Affiliation:
1VERDI Solutions GmbH, 1070 Vienna, Austria
Email: julianna.lisziewicz@verdi-solutions.com
ORCID: https://orcid.org/0000-0002-3349-4495
Affiliation:
1VERDI Solutions GmbH, 1070 Vienna, Austria
ORCID: https://orcid.org/0009-0009-5584-348X
Affiliation:
1VERDI Solutions GmbH, 1070 Vienna, Austria
ORCID: https://orcid.org/0000-0001-5105-4699
Affiliation:
1VERDI Solutions GmbH, 1070 Vienna, Austria
ORCID: https://orcid.org/0009-0007-9622-6993
Affiliation:
2Hospital Clínico Universitario Virgen de la Arrixaca and Biomedical Research Institute of Murcia (IMIB), 30120 Murcia, Spain
ORCID: https://orcid.org/0000-0003-2472-5893
Affiliation:
2Hospital Clínico Universitario Virgen de la Arrixaca and Biomedical Research Institute of Murcia (IMIB), 30120 Murcia, Spain
ORCID: https://orcid.org/0000-0001-9366-8410
Affiliation:
2Hospital Clínico Universitario Virgen de la Arrixaca and Biomedical Research Institute of Murcia (IMIB), 30120 Murcia, Spain
Affiliation:
2Hospital Clínico Universitario Virgen de la Arrixaca and Biomedical Research Institute of Murcia (IMIB), 30120 Murcia, Spain
ORCID: https://orcid.org/0000-0003-3307-1326
Affiliation:
2Hospital Clínico Universitario Virgen de la Arrixaca and Biomedical Research Institute of Murcia (IMIB), 30120 Murcia, Spain
ORCID: https://orcid.org/0000-0002-1471-8828
Affiliation:
1VERDI Solutions GmbH, 1070 Vienna, Austria
3Research Institute for Genetic and Human Therapy, 20132 Milano, Italy
ORCID: https://orcid.org/0000-0002-5928-172X
Affiliation:
2Hospital Clínico Universitario Virgen de la Arrixaca and Biomedical Research Institute of Murcia (IMIB), 30120 Murcia, Spain
ORCID: https://orcid.org/0000-0001-9080-1466
Affiliation:
2Hospital Clínico Universitario Virgen de la Arrixaca and Biomedical Research Institute of Murcia (IMIB), 30120 Murcia, Spain
Explor Immunol. 2025;5:1003221 DOI: https://doi.org/10.37349/ei.2025.1003221
Received: May 27, 2025 Accepted: September 12, 2025 Published: October 13, 2025
Academic Editor: Calogero Caruso, University of Palermo, Italy
T cell-based immunotherapies increasingly include personalized neoantigen vaccines that target tumor-specific mutations. However, despite their promise, current neoantigen vaccines show limited and unpredictable clinical benefit, with T cell responses observed in only a subset of patients. To overcome these limitations, we developed the VERDI (Vaccine Epitopes Ranked by Digital Intelligence) System—a cloud-based computational platform that integrates a patient’s human leukocyte antigen (HLA) class I and II genotype with selected tumor-associated antigens (TAAs), including cancer-testis antigens (CTAs), to identify peptides with high predicted immunogenicity and low risk of immune-related adverse events (irAEs). Using the VERDI System, we designed ten personalized peptide vaccines for a patient with metastatic signet ring cell carcinoma (SRCC), a rare and aggressive gastric cancer with limited treatment options. All ten VERDI vaccines were well tolerated and consistently induced tumor-specific T cell responses following a single administration, without the need for checkpoint inhibitors. The patient survived for 15 months—substantially longer than the reported median survival of 5.6 months in metastatic SRCC—highlighting the potential of this individualized, predictive vaccine platform to improve outcomes in advanced cancer.
Personalized medicine aims to tailor treatments to individual patients by considering their unique genetic and disease characteristics, rather than relying on population averages. In oncology, this has led to the development of personalized cancer vaccines—particularly neoantigen vaccines—which aim to stimulate immune responses against tumor-specific somatic mutations. These vaccines typically involve computational prediction of human leukocyte antigen (HLA)-binding epitopes derived from tumor exome data, followed by prioritization using methods such as mass spectrometry. The selected neoantigens are then incorporated into mRNA, DNA, or peptide vaccine platforms.
Despite their promise, current neoantigen vaccines face significant limitations. Only a small fraction of predicted neoantigens induce measurable T cell responses, even when combined with checkpoint inhibitors. Moreover, immunogenicity can only be confirmed post-treatment, making patient-level prediction of benefit uncertain. These issues limit the clinical utility of neoantigen vaccines and highlight the need for more reliable, individualized vaccine platforms.
To address these challenges, we developed the VERDI (Vaccine Epitopes Ranked by Digital Intelligence) System—a cloud-based computational pipeline that uses patient-specific HLA class I and II genotypes to select peptides from known tumor-associated antigens (TAAs), such as cancer-testis antigens (CTAs), that are predicted to elicit strong CD4+ and CD8+ T cell responses. Unlike neoantigen vaccines, VERDI does not rely on tumor mutations. Instead, it leverages shared antigens with known tumor specificity, while filtering out epitopes with expression in healthy tissues to reduce the risk of immune-related adverse events (irAEs). At the core of the VERDI System is a clinically validated diagnostic module (the VERDI test) that enables prediction of peptide immunogenicity at the individual level.
We used the VERDI System to develop personalized vaccines for a patient with metastatic signet ring cell carcinoma (SRCC), a rare and aggressive gastric cancer subtype with limited therapeutic options and poor prognosis. SRCC is characterized by diffuse infiltration, early metastasis to the peritoneum, and resistance to standard chemotherapy. With a median survival of just 5.6 months in advanced disease, there is an urgent need for effective personalized therapies.
This study aimed to evaluate the feasibility, safety, and immunogenicity of VERDI-designed peptide vaccines in a patient with treatment-refractory SRCC. We hypothesized that individualized vaccine design based on predicted HLA-restricted immunogenicity of shared TAAs would elicit robust tumor-specific T cell responses and contribute to prolonged survival.
The patient was diagnosed with advanced-stage gastric SRCC, an aggressive malignancy characterized by rapid invasion, chemoresistance, and peritoneal metastasis, associated with poor prognosis. He was a 70-year-old physician who had a previous medical history of smoking habit, well-controlled type 2 diabetes, prostatic syndrome, cholelithiasis, and left renal ureteral colic. He was admitted for evaluation of unexplained symptoms. Laboratory evaluation revealed elevated levels of carbohydrate antigen 19-9 (CA 19-9), suggesting a possible malignancy. Further clinical evaluation confirmed severe gastric inflammation due to Helicobacter pylori (H. pylori), and extensive gastric and intestinal metaplasia. Molecular studies indicated no loss of the DNA mismatch repair (MMR) mutL homolog 1 (MLH1) gene or MMR protein expression.
The VERDI vaccines were prepared as a magistral formulation, i.e., an individualized preparation compounded for a specific patient based on a medical prescription. Each VERDI vaccine consisted of a peptide of 20–50 amino acids in length, with a strength of 0.5 mg peptide per dose. Montanide ISA 51 VG (Seppic, France) was used as the adjuvant. The pharmaceutical form was an emulsion for subcutaneous injection, supplied in 1 mL syringes containing 0.7 mL of vaccine. Vaccines were administered in the axillary and inguinal regions, targeting the corresponding lymph node basins. A total of 10 VERDI vaccines were given as a single treatment course, delivered in two sequences: four vaccines followed by six vaccines, each administered as a single dose at 10 days apart. Each vaccine consisted of 0.5 mg peptide emulsified in Montanide ISA 51 VG and administered subcutaneously in the axillary or inguinal region, targeting regional lymph nodes.
Epitopes that most likely induce T cell responses were computed from the sequences of the selected target antigens using the VERDI test, the first HLA-genotype-based predictive immune diagnostic tool [1]. The input sequence data for the patient included 18 HLA alleles (both class I and II) and the target antigens. The 18 HLA allele sequences were obtained through high-resolution HLA genotyping of the patient. The VERDI test outputs the sequence data of the top-ranked epitopes representing the patient’s antigen-specific T cell repertoire and their predicted potency in stimulating T cells in the individual patient.
Recent studies have demonstrated the importance of stimulating both CD4+ T cells and CD8+ T cells for the treatment of established cancer [2, 3]. Therefore, we set the design criteria for the personalized VERDI vaccine to contain epitopes capable of providing strong antigen-specific stimulation to both CD4+ and CD8+ T cells. We selected the most immunogenic peptides from target antigens using the output of the VERDI diagnostic test. Peptides as long as 30 amino acids were employed to maximize the inclusion of both CD8+ and CD4+ epitopes with the highest predicted immunogenic potency.
All the immunogenic peptides selected as the VERDI vaccine candidate are subjected to safety tests. Checkpoint inhibitors, such as those targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed death-ligand 1 (PD-L1), often induce autoimmune side effects referred to as irAEs. To avoid the occurrence of irAEs with VERDI vaccines, the VERDI System filters out any epitope with significant expression in healthy tissues. This approach increases the likelihood that VERDI vaccines are designed to be not only immunogenic but also safe for the individual patient.
The active substance in each VERDI vaccine is a single peptide that must be manufactured to the highest quality standards. To ensure that each vaccine can be reliably produced and remains stable during shipping at room temperature, the VERDI algorithm includes a quality control step that filters out peptides for which quality and stability cannot be guaranteed [4].
T cell responses were quantified using a modified QuantiFERON® (QFN) ELISA assay (Qiagen Iberia, Barcelona, Spain) at several time points pre- and post-vaccination. Whole blood samples were collected and stimulated overnight with peptides from the VERDI vaccines. The quantity of interferon-gamma (IFN-γ) produced by T cells was measured by ELISA. The QFN test offers a high degree of sensitivity, with detection limits as low as 0.065 IU/mL for tuberculosis (TB) and 0.15 IU/mL for SARS-CoV-2 [5]. The QFN test provides a sensitive and specific assessment of vaccine-specific T cell responses without distinguishing CD4+ from CD8+ T cell responses.
Due to the unavailability of tumor samples for sequencing, the selection of vaccine targets was guided by data derived from peer-reviewed literature. Given that the patient had an active H. pylori infection and considering the gastric origin of the tumor, we identified CTAs linked with H. pylori-associated gastric cancer. CTAs are excellent targets for cancer vaccine development because they are typically restricted to tumor cells and germ cells of the testis, minimizing the risk of autoimmunity and maximizing the tumor specificity of the immune response.
We identified Kita-Kyushu lung cancer antigen-1 (KK-LC-1) as a promising target, as it demonstrates expression in approximately 80% of gastric cancers linked to H. pylori infection [6, 7]. Additionally, our selection included New York esophageal squamous cell carcinoma 1 (NY-ESO-1), encoded by the cancer/testis antigen 1B (CTAG1B) gene, known for its immunogenicity and expression in 24% of H. pylori-positive gastric cancers. NY-ESO-1 has been targeted in various vaccine studies, demonstrating its potential to elicit immune responses. We selected synovial sarcoma, X breakpoint 4 (SSX4), because it is expressed in 16% of H. pylori-positive gastric cancers and has been explored in previous cancer vaccine studies, highlighting its relevance as a target antigen. Furthermore, the inclusion of five additional CTAs was guided by a comprehensive transcriptome analysis of 375 gastric cancer specimens [8]. This selection process allowed us to assemble a panel of eight target CTAs with a high likelihood of expression in the patient’s tumor (Table 1). Although this approach was based on literature data rather than direct sequencing, it enabled a scientific evidence-based design strategy for the development of VERDI vaccines in this patient.
Evidence-based selection of target antigens expressed in H. pylori—positive gastric cancers.
| Targets | Rationale of target selection |
|---|---|
| KK-LC-1 | CTA is expressed in ~80% of H. pylori-associated gastric cancers. |
| NY-ESO-1 | Highly immunogenic CTA is expressed in ~24% of H. pylori-associated gastric cancers; it has been widely studied in cancer vaccine trials. |
| SSX4 | CTA is expressed in ~16% of H. pylori-associated gastric cancers; it has been previously investigated in cancer vaccine studies. |
| VCX2 | CTA expressed in gastric cancers; selected to broaden tumor-specific immune response. |
| MAGE-A3 | Highly immunogenic CTA expressed in gastric cancers; included in multiple vaccine trials. |
| MAGE-A6 | Highly immunogenic CTA expressed in gastric cancers; closely related to MAGE-A3 and selected for its immunogenic potential. |
| MAGE-A4 | CTA is expressed in gastric cancer, a known target of cancer vaccines. |
| MAGE-C1 | CTA is expressed in gastric cancer, a known target of cancer vaccines. |
CTA: cancer-testis antigen; KK-LC-1: Kita-Kyushu lung cancer antigen-1; MAGE-A3: melanoma antigen family A3; NY-ESO-1: New York esophageal squamous cell carcinoma 1; SSX4: synovial sarcoma, X breakpoint 4; VCX2: variable charge, X-linked 2.
The repertoire of epitopes that most likely induce T cell responses was computed from the sequences of the selected target antigens by the VERDI test, the first HLA-genotype-based predictive diagnostic tool [1]. The input sequence data included 18 HLA alleles from the patient, and the target antigens listed in Table 1. The VERDI test outputs the sequence data of the top-ranked epitopes representing the patient’s antigen-specific T cell repertoire and their predicted potency in stimulating T cells in the individual patient.
The VERDI test identified and ranked epitopes most likely to induce CD8+ cytotoxic T cell responses (Figure 1) and CD4+ helper T cell responses (Figure 2), prioritizing those with the highest predicted immunogenicity in the individual patient with SRCC.
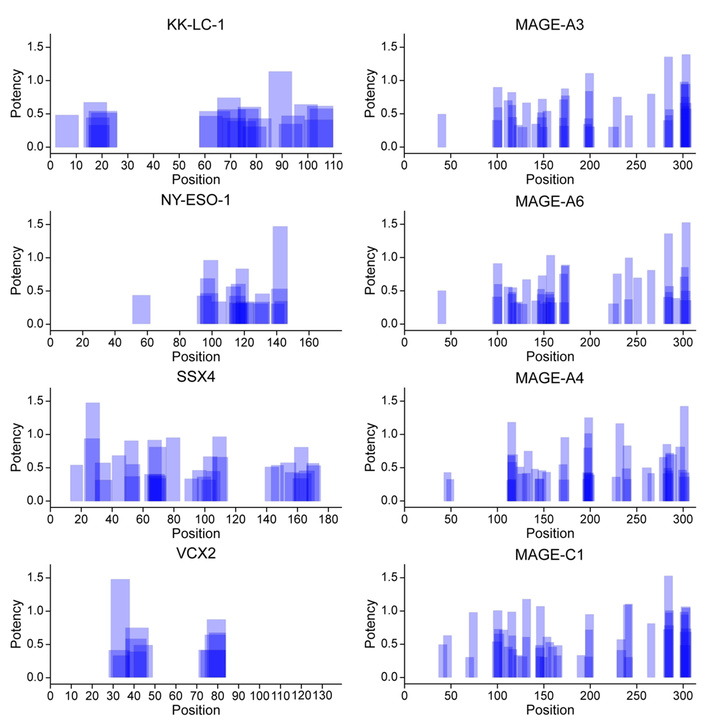
Predicted potency of CD8+ cytotoxic T cell activation by HLA class I—presented epitopes derived from eight tumor-specific antigens in a patient with SRCC. The VERDI diagnostic test was used to predict the patient-specific immunogenic epitope repertoire shown in the figure. Blue bars represent the positions of predicted epitopes along the amino acid sequences of the indicated antigens. Darker shading indicates overlapping epitopes, suggesting clusters of immunogenic regions within the antigen sequences. KK-LC-1: Kita-Kyushu lung cancer antigen-1; MAGE-A3: melanoma antigen family A3; NY-ESO-1: New York esophageal squamous cell carcinoma 1; SSX4: synovial sarcoma, X breakpoint 4; VCX2: variable charge, X-linked 2.
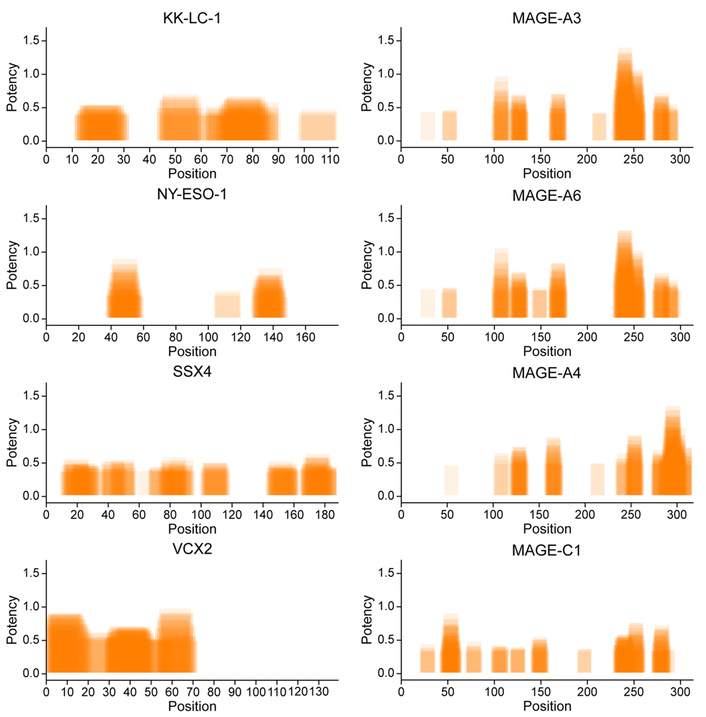
Predicted potency of CD4+ helper T cell activation by HLA class II—presented epitopes derived from eight tumor-specific antigens in a patient with SRCC. The VERDI diagnostic test was used to predict the patient-specific immunogenic epitope repertoire. Orange bars represent the positions of top-ranked predicted epitopes along the amino acid sequences of the indicated antigens. Darker shading indicates overlapping epitopes, suggesting clusters of immunogenic regions within the antigen sequences. KK-LC-1: Kita-Kyushu lung cancer antigen-1; MAGE-A3: melanoma antigen family A3; NY-ESO-1: New York esophageal squamous cell carcinoma 1; SSX4: synovial sarcoma, X breakpoint 4; VCX2: variable charge, X-linked 2.
We used the VERDI System to develop ten VERDI vaccines: two targeting KK-LC-1, two targeting SSX4, and one for each of the other targets. The KK-LC-1-specific VERDI vaccine (C1) contained the 9-mer epitope (RQKRILVNL) previously identified in lung cancer [9]. The other sequences do not have immunogenic epitopes reported in the literature. Immunization with multiple VERDI vaccines was crucial due to the patient’s metastatic cancer, which is characterized by a heterogeneous population of tumor cells [10]. This heterogeneity necessitates a multi-targeted approach to ensure that the immune system can recognize and attack several tumor cell variants [11].
We used the VERDI test to predict the potency of T cell responses in the SRCC patient, who was—and will remain—the sole recipient of these personalized vaccines [1]. Table 2 summarizes the selected vaccine peptides along with their predicted potency, which reflects the likelihood of eliciting CD4+ and CD8+ T cell responses in this individual. The predicted potency score is a relative, within-patient metric calculated by aggregating the immunogenicity of all predicted HLA class I (CD8+) and class II (CD4+) restricted epitopes contained within each vaccine peptide. For each epitope, the VERDI algorithm integrates multiple features—including HLA-binding affinity, antigen processing likelihood—to assign a numerical immunogenicity value. The potency score for each peptide is then derived by aggregating the immunogenicity values of all CD4+ or CD8+ T cell epitopes it contains, with higher scores indicating a greater predicted likelihood of inducing a T cell response in the patient. In this case, predicted potency scores ranged from 8.2 to 93.7 for CD4+ T cells and from 7.7 to 24.8 for CD8+ T cells, indicating a favorable likelihood of antigen-specific T cell activation.
Composition and predicted immunogenicity of ten VERDI vaccines developed for an SRCC patient.
| Vaccine ID | Target antigen | VERDI vaccine peptide sequence | Predicted potency | |
|---|---|---|---|---|
| CD4+ | CD8+ | |||
| C1 | KK-LC-1 | ILNNFPHSIARQKRILVNLSMVENKLVEL | 33.9 | 13.6 |
| C2 | KK-LC-1 | ALIVFWKYRRFQRNTGEM | 19.6 | 7.7 |
| C3 | NY-ESO-1 | TVSGNILTIRLTAADHRQLQLS | 37.1 | 14.1 |
| C4 | SSX4 | PRDDAQISEKLRKAFDDIAKYFSK | 25.3 | 12.8 |
| C5 | SSX4 | NQVERPQMTFGSLQRIFPKIMPKKPA | 8.2 | 11.5 |
| C6 | VCX2 | SDPKKKTTKVAKKGKAVRRGRR | 40.4 | 23.0 |
| C8 | MAGE-A3 | TFPDLESEFQAALSRKVAELVHFL | 11.9 | 13.5 |
| C9 | MAGE-A6 | KASDSLQLVFGIELMEVDPIGHVYIF | 24.3 | 16.6 |
| C10 | MAGE-A4 | KVLEHVVRVNARVRIAYPSLREAAL | 93.7 | 21.9 |
| C11 | MAGE-C1 | WGPRALVETSYVKVLHHLL | 15.7 | 24.8 |
KK-LC-1: Kita-Kyushu lung cancer antigen-1; MAGE-A3: melanoma antigen family A3; NY-ESO-1: New York esophageal squamous cell carcinoma 1; SSX4: synovial sarcoma, X breakpoint 4; VCX2: variable charge, X-linked 2; VERDI: Vaccine Epitopes Ranked by Digital Intelligence.
The predicted potency is patient-specific; the same peptide elicits different T cell responses in other individuals due to differences in their HLA genotype. The VERDI System ranks candidate vaccine peptides based on their predicted capacity to stimulate both CD4+ and CD8+ T cells in a given patient and selects one of the highest-ranked peptides as the vaccine candidate. The primary goal of this predictive selection strategy is to maximize the likelihood that each peptide administered will activate tumor-specific CD4+ and CD8+ T cells in the individual patient.
It is important to emphasize, however, that predicted potency does not necessarily correlate directly with the magnitude of T cell responses measured after vaccination. Ex vivo immune monitoring typically relies on peripheral blood-based assays that quantify cytokine production or surface activation markers (e.g., ELISA, ELISpot, or flow cytometry—based assays). The magnitude of these measured responses can be influenced by several factors, including the time of sampling, the extent of in vivo T cell stimulation by antigens expressed in the tumor, assay sensitivity, and experimental variables such as the duration and context of in vitro stimulation.
The clinical timeline of disease course and treatment is summarized in Figure 3. In detail, the patient was diagnosed with stage IV gastric SRCC and initiated first-line chemotherapy with a folinic acid, fluorouracil, oxaliplatin modified regimen 6 (FOLFOX6m), consistent with standard clinical guidelines [12]. After four cycles, disease progression was documented with worsening omental and mesenteric carcinomatosis and an elevation of CA 19-9 levels to 4,390 U/mL. According to current clinical recommendations, second-line chemotherapy with paclitaxel plus ramucirumab was initiated [13].
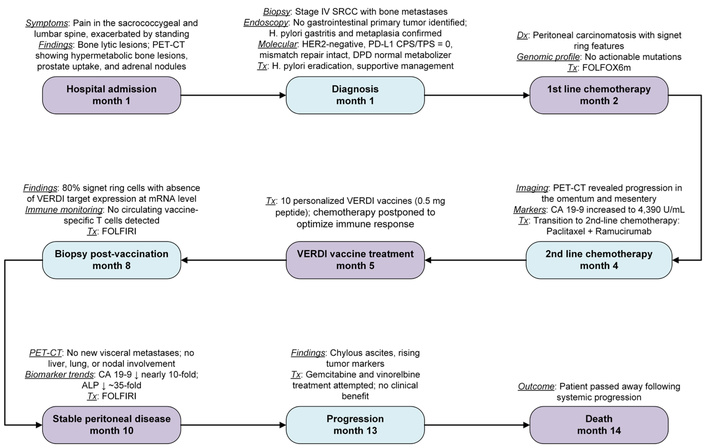
Clinical timeline of disease course and treatment in a patient with metastatic SRCC. CA 19-9: carbohydrate antigen 19-9; CPS/TPS: combined positive score/tumor proportion score; DPD: dihydropyrimidine dehydrogenase; FOLFIRI: folinic acid, fluorouracil, irinotecan; FOLFOX6m: folinic acid, fluorouracil, oxaliplatin modified regimen 6; HER2: human epidermal growth factor receptor 2; PD-L1: programmed death-ligand 1; PET-CT: positron emission tomography-computed tomography; SRCC: signet ring cell carcinoma; VERDI: Vaccine Epitopes Ranked by Digital Intelligence.
Considering the rapid progression and the well-documented poor prognosis of advanced SRCC [14, 15], a patient-specific treatment with personalized VERDI vaccines was prescribed based on their predicted safety and immunogenicity as determined by the VERDI report (Tables 1 and 2). The patient, himself a physician, was thoroughly informed of the rationale, potential benefits, and risks associated with VERDI vaccine therapy, and provided explicit written informed consent for both the treatment and the inclusion of his clinical data in this manuscript.
Scheduling the VERDI vaccinations posed a significant clinical challenge. Paclitaxel administration required premedication with dexamethasone (20 mg) to prevent potentially life-threatening infusion-related reactions [16]. However, dexamethasone, being a potent corticosteroid, is known to exert immunosuppressive effects, raising concerns about its potential negative impact on vaccine-induced immune responses [17, 18]. To mitigate this concern, the medical team implemented a personalized treatment schedule: single doses of the C2, C4, C5, and C10 VERDI vaccines were administered 10 days after chemotherapy, while the remaining six vaccines were given 24 days post-chemotherapy, resulting in the postponement of the subsequent chemotherapy administration to day 28. This individualized treatment schedule balanced the necessity of immunosuppressive premedication against the goal of optimizing vaccine efficacy, thus exemplifying a patient-centric therapeutic approach.
Clinical monitoring revealed discrete hypermetabolic bone foci suggestive of minimal recurrence while omental and mesenteric carcinomatosis remained stable. However, persistent neuropathy (likely attributable to oxaliplatin, a component of the initial FOLFOX regimen, or paclitaxel, employed as a second-line therapy) prompted initiation of third-line therapy with dose-reduced (80%) folinic acid, fluorouracil, and irinotecan (FOLFIRI), aiming to balance therapeutic efficacy and patient tolerance. After eight cycles of FOLFIRI, the patient experienced moderate disease progression, evidenced by increased metabolic activity in bone lesions, persistence of mild pleural effusion, and worsening multicompartmental ascites.
Subsequently, a fourth-line therapy regimen was initiated with gemcitabine and vinorelbine (GemVin), selected based on molecular sensitivities (OncoDeep report). Despite therapeutic efforts, disease progression continued, with new metastatic bone lesions, increased bilateral pleural effusions, and progressive ascites. The patient eventually passed away due to refractory chylous ascites. From initiation of therapy to his death, the patient’s overall survival was 15 months.
All ten VERDI vaccinations were excellently tolerated, with no observed hypersensitivity or skin reactions. However, a thrombotic event (month 7) presented a diagnostic challenge. The event was characterized by pronounced dilation of the left jugular vein, with a notable absence of contrast filling, extending from its cranial exit point at the jugular foramen to its bifurcation point at the left brachiocephalic venous trunk. Upon review of the patient’s medical history and the literature, it was hypothesized that the thrombosis was most likely associated with the underlying SRCC rather than the vaccinations. SRCC has been reported to present with internal jugular vein (IJV) thrombosis, potentially due to a hypercoagulable state related to the malignancy [19]. This hypothesis was supported by the patient’s elevated platelet count, which exceeded 350,000, fitting the criteria for prophylactic anticoagulation according to Khorana’s score.
Regarding the vaccination, thrombosis side effects are exceedingly rare and are primarily associated with vaccinations using adenovirus vectors and thrombocytopenia. As such, it seems unlikely that the VERDI vaccination contributed to the thrombosis. Given the timing and nature of prior chemotherapeutic exposures, and the lack of neurotoxic adjuvants in the VERDI vaccines, the persistent neuropathy is more likely attributable to oxaliplatin and paclitaxel than to vaccination.
Our safety findings confirmed the favorable safety profile of peptide vaccine treatments, and the absence of irAE indicated the value of our computational safety tests, excluding autoimmunity of VERDI vaccines in the patient.
Each VERDI vaccine elicited T cell responses compared to both baseline and negative controls (Figure 4). Although the dataset represented in Figure 4A was too small for statistical analysis, a biologically meaningful increase—exceeding threefold between trough and peak levels—was observed for peptides C2, C4, and C10. For the dataset shown in Figure 4B, a Wilcoxon signed-rank test demonstrated a statistically significant difference between baseline and Day 34 post-vaccination (p < 0.05). This difference remained significant at Day 70 (p < 0.05), despite a reduction in the magnitude of the T cell response.
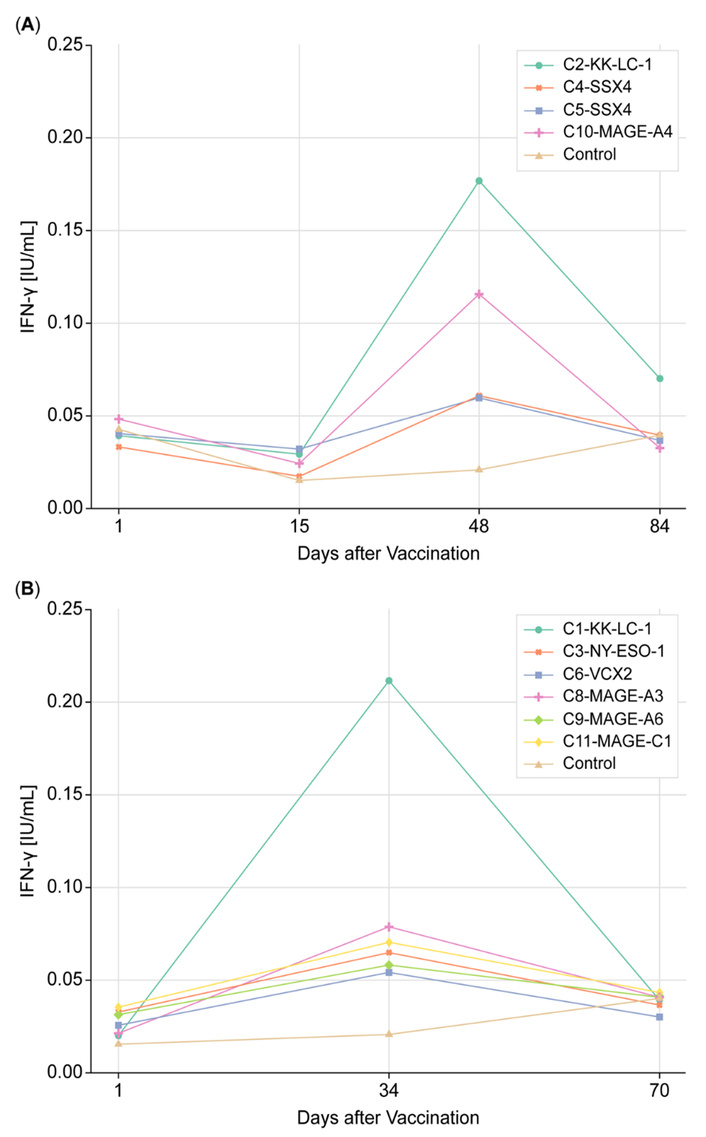
Tumor-specific T cell responses in the SRCC patient pre- and post-VERDI vaccine treatment. (A) Four vaccines targeting KK-LC-1, SSX4, and MAGE-A4 were administered 10 days (labeled as day 1) after paclitaxel and ramucirumab treatment. (B) Six vaccines targeting KK-LC-1, NY-ESO-1, VCX-2, MAGE-A3, MAGE-A6, and MAGE-C1 were given 24 days (labeled as day 1) after paclitaxel and ramucirumab. The subsequent paclitaxel and ramucirumab treatment was delayed by 24 days. KK-LC-1: Kita-Kyushu lung cancer antigen-1; MAGE-A3: melanoma antigen family A3; NY-ESO-1: New York esophageal squamous cell carcinoma 1; SRCC: signet ring cell carcinoma; VCX2: variable charge, X-linked 2; VERDI: Vaccine Epitopes Ranked by Digital Intelligence.
The decline in vaccine-induced T cell activity following peak response is attributed to the biological dynamics of effector T cells. Specifically, effector cells elicited by vaccination undergo senescence and apoptosis after fulfilling their cytotoxic function. Additionally, a proportion of these cells transition into memory T cell subsets, which are not detectable by the QFN assay. This shift contributes to the observed decrease in immune activity over time. While IFN-γ levels exceeded 0.1 IU/mL in only three vaccines, these responses were elicited after a single dose, without checkpoint inhibitors, and remained statistically significant at Day 34 and Day 70, indicating meaningful immunogenicity in the context of advanced cancer.
Two VERDI vaccines, both targeting the KK-LC-1 antigen (C1 and C2, Table 2), consistently induced high-magnitude T cell responses (0.17 and 0.21 IU/mL), despite being administered 10 days apart (Figure 4). Two additional vaccines (C4 and C5, Table 2) targeting the SSX4 antigen elicited lower but consistent responses (0.06 IU/mL). The reproducible induction of antigen-specific T cell responses by distinct peptides derived from the same antigen supports the predictive accuracy of the VERDI System to develop peptide vaccines capable of eliciting tumor-specific T cell responses in this individual.
The magnitude of T cell responses after a single dose of VERDI vaccines ranged from 0.05 to 0.21 IU/mL, comparable to responses seen in healthy individuals who received three doses of the BNT162b2 COVID-19 mRNA vaccine (0.1–0.9 IU/mL) using the same QFN assay [20]. The consistent induction of tumor-specific T cell responses following a single administration of 10 distinct peptides represents a significant achievement in the context of personalized cancer immunotherapy.
Before starting the personalized vaccine treatment, the patient experienced persistent pain that made walking difficult due to bone metastases. Following vaccine administration, his condition gradually improved. Six months later, he was able to walk 7 kilometers without pain and reported feeling stronger and more active. He did not experience any side effects from the vaccines. According to family and clinical observations, he expressed satisfaction with the treatment and experienced an improved quality of life during the months following vaccination.
We introduced the VERDI System to support the development of personalized cancer vaccines and demonstrated its predictive utility in a patient with metastatic SRCC. By matching patient-specific HLA genotypes with TAAs, the VERDI System enabled the selection of vaccine peptides with predicted immunogenicity and minimal risk of irAEs. The clinical findings, the consistent antigen-specific T cell responses following a single vaccine dose, and the absence of irAEs underscore the pipeline’s potential in developing safe and effective personalized vaccines for cancer patients (Figure 5).
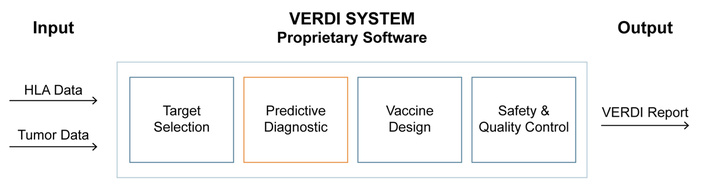
Computational pipeline of the VERDI System for personalized vaccine development. The VERDI System is a cloud-based computational pipeline designed to optimize the efficacy, safety, and quality of personalized VERDI vaccines. It prioritizes peptides predicted to elicit tumor-specific CD4+ and CD8+ T cell responses and filters out those with expression in healthy tissues to minimize the risk of irAEs. The output of the pipeline—the VERDI report—provides the selected peptide sequences along with their predicted potency scores for eliciting T cell responses in the individual patient. HLA: human leukocyte antigen; VERDI: Vaccine Epitopes Ranked by Digital Intelligence.
Current neoantigen vaccines typically include 10–40 HLA-restricted epitopes derived from several tumor mutations [21], yet only about 50% of patients exhibit measurable T cell responses, even after multiple doses and checkpoint inhibition [22]. In contrast, each VERDI vaccine contains several epitopes predicted to induce T cell responses against a single TAA and consistently elicited tumor-specific T cell responses following a single administration. The magnitude of these responses was comparable to those observed after three doses of the COVID-19 mRNA vaccine—an immunologically potent benchmark [20]. This consistent immunogenicity, achieved in the absence of checkpoint inhibitors, highlights a key advantage of the VERDI vaccines over conventional neoantigen vaccine approaches.
The rationale for VERDI vaccine therapy stems from the recognition that each patient presents a unique combination of tumor antigens and HLA alleles. As such, off-the-shelf cancer vaccines are limited by population-level generalization and rarely deliver meaningful clinical benefit [23]. In contrast, VERDI vaccines are designed to match the treatment to the individual, not the individual to the treatment. This represents a conceptual shift: rather than relying on population-based probabilities of benefit—as is common with approved immunotherapies such as checkpoint inhibitors, where individual response remains unpredictable—VERDI’s approach enables more predictable immune activation based on patient-specific immunogenomic data. Although the evidence is generated at the individual-patient level, it is mechanistically grounded and supported by observed T cell responses and clinical outcomes. In the case reported here, the patient with stage IV SRCC survived for 15 months after initiating therapy—substantially longer than the median survival of 5.6 months previously reported for metastatic SRCC [24]. While individual case outcomes must be interpreted with caution, the observed survival extension, along with consistent vaccine-induced T cell responses and maintained quality of life despite bone metastases, suggests potential clinical benefit from this individualized vaccine approach.
These results call for clinical trials to evaluate the efficacy of personalized oncology with VERDI vaccines, with the ultimate goal of improving the lives of cancer patients. Given that each VERDI vaccine is uniquely designed for an individual, conventional clinical trial models based on fixed products and population averages are inadequate. Instead, real-world evidence (RWE), longitudinal immune monitoring, and patient-centered outcome tracking will be essential to demonstrate the effectiveness of VERDI vaccine treatment at scale [25].
The integration of VERDI vaccines into clinical workflows also requires thoughtful planning regarding timing, treatment sequencing, and therapeutic combinations. While tumor-directed therapies such as chemotherapy or targeted agents may act synergistically with vaccine-induced immune responses, many antiproliferative agents can suppress the expansion of tumor-specific T cells and diminish the efficacy of vaccine therapy. In our case study, we delayed chemotherapy to allow vaccine-induced T cell proliferation, which may have contributed to enhanced tumor control. Additionally, the issue of intratumoral heterogeneity remains clinically significant, especially in metastatic settings where resistant subclones can escape single antigen targeting. To address this, we propose a combinatorial vaccination strategy involving twelve individualized VERDI vaccines, delivered in four-dose sets to different anatomical sites over multiple visits. This approach is intended to induce parallel T cell responses across multiple lymph nodes, thereby expanding tumor-specific T cell diversity and improving the likelihood of targeting heterogeneous tumor cell populations. Lastly, VERDI vaccines should not be reserved for metastatic patients. Given their favorable safety profile and minimal systemic toxicity, they are suitable for earlier administration, including neoadjuvant or adjuvant settings, where boosting of tumor-specific immunity may enhance the effectiveness of subsequent interventions and improve long-term disease control.
In conclusion, this study demonstrates the feasibility, safety, and potential clinical utility of personalized VERDI vaccines in a patient with advanced SRCC. The observed immune responses and prolonged survival support the core premise of the VERDI System: that individualized vaccine development based on tumor antigens and HLA genotype can overcome limitations of traditional vaccine platforms. Unlike current medicinal products, which remain static after approval, VERDI vaccines continuously evolve through patient-derived data, allowing for iterative improvement and precision-guided therapy. Further treatment of cancer patients using VERDI vaccines is warranted to validate and refine this approach in broader clinical settings.
CA 19-9: carbohydrate antigen 19-9
CTAs: cancer-testis antigens
FOLFIRI: folinic acid, fluorouracil, irinotecan
FOLFOX6m: folinic acid, fluorouracil, oxaliplatin modified regimen 6
HLA: human leukocyte antigen
IFN-γ: interferon-gamma
irAEs: immune-related adverse events
KK-LC-1: Kita-Kyushu lung cancer antigen-1
NY-ESO-1: New York esophageal squamous cell carcinoma 1
QFN: QuantiFERON®
SRCC: signet ring cell carcinoma
SSX4: synovial sarcoma, X breakpoint 4
TAAs: tumor-associated antigens
VERDI: Vaccine Epitopes Ranked by Digital Intelligence
The authors thank Sylva Petrocchi for editorial assistance and R. G. Lisziewicz for contributions to the peptide selection strategy. Language editing support was provided using ChatGPT (OpenAI).
JL: Conceptualization, Methodology, Writing—original draft, Writing—review & editing. JMM: Conceptualization, Writing—original draft, Writing—review & editing. AH: Software. AS: Formal analysis, Software, Visualization. TK: Software, Formal analysis. BGP: Supervision. MBB: Supervision. FL: Validation. CMMC: Resources. AM: Validation. MMV: Investigation. SM: Resources. All authors read and approved the submitted version of the manuscript.
JL is a shareholder of VERDI Solutions GmbH. Authors AS, TK, and AH are employed at VERDI Solutions GmbH. All other authors declare no conflicts of interest.
The personalized VERDI vaccine was administered under an individual healing attempt protocol in accordance with Article 37 of the Declaration of Helsinki, Version 2013. Approval by an institutional ethics committee was not required under applicable regulations.
Informed consent to participate in the study was obtained from the participant.
Not applicable.
The data presented in this study are available upon reasonable request from the corresponding author.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1616
Download: 27
Times Cited: 0
