts are exploring strategies to target these TAMs, including the use of CSF1R inhibitors, to mitigate immune evasion mechanisms [28]. A recent study has provided new insights into the roles of TAMs and proposed novel therapeutic approaches. Researchers utilized a unique reagent, FF-10101, characterized by its ability to form a covalent bond with CSF1R, thereby achieving prolonged inhibition. In preclinical animal models, treatment with FF-10101 led to a significant reduction in the population of immunosuppressive TAMs within the TME. Concurrently, there was a noticeable increase in both anti-tumor TAMs and tumor antigen-specific CD8+ T cells. These immunological shifts resulted in substantial suppression of tumor growth, evidenced by marked reductions in both tumor volume and growth rates in treated subjects compared to control groups [54].
Cancer cells reprogram their metabolism in order to suppress immune responses effectively. This reprogramming includes an increase in glycolysis and lactate production, which generates an acidic microenvironment that diminishes T cell functionality [55]. A recent study has revealed a previously unknown mechanism implicated in the pathogenesis of BC. Researchers found that BC cells actively secrete arginine, initiating a metabolic reprogramming in TAMs. This metabolic alteration leads to aberrant activation of the polyamine synthesis pathway within TAMs, notably marked by elevated spermine production. Spermine, through epigenetic regulatory mechanisms, fosters the polarization of macrophages towards an immunosuppressive phenotype [56]. Furthermore, tumor cells diminish the availability of crucial nutrients such as glutamine, thereby restraining T cell proliferation. To counter these deleterious effects, metabolic inhibitors, including indoleamine 2,3-dioxygenase (IDO) inhibitors, are currently under investigation [57].
The loss of MHC-I expression significantly diminishes tumor antigen presentation, thereby impeding T cell recognition. Mutations in beta-2 microglobulin (B2M), an essential component of MHC-I, have been identified as contributors to resistance against ICIs in conditions such as melanoma and lung cancer [58, 59]. In response, epigenetic therapies, notably HDAC inhibitors, are being pursued to restore effective antigen presentation.
In an effort to surmount resistance and enhance therapeutic efficacy, several innovative strategies are being explored, including:
Dual checkpoint blockade: This approach involves the combination of ICIs targeting multiple checkpoints, such as PD-1, CTLA-4, LAG-3, and TIM-3.
TME reprogramming: Strategies include the utilization of TAM-targeting agents, TGF-β inhibitors, and CSF1R inhibitors.
Epigenetic modulation: This involves the integration of DNMT and HDAC inhibitors to restore tumor immunogenicity.
Personalized immunotherapy: Employing multi-omics approaches and artificial intelligence to optimize patient selection and treatment personalization.
The advent of ICIs has revolutionized cancer treatment by rejuvenating anti-tumor immune responses. Despite the substantial efficacy demonstrated by PD-1/PD-L1 and CTLA-4 inhibitors, challenges such as resistance mechanisms, remodeling of the TME, and metabolic alterations continue to present significant obstacles. Enhancements in therapeutic regimens that combine diverse approaches, the discerning selection of patients based on specific biomarkers, and the discovery of novel immune targets are imperative for optimizing the clinical efficacy of ICIs. By addressing and overcoming these challenges, personalized immunotherapy strategies have the potential to improve patient outcomes and extend the benefits of ICIs to a wider spectrum of malignancies.
Hematological malignancies, encompassing lymphomas, leukemias, and MM, demonstrate unique immune evasion strategies that enable tumor cells to elude immune surveillance. In contrast to solid tumors, which often engage in spatially confined immune suppression, hematological malignancies arise from immune cell lineages and thus exhibit a particularly dynamic interaction with ICMs. The immune microenvironment of these malignancies is characteristically highly immunosuppressive, dominated by Tregs, MDSCs, and TAMs. The overexpression of immune checkpoints, including PD-1, CTLA-4, TIM-3, LAG-3, and TIGIT, plays a critical role in facilitating immune evasion and resistance to therapies (Table 4) [60]. While recent advances in immunotherapy highlight the potential of ICIs in treating hematological cancers, challenges such as primary and acquired resistance, immune-related adverse effects, and the need for effective biomarkers to tailor treatments persist [61].
The role of immune checkpoints in hematological malignancies.
| Classification | Checkpoint target | ICI drug | Notable trial |
|---|---|---|---|
| DLBCL | PD-1/PD-L1, CTLA-4, LAG-3, TIM-3, TIGIT | Pembrolizumab, nivolumab | None |
| PMBCL | PD-1/PD-L1, CTLA-4, LAG-3, TIM-3, TIGIT | Pembrolizumab | KEYNOTE-013, KEYNOTE-170 |
| HL | PD-1/PD-L1, CTLA-4, LAG-3, TIM-3, TIGIT | Nivolumab, pembrolizumab | KEYNOTE-087 |
| AML | PD-1/PD-L1, CTLA-4, LAG-3, TIM-3, TIGIT | Azacitidine, nivolumab | NCT02397720 |
| MM | PD-1/PD-L1, CTLA-4, LAG-3, TIM-3, TIGIT | Anti-TIGIT (BMS-986207), Anti-LAG-3 mAb (BMS-980616), pomalidomide, dexamethasone | NCT04150965 |
ICI: immune checkpoint inhibitor; DLBCL: diffuse large B cell lymphoma; PD-1: programmed death-1; PD-L1: programmed cell death ligand 1; CTLA-4: cytotoxic T lymphocyte-associated protein 4; LAG-3: lymphocyte activation gene-3; TIM-3: T cell immunoglobulin and mucin-domain containing protein 3; TIGIT: T cell immunoreceptor with Ig and ITIM domains; PMBCL: primary mediastinal large B cell lymphoma; HL: Hodgkin lymphoma; AML: acute myeloid leukemia; MM: multiple myeloma.
Lymphomas, particularly DLBCL and HL, are characterized by elevated levels of immune checkpoint expression, which contributes to immune suppression and disease progression [62, 63].
DLBCL, the most prevalent subtype of non-HL (NHL), features a diverse immune landscape. Notably, the activated B cell (ABC) subtype of DLBCL exhibits increased expression of PD-L1 due to the constitutive activation of the NF-κB pathway. This overexpression of PD-L1 enhances immune escape by inhibiting the function of cytotoxic T cells through the binding to PD-1 and the subsequent reduction in TCR signaling [64]. Furthermore, DLBCL tumors often contain high levels of Tregs, which express CTLA-4 extensively, thereby further dampening anti-tumor immune responses [65]. The coexistence of multiple immune checkpoints, such as LAG-3 and TIGIT, on tumor-infiltrating lymphocytes (TILs) exacerbates the immune dysfunction [66]. Although PD-1 inhibitors like pembrolizumab and rituximab have shown efficacy in R/R DLBCL, response rates are inconsistent, likely due to the presence of other immune checkpoints and the immunosuppressive TME [67]. Primary mediastinal large B cell lymphoma (PMBCL) is recognized as a rare and highly invasive subtype of NHL. Unlike other variants of DLBCL, PMBCL is distinguished by its unique pathological features, morphological patterns, and immunophenotypic profiles, which are associated with specific prognostic indicators. Despite its distinct characteristics, there remains a paucity of clinical evidence regarding PMBCL. Typically, PMBCL is linked with a significantly higher survival rate than that observed in DLBCL. In recent advancements, immunotherapeutic strategies employing PD-1 inhibitors have shown considerable efficacy in the treatment of R/R PMBCL. Nevertheless, these immunotherapies are not curative. Clinical trials, including KEYNOTE-170 and KEYNOTE-013, reveal that over half of the patients do not respond to these treatments, exhibiting a median PFS of only 5.5 to 10.4 months [68, 69].
In contrast, HL exhibits a particular vulnerability to PD-1 blockade. The defining feature of CHL is the presence of Hodgkin Reed-Sternberg (HRS) cells. These cells characteristically overexpress PD-L1 as a result of genetic amplifications on chromosome 9p24.1. Such genomic alterations lead to the persistent upregulation of PD-L1, thereby fostering an intensely immunosuppressive TME [70]. Consequently, PD-1 inhibitors, such as nivolumab and pembrolizumab, have demonstrated substantial efficacy in treating R/R HL, with clinical trials reporting response rates surpassing 70%. Nevertheless, resistance to ICIs can emerge over time. A proposed mechanism for this resistance involves the compensatory upregulation of the immune checkpoints TIM-3 and LAG-3 on exhausted T cells, which may diminish the long-term effectiveness of PD-1 blockade. Current research is exploring therapeutic approaches that target multiple checkpoints, including PD-1 and TIM-3, to potentially enhance treatment outcomes for HL patients [63].
AML poses a distinct challenge in the application of immune checkpoint-based therapies, attributed to its profoundly immunosuppressive bone marrow microenvironment. In contrast to lymphomas, which often exhibit high levels of PD-L1 expression on malignant cells, AML predominantly suppresses immune responses through interactions with immune cells within the bone marrow niche. AML blasts are known to upregulate PD-L1 in response to inflammatory cytokines such as IFN-γ, which contributes to Tex [71]. Furthermore, AML stem cells have been shown to evade immune surveillance by expressing TIM-3, which interacts with galectin-9 to induce T cell apoptosis [72]. Investigative studies into the TIM-3 inhibitor MBG453 have revealed that in patients with AML, leukemia stem cells with high TIM-3 expression are associated with increased T cell apoptosis. When combined with demethylating agents, such as azacitidine, some patients achieved complete remission. Moreover, bone marrow biopsy findings indicated a decrease in galectin-9 expression, suggesting that TIM-3 blockade might restore T cell function [73, 74].
In addition to PD-1 and TIM-3, AML leverages the CTLA-4 pathway to dampen immune responses. CTLA-4 is predominantly expressed on Tregs and is instrumental in maintaining immune tolerance. In AML, there is a notable expansion of Tregs that contributes to the suppression of effector T cell activity, thereby facilitating the survival of leukemia cells. The therapeutic blockade of CTLA-4 using ipilimumab, particularly post-transplantation, has been investigated as a strategy to enhance the graft-versus-leukemia (GVL) response. However, a significant concern with CTLA-4 inhibition in this context is the elevated risk of graft-versus-host disease (GVHD) following allogeneic stem cell transplantation [75].
Furthermore, the immune environment of AML is characterized by disrupted signaling pathways, notably JAK/STAT and NF-κB. Activation of JAK2/STAT3 in AML has been associated with increased PD-L1 expression, which further promotes immune escape [76]. Concurrently, NF-κB signaling contributes to the upregulation of various immune checkpoints, including PD-L1 and TIM-3. In light of these findings, therapeutic strategies that target both immune checkpoints and oncogenic signaling pathways are under investigation. Notably, the combination of hypomethylating agents, such as azacitidine, with PD-1 inhibitors has demonstrated potential in boosting immune responses in AML by augmenting tumor antigen presentation and diminishing immune suppression [77].
MM is a malignancy of plasma cells that proliferates within an immunosuppressive microenvironment in the bone marrow. In contrast to HL, which is highly amenable to PD-1 blockade, MM demonstrates intrinsic resistance to ICIs. This resistance is attributed to alternative mechanisms of immune evasion. A primary immune checkpoint implicated in the progression of MM is PD-L1, which is markedly upregulated not only on malignant plasma cells but also on bone marrow stromal cells [78]. Interaction between PD-1 and PD-L1 impedes the function of cytotoxic T cells, thereby promoting an environment favorable for MM cell survival. However, clinical trials assessing the efficacy of PD-1 inhibitors in MM have generally reported limited success, which is likely attributable to the activation of compensatory immune suppression pathways [79].
TIM-3 has been recognized as a critical immune checkpoint in MM, particularly concerning treatment resistance. This protein is predominantly expressed on exhausted T cells and MDSCs within the MM microenvironment. Its interaction with galectin-9 promotes T cell dysfunction and impedes anti-tumor immune responses [80]. Moreover, the expression of TIM-3 has been associated with resistance to immunomodulatory drugs (IMiDs), such as lenalidomide. This association suggests that targeting TIM-3 could potentially enhance the efficacy of current MM therapies [81].
TIGIT, another promising target in MM, is an inhibitory receptor that competes with CD226 for binding to CD155. Signaling through TIGIT attenuates the activation of T cells and NK cells, thereby diminishing the immune response against MM [82]. Recent investigations have proposed that a dual blockade of TIGIT and PD-1 might rejuvenate T cell functionality and amplify immune-mediated tumor eradication. Ongoing clinical trials are exploring the efficacy of anti-TIGIT antibodies, such as tiragolumab, in conjunction with PD-1 inhibitors to determine their potential impact on MM [83]. Furthermore, Richard et al. [84] conducted a study to evaluate the therapeutic effects of TIGIT and LAG-3 inhibitors on MM patients who had previously undergone extensive treatment. Initially, patients received only monoclonal antibodies during the first treatment cycle. From the second cycle onwards, pomalidomide and dexamethasone were incorporated into the treatment regimen. The preliminary results indicated that objective responses were achieved in 50% of the participants in the anti-TIGIT group and 33% in the anti-LAG-3 group [84].
Given the intricate immune landscape of MM, researchers are actively investigating combination therapeutic strategies. Techniques such as the dual blockade of PD-1 and TIM-3, the integration of ICIs with IMiDs, and targeting the immunosuppressive milieu of the bone marrow through myeloid-specific therapies are showing potential in enhancing treatment outcomes for MM patients. Additionally, advancements in single-cell transcriptomics and spatial proteomics are providing new insights into the immune microenvironment associated with MM, thereby aiding in the formulation of more precise and effective immunotherapeutic interventions [85].
Hematological malignancies utilize a variety of immune evasion strategies, rendering immune checkpoint blockade a promising yet challenging therapeutic strategy. Future advancements in combination therapies, biomarker-driven patient selection, and the development of novel immune targets will be essential for improving the clinical efficacy of ICIs.
BC is a heterogeneous disease characterized by distinct molecular subtypes, each exhibiting unique responses to ICIs. Unlike melanoma or lung cancer, which are marked by high tumor mutational burdens (TMB) and substantial immune cell infiltration, BC is typically considered an immunologically “cold” tumor. This characterization is partially due to the immunosuppressive activities of apolipoprotein E (APOE)+ macrophages during ICI treatment [86]. Nevertheless, recent advancements in the field of immunotherapy have shown that certain subtypes, notably TNBC, are responsive to ICIs, leading to the regulatory approval of PD-1/PD-L1 inhibitors for selected patients [87]. Despite these advancements, the effectiveness of ICIs in many BC patients remains limited by immune evasion mechanisms, including the upregulation of various immune checkpoints such as PD-L1, LAG-3, TIM-3, and TIGIT [88].
The deployment of ICIs in BC has been predominantly concentrated on TNBC, attributable to its pronounced immunogenicity and heightened expression of PD-L1. TNBC is distinguished by the absence of estrogen receptor, progesterone receptor, and HER2 expression, accounting for approximately 15% of all BC cases and associated with a dismal prognosis.
Expanding beyond TNBC, HER2+ BC has also been identified as a viable target for ICIs, especially when used in conjunction with HER2-directed therapies. Preclinical investigations indicate that HER2-targeted monoclonal antibodies like trastuzumab may bolster anti-tumor immunity through mechanisms such as antibody-dependent cellular cytotoxicity (ADCC) and the upregulation of PD-L1 expression [89].
Conversely, the majority of BC cases, which fall under the HR+ category, exhibit a limited response to ICIs. This subtype is typically characterized by a lower TMB, fewer TILs, and an immunosuppressive TME, which collectively contribute to reduced responsiveness to immune checkpoint blockade [90].
Figure 2 delineates the clinical efficacy of ICIs varies significantly among different BC subtypes. TNBC has shown the highest responsiveness.
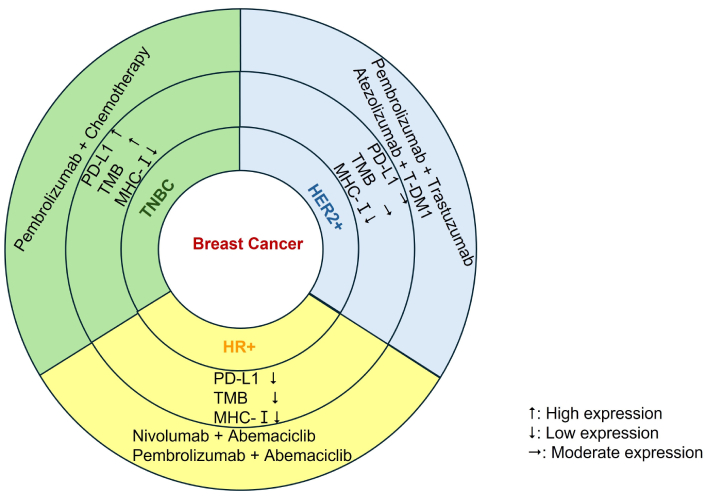
Clinical efficacy of ICIs varies significantly among different BC subtypes. ICIs: immune checkpoint inhibitors; BC: breast cancer; PD-L1: programmed cell death ligand 1; TMB: tumor mutational burdens; MHC: major histocompatibility complex; TNBC: triple-negative BC; T-DM1: trastuzumab emtansine; HER2+: human epidermal growth factor receptor 2-positive; HR+: hormone receptor-positive.
TNBC is recognized as the most immunogenic subtype of BC, characterized by elevated PD-L1 expression and extensive infiltration by immune cells. The KEYNOTE-522 trial examined the effects of neoadjuvant pembrolizumab combined with chemotherapy in early-stage TNBC, revealing a considerable enhancement in pathological complete response (pCR) rates compared to chemotherapy alone [91]. Conversely, the IMpassion131 trial, which studied atezolizumab combined with paclitaxel in metastatic TNBC, did not replicate the survival benefits observed in the earlier IMpassion130 trial. This discrepancy underscores the complexities associated with immunotherapy responses in TNBC [92].
Although HER2+ tumors generally exhibit lower overall PD-L1 expression than TNBC, preclinical studies suggest that HER2-targeted therapies may influence anti-tumor immunity. These therapies, including trastuzumab and lapatinib, have been shown to remodel the TME through various mechanisms. These include upregulating chemokines such as CXCL10 and CCL5 to attract cytotoxic T cells, enhancing antigen presentation by increasing MHC-I expression, and inducing immunogenic cell death. These mechanisms potentially enhance the efficacy of ICIs [93, 94]. The phase II PANACEA trial (NCT02129556) evaluated the efficacy of pembrolizumab in combination with trastuzumab in patients with PD-L1-positive, trastuzumab-resistant metastatic HER2+ BC. A modest ORR of 15% [76] underlines the therapeutic challenges encountered in drug-resistant scenarios, potentially arising from the pre-existing exhaustion of the TME. Notably, in patient subgroups characterized by high T cell infiltration, a higher ORR of 22% was observed, emphasizing the necessity to stratify patients based on their immune phenotypes [95]. The phase II KATE2 trial (NCT02924883) is currently evaluating the efficacy of combining atezolizumab with trastuzumab emtansine (T-DM1) in patients with metastatic HER2+ BC. Interim results, derived from a median follow-up period of 23.1 months, indicate a trend towards extended PFS in patients positive for PD-L1, with a hazard ratio of 0.77 and a 95% CI ranging from 0.54 to 1.09. The ORR for this cohort was 57.1%, compared to 51.3% observed in the group receiving T-DM1 monotherapy. The rationale for this combination therapy is grounded in the capacity of T-DM1 to induce immunogenic cell death, thereby facilitating the release of tumor antigens, while atezolizumab mitigates T cell suppression [96].
HR+ BC represents a particularly challenging subtype for the application of ICIs, likely due to its immunosuppressive TME. This environment is characterized by a low TMB and a high prevalence of Tregs and MDSCs. Despite these hurdles, innovative combination strategies, such as ICIs coupled with cyclin-dependent kinase (CDK)4/6 inhibitors, are under investigation to enhance immune responses. CDK4/6 inhibitors, including abemaciclib and palbociclib, not only induce cell-cycle arrest in HR+ tumors but also exhibit immunomodulatory effects. These effects include enhancing MHC-I expression on cancer cells, promoting neoantigen presentation, and inhibiting Treg proliferation by suppressing forkhead box P3 (FOXP3) transcription [97]. Such “immune priming” effects provide a theoretical foundation for their use in conjunction with ICIs. The phase II CheckMate 7A8 trial (NCT04075604) is currently evaluating the efficacy of nivolumab, a PD-1 inhibitor, in combination with abemaciclib for metastatic HR+ HER2– BC that has progressed during endocrine therapy. Interim results from this trial reveal an ORR of 16% across the overall patient cohort, with an enhanced response rate of 22% observed in patients exhibiting PD-L1-positive tumors (CPS ≥ 1). These findings underscore the significance of stratifying patients based on immune phenotypes [98]. Concurrently, the phase III MONARCH 2 extension study (NCT02107703) explored the combination of pembrolizumab with abemaciclib in a similar patient demographic. This study reported a 12-month PFS rate of 31% in the combination group, compared to 14% in the abemaciclib monotherapy group, though adverse effects such as diarrhea and fatigue were noted as concerns [99].
Immunophenotypic markers provide prognostic insights and aid in stratifying therapeutic responses. However, current clinical evidence indicates significant biomarker discordance between primary tumors and their metastatic counterparts. Comparative analyses reveal considerable heterogeneity in dynamic markers such as PD-L1, PD-1, and PD-L2, as well as in the density of TILs, with variance rates reaching 43–67% in clinical cohorts. This contrasts with the relative stability observed in TMB and microsatellite instability (MSI) across different disease sites, where consistency is maintained in over 80% of paired specimens. These findings highlight the critical need for comprehensive biomarker profiling to guide evidence-based therapeutic decisions in the management of checkpoint inhibitors [100]. In BC, the variation in biomarker profiles between primary and metastatic sites poses significant challenges for therapeutic decision-making. A notable example is HER2, a key immunophenotypic marker in directing treatment strategies. A comprehensive study covering 2,156 BC patients revealed a 25.5% rate of HER2 expression discordance between primary tumors and matched metastases, correlating with factors such as histological grade, hormone receptor status, metastatic site, and timing of metastasis onset [101].
Furthermore, subgroup analyses from the DESTINY-Breast03 trial demonstrated that trastuzumab deruxtecan (T-DXd) therapy yielded significant intracranial responses and reductions in central nervous system disease burden. Specifically, the intracranial ORR reached 63.9% with T-DXd, markedly exceeding the 33.4% observed with T-DM1 [102]. These findings highlight the considerable biomarker heterogeneity between primary and metastatic BC lesions. Consequently, comprehensive biomarker profiling across all tumor presentations is imperative. Exclusive reliance on biomarkers derived from the primary tumor for therapeutic decisions poses a risk of inaccurate assessments. For instance, if the metastatic foci exhibit high biomarker expression while the primary lesion shows low expression, discontinuing immunotherapy based on primary tumor findings would result in the forfeiture of potential therapeutic benefits. Conversely, administering immunotherapy unjustifiably in the absence of biomarkers in metastatic lesions could prove ineffective and increase toxicity. Moreover, the majority of clinical trials utilize primary tumor biomarkers as enrollment criteria; however, the heterogeneity inherent in metastatic lesions can prevent trial outcomes from accurately reflecting real-world clinical scenarios. To address these disparities, future research should explore various avenues, including refining biomarker detection methods, performing dynamic monitoring of tumor heterogeneity, and conducting targeted studies on specific populations. A noteworthy approach within the realm of optimizing biomarker detection is the application of multi-omics technologies, which may facilitate the identification of novel biomarkers and enable an exploration of how spatial heterogeneity in their expression impacts treatment strategies.
ICIs have revolutionized cancer treatment, offering durable responses in various malignancies, including melanoma, NSCLC, TNBC, and HL. Despite their success, ICIs encounter significant challenges, such as therapy resistance, irAEs, and the need for improved patient selection strategies. Additionally, emerging research suggests that integrating ICIs with TME modulation and metabolic reprogramming may enhance treatment efficacy [103, 104]. As the field evolves, exploring novel immunotherapeutic approaches, such as dual checkpoint blockade, chimeric antigen receptor (CAR)-T cell therapy, and oncolytic viruses, will be crucial for overcoming current limitations and extending the benefits of immunotherapy [105–107]. However, the application of ICIs in the treatment of BC and hematological malignancies frequently presents challenges, including high therapeutic costs, stringent infrastructure requirements for cell therapy and gene editing, and limited accessibility in low- and middle-income countries. Addressing these issues underscores the importance of optimizing treatment regimens and exploring more rational dosing schedules and treatment durations. For example, research indicates that extending the fixed-dose interval of ICIs may reduce costs without significantly compromising therapeutic efficacy. Moreover, combination therapies can enhance the effectiveness of ICIs, thereby reducing the necessity for high-dose or prolonged monotherapy. A case in point is that combining ICIs with targeted drugs might yield superior outcomes at a lower dosage [108].
One significant challenge in the field of immune checkpoint therapy is resistance to treatment, which can be broadly categorized into primary and acquired types. Primary resistance occurs when tumors inherently lack the necessary immune activation for ICIs to function effectively. This form of resistance may be due to a low TMB, inadequate antigen presentation, or the presence of an immunosuppressive TME [109]. For example, certain hematological malignancies and HR+ BC display limited responsiveness to ICIs, attributed to their low TMB and the substantial presence of Tregs and MDSCs, which hinder T cell activation [110, 111].
Acquired resistance develops after an initial positive response to ICIs but eventually leads to disease progression. The mechanisms driving acquired resistance include the upregulation of alternative immune checkpoints (e.g., TIM-3, LAG-3, TIGIT), loss of antigen presentation due to mutations in B2M, and metabolic adaptations that foster an unfavorable immune milieu [112]. Additionally, tumors may utilize epigenetic modifications to silence genes related to immune activation, thereby further diminishing the efficacy of ICIs [113]. To counteract these resistance mechanisms, research into combination therapies, including dual immune checkpoint blockade and the use of epigenetic modulators, is actively underway.
Apart from resistance, irAEs pose another significant challenge in ICI therapy. The inhibition of immune checkpoints can trigger toxicities that mimic autoimmune responses, affecting multiple organ systems, including the skin, gastrointestinal tract, liver, lungs, and endocrine glands. Prominent irAEs include colitis, pneumonitis, and hypothyroidism, with severe cases necessitating immunosuppressive interventions such as corticosteroids [114]. The grading of irAEs is crucial for guiding clinical management, and standardized frameworks such as the Common Terminology Criteria for Adverse Events (CTCAE, version 5.0) are widely used to classify these events from grade 1 (mild or asymptomatic, requiring no intervention) to grade 4 (life-threatening conditions necessitating urgent intervention or indicative of organ failure) [115]. Effective management of irAEs involves immunosuppressive measures and supportive care interventions. In severe cases, this may also require temporary or permanent discontinuation of ICI therapy [116].
The development of predictive biomarkers for irAEs, such as cytokine profiles and analyses of the gut microbiota, is essential for minimizing these side effects while maintaining anti-tumor immunity [117].
To augment the efficacy of ICIs, researchers are investigating innovative approaches that combine immune checkpoint blockade with modulation of the TME, metabolic reprogramming, and the application of advanced genetic engineering techniques.
TME exerts a significant influence on immune responses and impacts the effectiveness of ICI therapies. Numerous tumors foster an immunosuppressive milieu by recruiting Tregs, MDSCs, and TAMs, which collectively inhibit T cell activation and facilitate tumor proliferation [118]. Strategies aimed at targeting these immunosuppressive elements, such as employing TGF-β inhibitors or CSF1R inhibitors to deplete TAMs, have shown potential in augmenting the efficacy of ICIs [119, 120]. Moreover, the adjunctive use of ICIs with radiation therapy, VEGF inhibitors, or agents that induce regulated cell death may promote increased infiltration of immune cells and enhance overall treatment outcomes [121–123].
Another promising approach to counteract ICI resistance involves metabolic reprogramming. Tumors frequently modify their metabolic pathways to create a hostile environment that impairs immune cell function, characterized by increased glycolysis, depletion of crucial nutrients like glutamine, and the production of immunosuppressive metabolites such as lactate [124]. Recent research indicates that the inhibition of tumor metabolism through approaches like glycolysis inhibitors or metabolic checkpoint inhibitors, including IDO1 inhibitors, can rejuvenate exhausted T cells and boost the effectiveness of ICIs [125]. Investigations into metabolic pathways such as glutaminolysis and fatty acid oxidation are ongoing in both preclinical and clinical settings.
In the realm of genetic engineering, the advent of clustered regularly interspaced short palindromic repeats (CRISPR) based gene editing technologies offers unprecedented opportunities to enhance immune checkpoint therapies. CRISPR facilitates precise alterations in T cells, enabling enhancements in their anti-tumor functions, for example, by knocking out inhibitory receptors such as PD-1 or by upregulating co-stimulatory molecules [126]. Additionally, CRISPR allows for the creation of personalized neoantigen-targeting T cells, potentially increasing the specificity and durability of immune responses.
Beyond ICIs, several innovative immunotherapeutic strategies are under exploration to potentiate anti-tumor immune responses. Among the most notable are dual immune checkpoint blockade, adoptive cell therapies, and the application of oncolytic viruses.
Dual immune checkpoint blockade aims to surmount resistance by simultaneously targeting multiple inhibitory pathways. For example, the combination of PD-1 and CTLA-4 blockade (nivolumab combined with ipilimumab) has demonstrated superior efficacy in treating melanoma and NSCLC compared to monotherapy [127]. Concurrent targeting of PD-1 with emerging checkpoints such as LAG-3, TIM-3, or TIGIT is currently under clinical evaluation, with preliminary results indicating enhanced immune activation and improved tumor control [128].
Adoptive cell therapies, which include CAR-T and TCR-T cell therapies, have achieved significant success in hematological malignancies and are increasingly being investigated for solid tumors. CAR-T therapy, which involves engineering T cells to express CARs that recognize specific tumor antigens, has been particularly effective in B cell malignancies. However, its application in solid tumors faces obstacles such as inadequate T cell trafficking, antigen heterogeneity, and the immunosuppressive nature of the TME [129]. To address these challenges, researchers are developing next-generation CAR-T cells that are endowed with properties to resist immune checkpoints, adapt metabolically, and modulate the TME.
Oncolytic viruses constitute an innovative immunotherapy approach. These genetically engineered viruses selectively target and eliminate tumor cells while simultaneously enhancing immune responses. Certain oncolytic viruses are engineered to produce ICIs, such as antibodies that inhibit PD-L1, thereby augmenting their therapeutic potential. For instance, talimogene laherparepvec (T-VEC), an oncolytic herpesvirus that expresses granulocyte-macrophage colony-stimulating factor (GM-CSF), has been approved for treating melanoma and is currently under investigation in conjunction with ICIs for other cancers [130].
Although ICIs have dramatically transformed cancer therapy, their clinical utility remains constrained by several factors, including resistance mechanisms, irAEs, and variable patient responses. Addressing these limitations requires a multifaceted strategy that incorporates ICIs with TME modulation, metabolic reprogramming, and cutting-edge genetic engineering techniques. Emerging therapeutic modalities, such as dual checkpoint blockade, CAR-T cell therapy, and oncolytic viruses, show potential to extend the benefits of immunotherapy to a wider array of malignancies. However, the translation of these therapies into clinical settings faces considerable challenges. For instance, dual checkpoint blockade often increases toxicity risks, while CAR-T therapy is hindered by exorbitant manufacturing costs and stringent infrastructure requirements that restrict its availability in resource-limited settings. Additionally, the heterogeneity of trial outcomes, resulting from variations in patient populations, biomarker definitions, and therapeutic protocols, complicates the standardization of these strategies across different clinical contexts.
As our understanding of tumor-immune interactions deepens, developing personalized immunotherapies that are tailored to the unique profiles of individual tumors will be essential for optimizing therapeutic outcomes. Nevertheless, achieving this goal necessitates surmounting several obstacles, such as the lack of universally accepted predictive biomarkers and the complexities of combination therapies, which often increase toxicity without proportional efficacy gains. Future research should focus on conducting head-to-head comparisons of combination therapies to identify synergistic pairs, validating context-specific biomarkers, and exploring cost-efficient manufacturing techniques to improve accessibility. Moreover, long-term real-world data concerning the durability of responses and late-stage toxicities are vital for refining these approaches to encourage broader clinical adoption. Continued research efforts are imperative to identify reliable predictive biomarkers, optimize combination therapies to balance efficacy and safety, and leverage innovative technologies to increase the durability and specificity of anti-tumor immune responses.
AML: acute myeloid leukemia
APCs: antigen-presenting cells
B2M: beta-2 microglobulin
BC: breast cancer
CAR: chimeric antigen receptor
CDK: cyclin-dependent kinase
CHL: classic Hodgkin lymphoma
CI: confidence interval
CPS: combined positive score
CRISPR: clustered regularly interspaced short palindromic repeats
CSF1R: colony-stimulating factor-1 receptor
CTLA-4: cytotoxic T lymphocyte-associated protein 4
CTLs: cytotoxic T lymphocytes
DLBCL: diffuse large B cell lymphoma
DNMTs: DNA methyltransferases
HDAC: histone deacetylase
HER2+: human epidermal growth factor receptor 2-positive
HL: Hodgkin lymphoma
HR+: hormone receptor-positive
ICIs: immune checkpoint inhibitors
ICMs: immune checkpoint molecules
IDO: indoleamine 2,3-dioxygenase
IFN-γ: interferon-gamma
IMiDs: immunomodulatory drugs
irAEs: immune-related adverse events
LAG-3: lymphocyte activation gene-3
MDSCs: myeloid-derived suppressor cells
MHC: major histocompatibility complex
MM: multiple myeloma
NHL: non-Hodgkin lymphoma
NK: natural killer
NSCLC: non-small cell lung cancer
ORR: objective response rate
PD-1: programmed death-1
PD-L1: programmed cell death ligand 1
PFS: progression-free survival
PMBCL: primary mediastinal large B cell lymphoma
R/R: relapsed or refractory
TAMs: tumor-associated macrophages
TCR: T cell receptor
T-DM1: trastuzumab emtansine
T-DXd: trastuzumab deruxtecan
Tex: T cell exhaustion
TIGIT: T cell immunoreceptor with Ig and ITIM domains
TILs: tumor-infiltrating lymphocytes
TIM-3: T cell immunoglobulin and mucin-domain containing protein 3
TMB: tumor mutational burdens
TME: tumor microenvironment
TNBC: triple-negative breast cancer
Tregs: regulatory T cells
VEGF: vascular endothelial growth factor
QB, HC, and SW: Writing—original draft. ZT and HT: Conceptualization, Supervision, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Luis Cabezón-Gutiérrez ... Vilma Pacheco-Barcia
Raffaele Pellegrino ... Antonietta Gerarda Gravina
Lingli Zhao ... Gaoli Niu
Rawaa AlChalabi ... Ahmed AbdulJabbar Suleiman
Fakher Rahim ... Issenova Balday
Neha Kannan ... Giuseppe Minervini
