126 results in Exploration of Neuroscience
Latest
Sort by :
- Latest
- Most Viewed
- Most Downloaded
- Most Cited
Open Access
Review
Innovations in hydrocephalus modeling: bridging animal models, bioengineering platforms, and precision therapies
Ioannis Angelopoulos
Published: March 10, 2026 Explor Neurosci. 2026;5:1006126

Open Access
Retraction
Retraction: Extracellular vesicles in neurological disorders: emerging roles and underlying molecular mechanisms
Mst. Afsana Mimi, Md. Mahmudul Hasan
Published: February 08, 2026 Explor Neurosci. 2026;5:1006125

Open Access
Original Article
Tissue transglutaminase modulates pain but not neuronal survival after nerve injury
Gong-Wei Lyu ... Tie-Jun Sten Shi
Published: February 05, 2026 Explor Neurosci. 2026;5:1006124
Open Access
Original Article
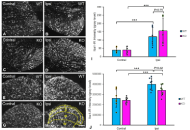
Restorative effects of Aframomum melegueta and Aframomum danielli-supplemented diets on sperm quality and testicular health following scopolamine-induced neurotoxicity in rats
Odunayo M. Agunloye ... Ganiyu Oboh
Published: February 01, 2026 Explor Neurosci. 2026;5:1006123
This article belongs to the special issue Medicinal Plants and Bioactive Phytochemicals in Neuroprotection (Vol II)

Open Access
Perspective
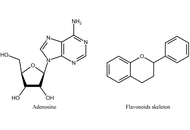
Perspectives on the use of flavonoids in glioblastoma treatment by targeting adenosine receptors
Katrin Sak
Published: January 22, 2026 Explor Neurosci. 2026;5:1006122
This article belongs to the special issue Current Approaches to Malignant Tumors of the Nervous System
Open Access
Review
Cerebral amyloid angiopathy: updates on pathophysiology, diagnosis, and management
Trinity Willsey ... Mo-Kyung Sin
Published: January 05, 2026 Explor Neurosci. 2026;5:1006121

Open Access
Case Report
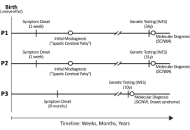
First report of SCN8A-related neurodevelopmental disorder and a case of SCN1A-related Dravet syndrome in Libya
Anwaar M. Bennour ... Heba A. El-Zawawi
Published: December 23, 2025 Explor Neurosci. 2025;4:1006120
This article belongs to the special issue Advances in Epilepsy Research
Open Access
Review
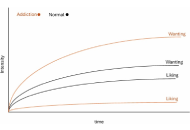
Rethinking computational models of addiction: toward context-sensitive and psychologically grounded frameworks
Anamaria Madeliene Manu
Published: December 09, 2025 Explor Neurosci. 2025;4:1006119
Open Access
Review
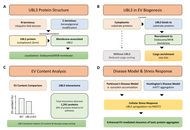
Retracted: Extracellular vesicles in neurological disorders: emerging roles and underlying molecular mechanisms
Mst. Afsana Mimi, Md. Mahmudul Hasan
Published: December 01, 2025 Explor Neurosci. 2025;4:1006118
Open Access
Case Report
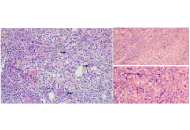
Isolated primary CNS lymphoma: a case report and a contemporary review
Ali Msheik ... Abdelnaser Thabet
Published: December 01, 2025 Explor Neurosci. 2025;4:1006117
Open Access
Systematic Review
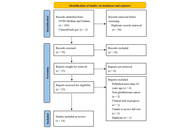
Bi-, tri-, and multi-specific T-cell engager therapies in glioblastoma: a decade of preclinical innovation
Adam H. Lapidus, Malaka Ameratunga
Published: November 28, 2025 Explor Neurosci. 2025;4:1006116
This article belongs to the special issue Current Approaches to Malignant Tumors of the Nervous System
Open Access
Original Article
The association of brain-derived neurotrophic factor Val66Met polymorphism with stroke outcomes: a cross-sectional pilot study
Eduard Tiozzo ... Tatjana Rundek
Published: November 11, 2025 Explor Neurosci. 2025;4:1006115

Open Access
Review
Pain management in Guillain-Barré Syndrome: a literature review
Kyla D. Groves ... Kunal Aggarwal
Published: October 28, 2025 Explor Neurosci. 2025;4:1006114

Open Access
Case Report
Cyclic vomiting syndrome (CVS) responsive to single-dose olanzapine: a case report
Sara Sipilä ... Jussi O.T. Sipilä
Published: October 22, 2025 Explor Neurosci. 2025;4:1006113

Open Access
Review
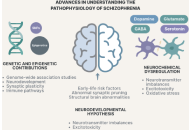
Advancing our understanding of schizophrenia: insights from recent research, emerging therapies, and future directions
Tolutope Adebimpe Oso ... Don Eliseo Lucero-Prisno
Published: October 12, 2025 Explor Neurosci. 2025;4:1006112
Open Access
Review
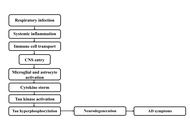
Respiratory pathogen to brain degeneration: a review of Chlamydia pneumoniae’s role in Alzheimer’s disease
Atif Salim Khatib ... Daniya Tasnim
Published: September 23, 2025 Explor Neurosci. 2025;4:1006111
Open Access
Editorial
Editorial: novel therapeutic approaches for the treatment of depression
Ayan Mohamud Yusuf, Dirk M. Hermann
Published: September 16, 2025 Explor Neurosci. 2025;4:1006110
This article belongs to the special issue Novel Therapeutic Approaches for the Treatment of Depression

Open Access
Original Article
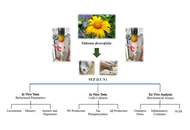
Tithonia diversifolia (Hemsl.) extract attenuates cognitive dysfunction, oxidative stress and neuroinflammation in a model of sporadic Alzheimer’s disease induced by streptozotocin
Graziella Martins Guimarães ... Márcia Maria de Souza
Published: September 10, 2025 Explor Neurosci. 2025;4:1006109
Open Access
Original Article
Behavioral effects of Gabrb2 knockout and an anti-inflammatory herbal formula on aged mice
Manel Barki ... Hong Xue
Published: September 09, 2025 Explor Neurosci. 2025;4:1006108

Open Access
Original Article
Impact of neurology staff’s adherence to management guidelines on seizure freedom in epilepsy patients
Rodolfo Cesar Callejas-Rojas ... Ildefonso Rodriguez-Leyva
Published: August 14, 2025 Explor Neurosci. 2025;4:1006107
This article belongs to the special issue Advances in Epilepsy Research

Journal Information
 Previous
Previous