Affiliation:
Department of Psychiatry, University of Melbourne, Austin Hospital, Heidelberg 3084, Australia
Email: trevorrn@unimelb.edu.au
ORCID: https://orcid.org/0000-0003-2903-7096
Explor Neurosci. 2025;4:1006105 DOI: https://doi.org/10.37349/en.2025.1006105
Received: April 10, 2025 Accepted: July 14, 2025 Published: August 07, 2025
Academic Editor: Ayan Mohamud Yusuf, University of Duisburg-Essen, Germany
The article belongs to the special issue Novel Therapeutic Approaches for the Treatment of Depression
Treatment resistant depression (TRD) is frequently encountered in clinical practice. The lack of response of the condition to conventional medications and augmentation strategies has spawned the search for novel treatment approaches. Psychedelic medications used in conjunction with intensive psychotherapy, so-called psychedelic-assisted psychotherapy (PAP), have been evaluated in a limited number of studies as an alternative tactic. This psychedelic renaissance has seen psilocybin, a naturally occurring, potentially hallucinogenic substance occurring in some species of mushrooms, used as one exemplar. The definition of “treatment resistance” varies between different authorities, but there is general agreement that a minimum standard is failure to respond to at least two pharmacological agents from different classes used at a therapeutic dose for an adequate length of time. In the studies to date, more stringent definitions have mostly been applied. Each of the clinical evaluations finds that the addition of a single dose of psilocybin to the psychotherapeutic regimen produces a rapid and clinically significant decline in depressive symptomatology, which is mostly retained in follow-up evaluations out to 12 weeks or longer. Psilocybin was well tolerated with mostly mild to moderate side effects of elevated blood pressure, fatigue, lack of concentration, headache, lethargy, vertigo, feeling of physical or emotional weakness, decreased appetite, nausea, feeling dull, and being easily exhausted, which were transient. Hallucinogen persisting perception disorder (HPPD) has occasionally been reported, while there were few reports of suicidal ideation and behaviour. Psilocybin appears to offer the promise of rapid alleviation of resistant depressive symptoms, but further controlled evaluations are necessary before the drug can be given routinely.
The contemporary resurgence of interest in the therapeutic potential of psychedelic substances for mental health treatment might be referred to as a “psychedelic renaissance”. Momentum for the revival of interest in a range of compounds and their use in psychiatric disorders stems from a growing body of research and the recognition of the limitations of current treatments for conditions such as major depression, post-traumatic stress disorder (PTSD), and substance use disorders. There is a history of the use of these substances dating back to the mid twentieth century, when both psilocybin and lysergic acid diethylamide (LSD) were synthesised in the laboratories of Sandoz by Albert Hofmann and colleagues [1]. They were soon taken up by the psychiatric community as they appeared to offer a “consciousness expansion” which was considered to be useful in psychotherapy [2]. The apparent efficacy of LSD in alcohol use disorder was lauded [3], but by the early 1970s, the drug was declared a prohibited substance in the United States and similar bans soon followed in other countries. Revival of interest in the current era has focused on studies of the use of ketamine, psilocybin, and ecstasy [3,4-methylenedioxymethamphetamine (MDMA)]. Of particular interest has been the assessment of the efficacy of psilocybin in “treatment resistant” major depression. Accordingly, the use of psilocybin in conjunction with psychotherapy, so-called “psychedelic-assisted psychotherapy” in this difficult to treat depression, is reviewed.
The author searched the PubMed database (National Library of Medicine) and the Web of Science (Clarivate Analytics PLC) using the terms “treatment resistant depression”, “psilocybin”, and “clinical trial”. A database of 81 articles was returned from PubMed and 78 articles from Web of Science. After removal of duplicates, each abstract was reviewed by the author, and articles that did not specifically include the term “treatment resistance” were manually excluded, as were articles describing psilocybin treatment of depression. Review articles were also excluded. From the “final” set of articles obtained, the reference lists were screened manually for any additional references not included in the two overlapping searches.
Depression that is resistant to treatment is frequently encountered in clinical practice and represents a challenge to the treating practitioner. Although well recognised at a practical level, a consensus definition is lacking [4]. One definition proposed by several authors is depression that does not respond to standard treatments, including at least two different antidepressant medications taken at adequate doses for a sufficient duration [5–7]. Inherent in this definition is the often-vexed question of what constitutes adequate doses of medication and what is a sufficient duration of treatment. Furthermore, the definition focuses solely on pharmacological treatments and does not recognise the potential utility of psychotherapeutic approaches. Alternative definitions have been proposed, such as using staging criteria to determine a level of resistance based on responses to escalating treatment modalities [6]. Thase and Rush [7] proposed a five-stage model of resistance with the lowest level failure to respond to a single class of antidepressant up to the highest stage failure to respond to electroconvulsive therapy (ECT) in addition to failure to respond at each of the lower stages. This hierarchical definition has the virtue that it can be readily applied and aligns with clinical practice. On the other hand, some shortcomings of the definition have been identified, most critically the implicit assumption of equivalence between pharmacological treatments [6]. Several other staging definitions of treatment resistant depression have been developed in an attempt to obviate the shortcomings of the Thase and Rush criteria [7]. These include the Maudsley Staging Model [8], the European Group for the Study of Resistant Depression [9], the Dutch Measure for Quantification of Treatment Resistant Depression Model [10], and the Massachusetts General Hospital Staging Model [11]. The details of these schemas are not discussed here, but essentially, they expand upon the Thase and Rush criteria [7] with more detailed explanations of the various stages of resistance. Despite the existence of the various frameworks, few have been widely applied in clinical trials, while they have failed to contribute significantly to any underlying biological aetiology of the emergence of treatment resistance.
The medicines regulatory agencies, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have adopted as a definition of treatment resistant depression that which is most widely used, i.e., an inadequate response to at least two antidepressants, despite adequate treatment duration and adherence. In the context of the studies described here on the efficacy of psilocybin, this definition has been employed in the majority of studies.
Psilocybin is a classic hallucinogenic substance belonging to the tryptamine class, naturally occurring in over 200 species of fungi. The most potent varieties are found within the Psilocybe genus, particularly P. azurescens, P. semilanceata, and P. cyanescens [12]. This compound was isolated by Albert Hofmann in 1958 from the P. mexicana mushroom, which the Aztecs referred to as teonanacatl [13]. As a classic hallucinogen, psilocybin’s psychedelic effects have been primarily attributed to its action as a 5HT2A agonist (see further discussion below). Its chemical structure closely resembles that of serotonin (see Figure 1). The psychoactive effects of psilocybin are largely due to its rapid metabolic conversion to psilocin (see Figure 1). While psilocybin itself may not be pharmacologically active, its dephosphorylation upon ingestion occurs swiftly, making it challenging to evaluate its individual effects (see further discussion below).
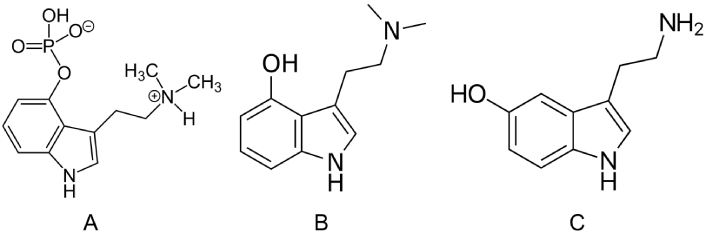
Chemical structures, IUPAC name, and common names of: (A) psilocybin, [3-[2-(dimethylamino)ethyl]-1H-indol-4-yl] dihydrogen phosphate; (B) psilocin, 3-[2-(dimethylamino)ethyl]-1H-indol-4-ol; (C) serotonin, 5-hydroxytryptamine, 3-(2-aminoethyl)-1H-indol-5-ol. IUPAC: International Union of Pure and Applied Chemistry. Sources of the images: (A) https://zh.wikipedia.org/wiki/%E8%B5%9B%E6%B4%9B%E8%A5%BF%E5%AE%BE; (B) https://en.wikipedia.org/wiki/Psilocin; (C) https://en.wikipedia.org/wiki/Serotonin
Studies in vivo have shown that psilocybin rapidly loses its phosphate group and is converted to its active metabolite psilocin, which is formed in the acidic environment of the stomach or by the action of alkaline phosphatase in the intestine and kidney [14]. Psilocybin can thus be considered a “pro-drug” for psilocin. Pharmacokinetic studies following psilocybin administration reflect the properties attributable to the metabolite rather than the parent drug. No psilocybin was detected in plasma or urine after ascending oral doses of the drug, with psilocin appearing in plasma within 20–30 min [15, 16]. Maximum concentrations of psilocin were observed within 3 h of oral dosing of psilocybin [15, 16]. In a single dose study, psilocin demonstrated linear pharmacokinetics in the range 0.3 to 0.6 mg/kg psilocybin based on the maximum drug concentration (Cmax) and area under the plasma concentration time curve [15]. A more extensive study in healthy volunteers reported similar results in the dose range 15 to 30 mg psilocybin (i.e., similar dose range to the previous study based on average body weight) [17]. The apparent volume of distribution calculated for psilocin exceeded that of total body water, indicating extensive tissue distribution [17]. The absolute bioavailability of psilocin was about 52% after oral administration in 3 human volunteers [18]. Despite the extensive distribution to tissue psilocin has a relatively short half-life, with a mean of 3 ± 1.1 h determined in one study [18]. On the other hand, highly variable plasma elimination half-lives of more than twice this mean value have been reported [19]. This variance probably reflects individual enzyme polymorphisms responsible for metabolic pathways.
Psilocin undergoes extensive first pass metabolism in the liver and is excreted principally by the kidneys [20]. Several metabolites have been identified in plasma and urine, but none are considered pharmacologically active [21, 22]. About 2% to 4% of psilocin is excreted unchanged in the urine [20]. Metabolism of psilocin occurs via extensive glucuronidation by UDP glucuronosyltransferases to form psilocin-O-glucuronide, which is excreted in the urine and faeces [23]. Unconjugated psilocin is metabolised to inactive 4-hydroxyindole-3-acetic acid (4-HIAA) via monoamine oxidase and aldehyde dehydrogenase [23]. In plasma, the psilocin metabolites are present at concentrations exceeding those of psilocin [17].
Like other traditional psychedelic agents, at least some effects of psilocybin can be explained by an agonist action of the drug at 5HT2A receptors. Hallucinogenic activity of psilocybin and other indoleamine hallucinogens, for example, is highly correlated with the effective dose producing the intended pharmacological effect in 50% of the population (ED50) for binding at this receptor subtype [24]. Following single oral doses of psilocybin (3–30 mg), the 5HT2A receptors were occupied in a dose dependent manner. The half maximal effective concentration (EC50) for receptor occupancy was calculated as ~2 μg/L. Furthermore, it has been demonstrated that the 5HT2A antagonist, ketanserin, can block the hallucinogenic effect of psilocybin [25]. An agonist action at the 5HT2A receptor initiates a series of “downstream” effects through the action of second messenger systems. Differences in these second messenger pathways between hallucinogenic and non-hallucinogenic 5HT2A agonists have been demonstrated [26]. The difference in G-protein activation and specific signalling pathways or “biased agonism” has been proposed as an explanation of the psychoactive differences between hallucinogen and closely related non-hallucinogen 5HT2A receptor agonists [27]. Psychedelic agents activate both 5HT2A-Gq/11 and β-arrestin2 transducers, but recent studies suggest that recruitment of the 5HT2A-Gq predicts psychedelic potential [28]. Furthermore, a threshold level of activation of 5HT2A-Gq is required to induce psychedelic effects, which explains why 5HT2A partial agonists are non-psychedelic [28]. Binding at other 5HT2 receptors as well as to non-5HT2 receptors may contribute to the mediation of the actions of psilocybin [27]. Thus, it has been proposed that hallucinogenic agonists (but not non-hallucinogenic agonists) bring about their effects through the formation of a complex between 5HT2A and a metabotropic glutamate-2 (mGlu2) receptor [27]. Multiple early growth response genes are also activated by hallucinogens, which are related to synaptic enhancement, which in turn may be related to the antidepressant effects of psilocybin [29]. Psilocybin effects on other receptor subtypes within the serotonergic system, for example, 5HT1A partial agonist actions, inhibition of the serotonin transporter, may contribute to the behavioural effects of the compound. Similarly, there are actions at the dopamine D2 receptor and the glutamatergic system, which are thought to influence drug effects [26]. In common with conventional antidepressants [such as selective serotonin reuptake inhibitors (SSRIs)], psilocybin, through its actions at these receptors, has been demonstrated to promote a rapid increase in brain derived neurotrophic factor (BDNF) concentrations in the brain [29]. Serotonergic psychedelics have been shown to promote rapid dendritic and axonal remodelling. Increased dendritic arbor complexity following treatment of cortical neurons with serotonergic psychedelics has been demonstrated. This appeared to arise from an increase in the number of dendritic branches and the total length of branches [30, 31]. Similar effects have been shown to occur with conventional antidepressant medications [32]. Taken together, it is proposed that the antidepressant effects of psychedelics and conventional medications bring about their effects through a common neurobiological mechanism.
Neuroimaging studies have examined the effect of psilocybin on the “so-named” default mode network (DMN), several interconnected brain regions, including the medial prefrontal cortex, posterior cingulate cortex, precuneus, and angular gyrus [33]. The DMN is active when the brain is not focused on a specific, external task. It is characterized by increased activity during states of rest, mind-wandering, and internally focused thought, such as recalling memories or planning for the future. Conversely, DMN activity typically decreases when an individual is engaged in focused attention on external stimuli or demanding tasks. Altered functional connectivity within the DMN has been implicated in several psychiatric disorders, including major depression [33]. Therapeutic effects of psychedelic agents are proposed to reduce the functional connectivity within the DMN while at the same time increasing the connectivity of the DMN to other networks, which is related to the altered states of consciousness experienced by users and the therapeutic outcomes resulting from the interaction [33].
The user experience of psilocybin is highly variable and depends on the mindset and environment in which the user has the experience, factors commonly referred to as set and setting, a theme in common with all psychedelic agents [34]. Qualitatively, the effects of psilocybin are similar to those of LSD, except that the duration of effect is usually shorter. Psilocybin “trips” last anywhere from 4 to 6 h, with effects felt within 20 to 40 min after ingestion and peaking around the 2- to 3-h mark, i.e., approximating the peak psilocin concentrations in plasma [35]. After ingesting psilocybin, the user may experience a wide range of emotional effects which can include disorientation, lethargy, giddiness, euphoria, joy, depression, transient paranoia, hallucinations, and synaesthesia [35]. Common physiological responses include pupil dilation, changes in heart rate (mostly increases), changes in blood pressure both hypotension and hypertension, general instability, nausea, and tremor [36]. Patients with a pre-existing diagnosis of schizophrenia or psychotic episodes are excluded from clinical trials due to concerns about the risk of triggering or worsening psychosis, although the necessity for the exclusion of such patients has recently been questioned [37]. Tolerance to all of the effects of psilocybin can develop on repeated use, but after several weeks of withdrawal from the drug, recovery from tolerance is observed [38]. No apparent physiological dependence, as evidenced by withdrawal symptoms, has been documented in humans or in pre-clinical studies [39].
The use of psilocybin under controlled conditions is generally regarded as safe, although further information is required [40]. A meta-analysis of six double-blind trials involving 528 patients found that headache (2–66%), nausea (4–48%), anxiety (4–26%), dizziness (6–8%), and alterations of blood pressure (up to 76%) occurred significantly more often with psilocybin than with comparator agents [41]. Most effects were regarded as tolerable and subsided within 48 h of the single doses. Few required medical intervention. In a review of studies of healthy participants administered psilocybin (n = 659), no immediate serious adverse events were reported. Similarly, no outpatient participants in psilocybin trials (n = 618) experienced an immediate serious adverse event [42]. On the other hand, delayed serious adverse events, although occurring relatively rarely (3.9% of 584 outpatient participants), included suicidal behaviours and increased suicidal ideation [42].
In terms of toxicity, the lethal dose for humans has been estimated at 6 g or approximately 200 times greater than the highest dose (30 mg) used in clinical studies [43].
The role of psychotherapy is regarded as critical to a positive outcome from the psychedelic experience. However, whether the psychotherapy provided is “therapeutic” or not has been the subject of debate. It has been posited that any therapeutic effects are derived from the drug alone as the “psychotherapy” usually provided does not conform to any recognised schema and that the nondirective counselling employed is not effective for the conditions (such as treatment resistant depression) being treated [44]. Furthermore, since psilocybin showed a clear dose-response effect, the sine qua non of pharmacological action, in the treatment of resistant depression [45] the therapeutic effect is therefore purely pharmacological. Based on these premises, the authors have argued that the treatment modality should not be referred to as “psychedelic-assisted psychotherapy” but rather simply as “psychedelic treatment”. The counterargument is that the extensive use of supportive therapy provided by “experienced” psychotherapists before, throughout, and after the experience is indeed “therapeutic” [46]. Scores on the Mystical Experience Questionnaire, a self-report measure of the acute effects of psilocybin, have been demonstrated to be predictive of beneficial outcomes from psychedelics administered in experimental contexts [47]. Regardless of the merits or otherwise of these arguments, it is clear that the studies are consistent with respect to some aspects of the provision of counselling pre-drug regarding the use of psilocybin, particularly to drug-naive subjects. Prior to the psychedelic medication, patients meet with a therapist, usually at least three times, to build trust, receive psychoeducation, and prepare for the psychedelic experience. During the drug session, which typically lasts six to eight hours, the therapist and an assistant therapist are present throughout. Sessions are typically conducted in a setting which is designed to be as non-clinical as possible, while patients listen to music of their own choice selected to be calming. Following the drug session, further psychotherapy sessions are held to debrief about and to take away understanding from the experience.
Despite this uniformity of approach, there is an absence of any manualised approach to psychotherapy employed across the studies, with each study using an individually determined methodology and a flexible number of pre- and post-drug sessions [44]. Indeed, little research into the psychotherapy provided during psychedelic therapy has been conducted, leaving the question of necessary and sufficient components unanswered [48]. One psychological support model, as used in trials of treatment resistant depression, has been described as a potential framework for further research [49]. The model also outlines suggested requirements for therapist training. One caveat of this proposal is that it was wholly developed by a pharmaceutical company (Compass Pathways) developing a psilocybin product, which does not necessarily detract from the merit of the proposal, but it is likely to reflect the company’s concerns and biases.
Indications for the effectiveness of psilocybin in depression came from studies of the drug in patients with terminal illnesses. For example, in patients with life-threatening cancer who developed clinically significant symptoms of depression and anxiety, psilocybin (22 or 30 mg/70 kg) was more effective in reducing the symptoms than a low dose of the drug (1 or 3 mg/70 kg) [50]. The low dose was regarded as a placebo-like dose of the agent. Other studies, with relatively small sample sizes and utilising different doses of psilocybin, reported similar results in patients with life-threatening conditions [51, 52] while a Cochrane review concluded that the drug was probably effective for use in such patients [53]. Similarly, in severe major depressive disorder (MDD), several studies attest to the effectiveness of psilocybin assisted psychotherapy [54–56]. Given the importance of resistant cases in the landscape of major depression treatment, it is not surprising that the efficacy of psilocybin in specifically defined cases has begun to be evaluated.
Evaluations of psilocybin in treatment resistant cases to date are summarised in Table 1. Psilocybin has been evaluated in six studies of resistant MDD comprising 299 unique patients, with one study contributing the majority (n = 233). Psilocybin is typically used in a single dosing session but has also been evaluated in repeated dosing sessions separated by intervals of one week or more. In the studies conducted to date, the majority of patients have been free of other psychotropic medications. Furthermore, certain psychiatric exclusion criteria, for example previous psychotic disorders, are a stringent requirement of participation in the studies. In general, patients in the studies have been medically well with no evidence of serious somatic disorders.
Clinical trials of psilocybin in treatment resistant major depression
| Patients | Psilocybin dose | Number of sessions | Primary outcome measure | Methodology | Outcome | Reference |
|---|---|---|---|---|---|---|
| Unipolar major depression | ||||||
| DSM-5 MDD; failure to respond to 2 to 4 treatments | 1 mg (n = 79)10 mg (n = 75)25 mg (n = 79) | Single session | MADRS | Multicentre, double-blind; MMRM for analysis of change from baseline to week 3 | 25 mg superior to 1 mg at week 3; patient-reported depression severity, anxiety, affect, and functioning improved at week 3 | [45, 59] |
| MDD confirmed by MINI; failure to respond to 2 treatments | 10 mg (n = 12)25 mg (n = 12) | Two dosing sessions separated by 1 week | QIDS | Single site, open label | Reduced depressive symptoms 1 week and 3 months after high dose | [57] |
| MDD confirmed by MINI; failure to respond to 2 treatments | 10 mg (n = 20)§25 mg (n = 20)§ | Two dosing sessions separated by 1 week | QIDS | Single site, open label | Reduced depressive symptoms at 1 week, 3- and 6-month follow-ups | [58] |
| DSM-5 MDD; failure to respond to 2 to 4 treatments | 25 mg (n = 19) | Single session | MADRS | Open label study; adjunct to SSRI treatment | Mean change from baseline to week 3 MADRS score 14.9; significant clinical improvement | [60] |
| DSM-5 MDD; insufficient response to at least five treatments | 25 mg (n = 12) | Single session | MADRS | Single site, open label | Statistically significant decline in MADRS scores from baseline to week 3, 12 (RMANOVA) | [61] |
| DSM-5 MDD; non-response to ≥ 5 treatments; military veterans | 25 mg (n = 15) | Single session | MADRS | Single site, open label | MADRS score decreased significantly from baseline across weeks 1, 3, and 12 (MMRM) | [62] |
| Bipolar II depression | ||||||
| Clinical assessment MDD or BPD II depression; multiple medication failures | 25 mg (n = 30) | 1, 2, or 3 sessions depending on response | MADRS | Randomised wait list controlled | Reduced depressive symptoms after one dose; further reductions after repeated doses | [63] |
| DSM-5 BPD II with insufficient response to two or more pharmacological treatments | 25 mg (n = 15) | Single session | MADRS | Single site, open label | Mean change of 24 points on MADRS from baseline to week 3 | [64] |
§ includes 12 patients from 2016 report [57]. DSM: Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association; MDD: major depressive disorder; MADRS: Montgomery-Åsberg depression rating scale; MMRM: mixed models for repeated measures; MINI: Mini International Neuropsychiatric Interview; QIDS: Quick Inventory of Depressive Symptomatology; SSRI: selective serotonin reuptake inhibitor; RMANOVA: repeated measures analysis of variance; BPD II: bipolar disorder type II
An open-label, feasibility study of the utility of psilocybin in the target population was performed using a two-dose regimen of the drug [57]. Diagnosis of treatment resistance was based on clinical interview using the Mini International Neuropsychiatric Interview (MINI) and no improvement in symptoms in the current episode despite two adequate courses of antidepressant treatment of different pharmacological classes lasting at least 6 weeks. The study was performed in 12 patients from an initial group of 72 who expressed interest in the trial, the majority being excluded due to insufficient severity of illness or not meeting the entry criteria. Patients received a low oral dose of psilocybin 10 mg on the first dosing day and a high oral dose of psilocybin 25 mg on a second dosing day, separated by 1 week. For monitoring of treatment outcome, the Quick Inventory of Depressive Symptomatology (QIDS) was used. Mean depression rating score decreased from baseline at 1 week after the high dose session of psilocybin and was maintained up to three months. The effect size for the difference between baseline and each rating point was calculated using Hedge’s g. At weeks 1, 2, 3, 5, and 3 months, the values were statistically significant and are regarded as indicative of a large effect size (values ranged from 3.2 to 2.0). Based on Hedge’s g, there appeared to be a waning of the effect of the drug at 3 months, but the value of g was still large (2.0; P = 0.003). As discussed above, psychotherapy sessions were provided to participants throughout the study. Based on a score of ≤ 9 on the Beck Depression Inventory (BDI), eight patients (67%) achieved a remission at 1 week. At the three-month follow-up visit, seven patients (58%) met criteria for a response (50% reduction in BDI score from baseline) while five of these patients (42%) were still in remission. This pilot study was extended to include an additional eight patients, with the follow-up period after psilocybin dosing increased to six months [58]. In terms of the primary outcome measure, the QIDS, the addition of the extra patients made no difference to the findings: psilocybin (with psychotherapy) resulted in a rapid improvement in depressive symptoms, which was maintained up to six months. The calculated effect sizes were large (Cohen’s d ranged from 1.5 to 2.3). The waning of effect at three and six months, based on the calculated effect sizes, was evident as in the previous report. Depressive symptoms were reduced for the first 5 weeks post-treatment (Cohen’s d = 2.2 at week 1, 2.3 at week 5; both P < 0.001). Of the nineteen patients who completed all phases of the study, nine and four patients, respectively, met the criteria for response and remission at week 5. At the three- and six-month visits, depressive symptoms were reduced compared to baseline measures (Cohen’s d = 1.5 and 1.4, respectively, both P < 0.001). Of the responders at 5 weeks, three of the nine had relapsed at six months, but the remaining patients maintained response.
A multi-centre, dose-response study of the effect of psilocybin for treatment resistant depression was performed as a randomised, double-blind trial in 233 patients meeting Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association (DSM)-5 criteria for MDD, diagnosed with the MINI, and treatment resistance defined by the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire (MGH ATRQ) [45]. Patients were randomly assigned to a single dose of 1, 10, or 25 mg of psilocybin together with psychological support. The 1 mg dose was regarded as ineffective and could be viewed as a pseudo-placebo. The primary outcome measure of the study was the change from baseline to week 3 in the Montgomery-Åsberg depression rating scale (MADRS) with patients followed up to 12 weeks after treatment. The primary efficacy variable was evaluated using a mixed models for repeated measures (MMRM) analysis comparing the 25 mg dose with the 1 mg dose and the 10 mg dose with the 1 mg dose. A statistically significant difference was observed for the 25 mg dose compared to the 1 mg dose, but not for the 10 mg dose compared to the 1 mg dose. The effect of the treatment on MADRS scores was generally retained out to 12 weeks, but as in the studies of Carhart-Harris and colleagues [57, 58], there was a waning of the intensity of the response. Response rates (defined as ≥ 50% decrease in MADRS from baseline) were 37% in the 25 mg, 19% in the 10 mg, and 18% in the 1 mg groups at week 3. Response was maintained by 20%, 5%, and 10% in the 25 mg, 10 mg, and 1 mg groups, respectively at week 12. Fewer patients achieved remission (MADRS score ≤ 10) at week 3: 29%, 9%, and 8% for the 25 mg, 10 mg, and 1 mg groups, respectively. Remission rates at week 12 were not reported but are likely to be lower than for week 3, consistent with a waning of efficacy. Patient measures of depression, anxiety, and functioning were also taken in this study and reported as a separate analysis [59]. In general, the patient-rated outcomes were consistent with the clinician-rated data: the 25 mg dose was superior to either the 10 mg or 1 mg dose. In addition to decreases in self-rated depression and anxiety scores, patients reported an improvement in quality of life, functioning, and cognition.
In the previous studies of treatment resistant depression, patients were weaned off all other psychotropic medications before psilocybin treatment. To examine the potential interference of antidepressants with the efficacy of a fixed dose of psilocybin, an open-label study was conducted in 19 patients with resistant depression taking a range of SSRI antidepressants [60]. Patients met DSM-5 criteria for major depression as well as having failed to respond to an adequate dose and duration (> 8 weeks) of two to four pharmacological treatments for the current episode, including their current SSRI. All patients received a single 25 mg dose of psilocybin and psychological support. The primary efficacy end point was the change in MADRS total score from baseline to three weeks post-psilocybin administration. No formal statistical analysis of change in scores was performed, but the mean decline in the MADRS from baseline to week 3 was 14.9, a clinically meaningful improvement from the baseline score of 31.7. Remission of symptoms (defined as a MADRS total score ≤ 10) during the follow-up period were 52.6% at day 2, 47.4% at week 1, 42.1% at week 2, and 42.1% at week 3. Response rates [based on the proportion of participants with a score on the Clinical Global Impression Scale—severity of 1 (“normal, not ill at all”) or 2 (“borderline mentally ill”)] were 42.1% on day 2, 63.2% at week 1, 57.9% at week 2, and 52.6% at week 3. The results suggest that psilocybin might be used as an adjunct to ongoing antidepressant treatment without any severe adverse reactions, although more extensive studies are necessary.
A similar open-label study was conducted in 12 patients with severe treatment resistant depression [61]. Patients had a diagnosis of single or recurrent episode of MDD (DSM-5 criteria) with at least five treatment failures during the current episode. The primary outcome measure was the change in the MADRS score from baseline to 3 weeks post-dosing. Psilocybin was administered as a single oral dose of 25 mg with standardised psychotherapy as in other trials of psilocybin. In the majority of patients, there was a significant decline in the MADRS score from baseline one week after psilocybin (mean change 19.4), which was maintained at week 3 (mean change 15.8) and week 12 (mean change 17.2). The changes were both statistically (MMRM analysis of change from baseline) and clinically significant. At week 1, 75% of patients met response (≥ 50% decline in baseline MADRS score) and remission (MADRS score ≤ 10) criteria. Corresponding data for week 3 were 66.7% and 41.7% of participants, respectively. At week 12, seven out of 12 patients (58.3%) met response criteria and three patients (25%) were in remission. In this study, most patients had co-morbid diagnoses. Of particular interest was PTSD. Exploratory analysis of data in this trial suggested that the antidepressant response might be attenuated in patients with this co-morbidity. This finding prompted an additional study in military veterans with PTSD using the same methodology [62]. Patients in this study met the diagnostic criteria of severe resistant comorbid depression, defined as MDD that failed to respond to ≥ 5 treatments during the current episode, or a current episode persisting > 2 years. Diagnosis was confirmed using the MINI interview and met DSM-5-TR criteria for major depression. The results of this study were largely in agreement with those of the previous trial, namely, psilocybin was associated with a significant decline in MADRS scores at one week from baseline. Response and remission were defined as in the previous trial. At week one, 67% of patients met response criteria and 47% remission. By week three, 60% were responders and 53% were in remission, while at week 12, the corresponding proportions were 47% and 40%, respectively. The study confirms the earlier exploratory analysis, namely that comorbid PTSD does not significantly affect depression outcomes in psilocybin assisted psychotherapy.
A randomised study examined the effects of a fixed dose of psilocybin (25 mg) in treatment resistant patients with either MDD or depression in the context of a bipolar II diagnosis [63]. Treatment resistance was defined as having failed to respond to an adequate dose and duration of at least two pharmacological treatments for the current episode of depression, as determined by the MGH ATRQ. Patients were randomly assigned to either immediate treatment with psilocybin or a wait list control. All patients eventually received psilocybin with adjunctive psychotherapeutic support. The primary outcome measure was the decrease in MADRS score from baseline, measured at 2 weeks after the initial dose of psilocybin. A two-tailed t-test was used to compare changes in mean MADRS scores between groups (immediate treatment and wait list). Patients had one, two, or three psilocybin sessions (25 mg psilocybin). Each dose was accompanied by one preparatory therapy session, a supportive dosing session, and two integration therapy sessions. Patients were evaluated at multiple intervals up to six months after the initial treatment. Patients assigned to the wait list group had a small decline in MADRS score over two weeks, whereas those in the immediate treatment group achieved a significant decline in depressive symptoms (3.0 versus 9.6 mean change for wait list and immediate group, respectively). The effect size of 1.1 (95% confidence interval: 0.3–1.8; P = 0.005) based on Hedge’s g is considered to be large. Over the six-month follow-up period, depressive symptoms improved as judged by the decline in mean MADRS scores. Response and remission rates in this study were not reported. Where patients showed signs of relapse of depression, additional doses of psilocybin (up to three doses in total as noted above) could be provided. A total of seventeen patients received a second dose of psilocybin, while five patients received a third dose. Findings in this study suggest the feasibility of a multiple dose approach for relapsing conditions.
The efficacy of a single dose of psilocybin (25 mg) in 15 patients with bipolar II depression was performed as an open-label study [64]. Participants met DSM-5 criteria for bipolar II disorder based on the MINI interview. Treatment resistance during the current depressive episode met the MGH ATRQ criteria. After the psilocybin session, patients were followed up at regular intervals for 12 weeks. The primary outcome was the change in the MADRS total score from baseline visit to week 3 follow-up. All participants had a lower MADRS score at week 3 (statistically significant mean change of 24). A one-way repeated measures analysis of variance (RMANOVA) on the raw scores at baseline, weeks 1, 3, and 12 showed a statistically significant effect of time. At each post-treatment assessment, MADRS scores were significantly decreased from baseline (week 1: Cohen’s d = 4.78, P < 0.001; week 3: Cohen’s d = 4.08, P < 0.001; week 12: Cohen’s d = 3.39, P < 0.001). Mean MADRS scores were not significantly different among weeks 1, 3, and 12, suggesting stability of posttreatment effects. However, examination of the raw scores for individual patients showed that for some, the initial improvements in depression scores at week 1 were not uniformly maintained across the 12-week treatment period. At least three patients’ MADRS scores exceeded the criterion for remission and could be considered to have relapsed. At week 3 (the primary outcome assessment), 80% of patients (n = 12 of 15 who completed the study) met the criterion for response (≥ 50% decline in MADRS from baseline), and 11 met the remission criterion (MADRS score ≤ 10). At week 12 (study end point), 12 patients met both response and remission criteria.
When used in single doses under controlled conditions, as in the studies described here, psilocybin is regarded as safe with relatively minor adverse events reported. The potential for lethality due to overdose of psilocybin is considered to be low [19]. A lethal dose of psilocybin has been estimated as at least five hundred times greater than the therapeutic dose range of 15 to 30 mg [43]. At high doses, psilocybin is capable of inducing symptoms not dissimilar to those of psychosis but with more intense visual effects [65]. As with other agents producing visual hallucinations and illusions, the effects appear to be mediated via an agonist action at 5HT2A receptors [24].
One particular concern with the use of hallucinogenic substances generally is the occurrence of hallucinogen persisting perception disorder (HPPD). The condition is better known by the term “flashbacks” and consists of ongoing hallucinations of varying intensity long after the initial experience with the drug [65]. Following psilocybin administration, the phenomenon is thought to be rare [66]. Nevertheless, some case reports have emerged of HPPD in subjects using psilocybin in a recreational setting in the context of multiple drug use, particularly with cannabis and ecstasy [67, 68]. The contribution of psilocybin to the visual effects experienced in these cases is difficult to ascertain. The mechanism of HPPD at the neurochemical or brain circuitry level, irrespective of the responsible agent, remains speculative [69]. At a neurochemical level, hallucinogen use may lead to the destruction of GABAergic cortical serotonergic inhibitory interneurons, which can cause a breakdown in the mechanisms that filter unnecessary stimuli [69]. A reverse tolerance or sensitisation theory has been proposed to account for flashbacks even after the stimulus has been removed [70]. The lateral geniculate nucleus (LGN), an area of the thalamus involved in visual perception pathways, is also thought to be involved in HPPD with flashbacks as a type of visual seizure [71]. An alternative perspective posits that symptoms in predisposed individuals may arise from an overactivation of neural-visual pathways following exposure to certain stimuli [72]. Additionally, some researchers argue that environmental triggers resembling the original experience can prompt flashback episodes [73]. Others contend that heightened anxiety, coupled with inherent vulnerabilities in perceptual processing, contributes to these phenomena [72].
Several systematic reviews and meta-analyses of the adverse events associated with psychedelic drug use, including psilocybin and other agents, such as LSD, have been performed [19, 74–77]. These reports suggest that the agents are well tolerated with few serious adverse events and minor increases in suicidal ideation. Importantly, no verifiable deaths associated with the individual psychedelic agents have been reported [77]. The safety profile of psilocybin administered to healthy volunteers was available from a pooled analysis of three single dose studies [78]. In these double-blind, placebo-controlled, crossover studies, 85 healthy volunteers received psilocybin 15, 20, 25, or 30 mg. Subjective effects of the drug were measured with visual analogue scales throughout the experience, while physiological measures such as blood pressure, heart rate, and body temperature were obtained as repeated measures. Adverse effects were assessed with a standardised rating scale. The most frequent acute adverse effects included fatigue, lack of concentration, headache, lethargy, vertigo, feeling of physical or emotional weakness, decreased appetite, nausea, feeling dull, and being easily exhausted. In this analysis, there did not appear to be any difference in side effects across dose groups. Renal and hepatic function, as well as blood cell counts measured 28 (± 18) days after psilocybin administration, remained within normal limits. Body temperature and blood pressure were slightly increased during the psilocybin experience, while tachycardia was observed in < 10% of participants. The effects did not appear to be dose dependent. In these studies, five subjects reported flashbacks occurring within 72 h of the psilocybin dose, while in one subject, the flashbacks persisted for several months.
A comprehensive review of adverse experiences with psilocybin in clinical trials across all indications for which the drug has been used reported similar experiences for patients as the healthy volunteers and included elevated blood pressure, headaches, nausea, vomiting, fatigue, and anxiety [76]. Suicidal ideation and behaviour were also noted in some patients, but principally in subjects with a past history. The review supported the notion that psilocybin use is generally safe.
Lowering of the seizure threshold has been suggested as an infrequent adverse effect, particularly in times of heightened stress [65]. Seizures may occur when psilocybin is used concomitantly with tramadol, an opioid receptor agonist.
Prolongation of the corrected QT interval (QTc) from baseline after psilocybin administration was reported in two subjects receiving 25 mg of the drug [45]. In both subjects, the QTc interval had returned to within the normal range at follow-up. The effect of psilocybin on QT interval prolongation remains largely unknown, as ECGs were obtained only at baseline in the studies performed to date [79]. A theoretical possibility exists of valvulopathy with chronic use of “microdoses” of psilocybin because of strong agonism at the 5HT2B receptor. Echocardiographic monitoring should be implemented to detect any cases under chronic use.
Treatment with psilocybin and psychological support for various psychiatric disorders is an active area of research. A cursory search of the website ClinicalTrials.gov with the search term “psilocybin” noted more than 200 trials registered across multiple conditions. Combined with “treatment resistant depression”, 27 such trials were retrieved. At present, psilocybin assisted psychotherapy for treatment resistant depression has been evaluated in a few trials and in predominantly small groups of patients. Efficacy has been evaluated in populations of patients with well-defined resistance using relatively consistent criteria between studies, making the data comparable. Each of the studies is consistent in finding a rapid onset of antidepressant effect after a single dose of psilocybin. While the minimum effective dose is yet to be precisely defined, the majority of studies have utilised 25 mg as an effective dose, while the lower doses investigated to date (1 mg, 10 mg) appear to be much less effective or not effective at all. The upper threshold of doses is yet to be defined but is likely to be tempered by safety concerns, particularly the risk of drug induced psychotic symptoms. While the combination of psilocybin with psychotherapy is seen as crucial for maximizing the therapeutic effects of the drug, the specific effectiveness of the psychotherapy has been questioned. It is claimed that therapeutic settings that facilitate openness and emotional exploration during the psilocybin experience enhance benefits for patients with treatment resistance, yet specific manualised approaches to psychotherapy have not been evaluated. Indeed, psychotherapies accompanying the use of psychedelic agents in general have been the subject of limited research, if at all [48]. As more studies affirm the efficacy of psilocybin in treatment resistant depression and other mental health conditions, integrating these therapies into mainstream mental health care will pose both logistic and ethical challenges. Furthermore, little attention has been applied to the specific training of therapists. While most studies have utilised psychiatrists or psychologists to provide the therapy throughout a course of psychedelic therapy, there are no mandated requirements. An aspect of this training has been whether the therapist should have a personal psychedelic experience [80, 81]. An analysis of the issues involved suggested that a requirement for a personal experience with psychedelics is not justified. On the other hand, a personal experience with a low risk profile serotonergic psychedelic should be offered to therapists during training [81].
There is a growing shift towards the acceptance of psychedelic substances for therapeutic use. This inevitably will lead to the re-examination of the regulatory status of such compounds. In 2023, the Therapeutic Goods Administration (TGA) of Australia announced that MDMA and psilocybin would be reclassified from Schedule 9 prohibited substances to Schedule 8 controlled drugs, and they would be legally accessible in specific therapeutic contexts under the Authorised Prescriber Scheme [82]. The site noted that:
“The Therapeutic Goods Administration (TGA) will permit the prescribing of MDMA for the treatment of post-traumatic stress disorder and psilocybin for treatment-resistant depression. These are the only conditions where there is currently sufficient evidence for potential benefits in certain patients.”
Under the new regulations, the prescribing psychiatrist must be authorised by the TGA to prescribe the drugs, must supervise their patients when they take the medicine, must have experience relevant to the specific condition and the clinical use of psychedelic therapies, must use an evidence-based treatment protocol reflecting those used in contemporary clinical research settings, and must obtain approval of the treatment protocol from a registered human research ethics committee. The scheme is monitored by the TGA for efficacy and for adverse events.
Several questions remain about the use of psilocybin in treatment resistant depression and in psychiatric disorders in general. The conundrum of what constitutes a placebo control and how to ensure blinding of participants is difficult to overcome. Although the use of a conventional placebo can lead to unblinding of participants, effect sizes in such studies are large and generally exceed those of non-psychedelic treatments (with or without formal psychological support) [83]. To overcome this problem, an active placebo, designed to mimic some of the physical effects of the psychedelic, has been proposed [84]. The use of low, ineffective doses of the psychedelic agent may also suffice as a placebo, but further studies are necessary.
As noted earlier in this review, the majority of studies to date have included relatively small groups of patients, at least for treatment resistant depression. Clearly, studies in larger, well-defined populations of patients are required to better understand the efficacy of the treatment. Such studies should consider the issues raised here, namely the placebo problem, blinding to treatment, and the standardisation of the psychological support. Comparisons with conventional methodologies for resistant depression, such as antidepressant combinations, ECT, repetitive transcranial magnetic stimulation (rTMS), or augmentation with lithium or atypical antipsychotic medications, would aid in establishing the place of psychedelic therapy as a modality in these patients. Furthermore, such studies may also help to establish the nature of patients best suited to the treatment. The centrality of the hallucinogenic experience of psilocybin to the therapeutic effect has not been investigated. In this context, the proposal [85] to conduct a proof-of-concept trial combining psilocybin and risperidone in adults with treatment resistant depression could, when completed, shed light on the concept. It is known that the hallucinogenic effects of psilocybin can be blocked in humans by co-administration of ketanserin or risperidone. Further, a pre-clinical study in a mouse model of anhedonia showed that psilocybin reversed the anhedonic responses, an effect that was not prevented by pre-treatment with ketanserin [86]. The study would suggest that psilocybin is an antidepressant of itself and opens the possibility of producing rapid acting antidepressant agents acting through a mechanism similar to psilocybin but without the hallucinogenic effects.
The studies conducted thus far indicate that psilocybin can elicit antidepressant effects after just one dose for many patients. This is particularly notable considering the gradual onset associated with traditional medications. For patients who do not respond adequately, there is some evidence suggesting that additional doses could lead to the desired alleviation of symptoms. This raises important questions regarding both the short-term safety of this approach and the long-term implications, especially in light of documented tolerance to psilocybin’s effects.
The promise of rapid symptom relief has been, until now, an unattainable goal of pharmacotherapeutic approaches to the disorder of major depression. Psilocybin and perhaps other psychedelic agents may put that goal within reach.
BDI: Beck Depression Inventory
DMN: default mode network
DSM: Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association
ECT: electroconvulsive therapy
HPPD: hallucinogen persisting perception disorder
LSD: lysergic acid diethylamide
MADRS: Montgomery-Åsberg depression rating scale
MDD: major depressive disorder
MDMA: 3,4-methylenedioxymethamphetamine
MGH ATRQ: Massachusetts General Hospital Antidepressant Treatment Response Questionnaire
MINI: Mini International Neuropsychiatric Interview
MMRM: mixed models for repeated measures
PTSD: post-traumatic stress disorder
QIDS: Quick Inventory of Depressive Symptomatology
QTc: corrected QT interval
SSRIs: selective serotonin reuptake inhibitors
TGA: Therapeutic Goods Administration
TRN: Conceptualization, Investigation, Writing—original draft, Writing—review & editing.
The author declares that he has no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Hans-Klaus Goischke
Behrooz Afshari
Claudia Vollbracht, Marc Werner
Bobbie Posmontier ... Tony Ma
Eloisa Ruiz-Marquez
Eloisa Ruiz-Marquez
Ayan Mohamud Yusuf, Dirk M. Hermann
