Affiliation:
1School of Pharmacy, Desh Bhagat University, Mandigobindgarh 147301, Punjab, India
Email: dikshadalal18@gmail.com
ORCID: https://orcid.org/0000-0003-1707-6135
Affiliation:
2University Institute of Pharma Sciences, Chandigarh University, Mohali 140413, Punjab, India
Affiliation:
1School of Pharmacy, Desh Bhagat University, Mandigobindgarh 147301, Punjab, India
Email: anishsingh7421@gmail.com
ORCID: https://orcid.org/0000-0002-8547-2263
Explor Neurosci. 2025;4:100698 DOI: https://doi.org/10.37349/en.2025.100698
Received: April 18, 2025 Accepted: June 09, 2025 Published: July 04, 2025
Academic Editor: Marcello Iriti, Milan State University, Italy
Parkinson’s disease is typified by Lewy bodies and the selective death of dopaminergic neurons in the substantia nigra. α-Synuclein aggregation, neuroinflammation, mitochondrial dysfunction, and oxidative stress are key components of its pathophysiology. The neuroprotective potential of natural substances with anti-inflammatory and antioxidant qualities has drawn attention in recent years. A naturally occurring isoflavone that is mostly present in red clover and other legumes, biochanin A has shown promise as a treatment option for Parkinson’s disease. Preclinical research has shown that biochanin A uses a variety of methods to provide notable neuroprotective benefits. By activating the Nrf2/ARE pathway, it scavenges reactive oxygen species (ROS), upregulates antioxidant defense enzymes, and inhibits pro-inflammatory mediators by modifying the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling cascade. Additionally, it has been demonstrated that biochanin A preserves neuronal integrity in Parkinson’s disease models by reducing dopaminergic neuronal death, inhibiting microglial activation, and mitigating mitochondrial dysfunction. Its potential as a neurotherapeutic agent is increased by its capacity to pass the blood-brain barrier. To investigate its safety, bioavailability, and effectiveness in people, more translational and clinical research is necessary. Biochanin A’s incorporation with neuroprotective techniques may pave the way for novel supplementary treatments for Parkinson’s disease. Therefore, the current review aims to present a thorough investigation of the molecular basis of biochanin A’s anti-Parkinson properties in Parkinson’s disease, building on the body of existing research that explains these properties.
A progressive neurodegenerative disease, Parkinson’s disease (PD) is marked by the selective death of dopaminergic neurons in the substantia nigra pars compacta (SNpc). This condition causes non-motor symptoms like cognitive decline and autonomic dysfunction in addition to the classic motor symptoms of bradykinesia, rigidity, and tremor [1]. Its global frequency is believed to be around 1% in those over 60 and up to 4% in people over 80. Its prevalence rises sharply with age, especially after the age of 60 [2, 3]. Rising life expectancy, better diagnostic tools, and population aging are all contributing to the gradually growing burden of PD, with estimates suggesting that by 2040, the number of affected people may double [4]. Additionally, the illness exhibits a small male preponderance, and its complicated etiology and epidemiological patterns are influenced by both hereditary and environmental variables [5]. The main characteristic of this disease is the loss of dopamine in the striatum due to the degeneration of dopaminergic neurons in the SNpc, which disrupts the circuitry of the basal ganglia and causes the classic motor symptoms, such as bradykinesia, rigidity, resting tremor, and postural instability [6]. Lewy bodies, which are intracytoplasmic inclusions mostly made of misfolded and aggregated α-synuclein protein, are a major pathogenic feature of PD [7]. The core pathophysiology of PD includes oxidative stress, mitochondrial failure, neuroinflammation, and aberrant protein aggregation [8]. Excessive reactive oxygen species (ROS) and impaired adenosine triphosphate (ATP) generation are caused by mitochondrial malfunction, which also causes oxidative damage and apoptosis [9]. Dopamine metabolism itself, which generates hydrogen peroxide and other hazardous intermediates, aggravates oxidative stress even more [10]. Furthermore, it is becoming more widely acknowledged that neuroinflammatory processes are mostly driven by activated microglia and increased pro-inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), important causes of dopaminergic neuron degradation in PD [11]. Additionally, there is proof that α-synuclein aggregates propagate from cell to cell in a prion-like manner, which contributes to the disease’s progressive character and anatomical spread across the nervous system [12]. A naturally occurring isoflavone mostly present in red clover (Trifolium pratense), soy, and other legumes, biochanin A has garnered increasing interest because of its potential for neuroprotection [13]. A phytoestrogen with anti-inflammatory, anti-apoptotic, and antioxidant properties, biochanin A, may be able to lessen the intricate pathophysiology of neurodegenerative diseases like PD [14]. By scavenging ROS, decreasing lipid peroxidation, and upregulating endogenous antioxidant defenses such as glutathione (GSH), catalase (CAT), and superoxide dismutase (SOD), biochanin A has shown strong antioxidant activity [15]. Moreover, biochanin A inhibits nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling and microglial activation, which lowers the expression of inflammatory mediators [16]. Furthermore, it modulates mitogen-activated protein kinase (MAPK) signaling pathway to inhibit apoptosis and oxidative stress mediated inflammatory stress to confer anti-Parkinson’s activity. In light of these diverse impacts, biochanin A is an intriguing medicinal phytochemical that demands more research in the context of PD. The objective of this review is to provide an overview of the current understanding of biochanin A’s neuroprotective processes and possible applications in the treatment and prevention of PD. Trifolium repens, commonly known as white clover, is a herbaceous perennial plant belonging to the Fabaceae family. It is widely cultivated as a forage crop and is recognized for its trifoliolate leaves and small, white flowers. White clover contains various bioactive compounds, including flavonoids, isoflavones, phenolic acids, cyanogenic glucosides, saponins, and condensed tannins [17]. These molecules contribute to its medicinal properties, such as antioxidant, anti-inflammatory, and antimicrobial effects. Additionally, it plays a role in nitrogen fixation, improving soil fertility. Red clover (Trifolium pratense) contains a diverse range of bioactive molecules with medicinal, ecological, and agricultural significance [18, 19]. Here are some examples: formononetin, biochanin, quercetin, kaempferol, linamarin, lotaustralin, scopoletin, resveratrol, coumestrol, etc., as shown in Figure 1 [20]. According to several research conducted to date, Trifolium pratense has a variety of therapeutic properties, including neuroprotection. Several Chinese herbs, including the leaves and stems of Trifolium pratense, contain the isoflavonoid biochanin A. It has a number of therapeutic effects, including hepatoprotective, anti-inflammatory, antioxidant, and anticancer properties [20]. Research has shown that biochanin A may have neuroprotective properties, such as lowering inflammation and preventing the generation of ROS, which might aid in the treatment of AD and cerebral ischemic stroke. The isoflavone biochanin A, which is derived from Trifolium pratense, thus has neuroprotective properties [21].
Biochanin A is lipophilic and has good permeability; therefore, it is rapidly absorbed into the gut. In the liver, biochanin A is extensively metabolized. Research has shown that hydroxylation, glucuronide conjugation, and sulfate conjugation transform it into bioactive metabolites [22]. One of the active metabolites of biochanin A is genistein, which also goes through phase I and phase II interactions with hydroxy-biochanin A in the liver. Human oral absorption and water solubility have been reported to be inadequate [23]. The bioavailability of biochanin A in rats was noted by Moon et al. [24]. Rat bile and plasma were found to contain greater levels of conjugates of biochanin and its metabolite genistein. In plasma, biochanin was only 1.5% present as a free fraction. Furthermore, the apparent volume of distribution is greater, and the clearance of biochanin A and its metabolite genistein is higher [24].
Numerous studies show that oxidative stress is a key factor in the development of Parkinson’s [25–27]. Reduced levels of antioxidants like CAT, GSH, glutathione peroxidase (GPx), SOD have been shown to contribute to oxidative stress in renal tissues [28]. Numerous cell-line and in vivo studies have investigated the anti-Parkinson’s effects of biochanin A, highlighting its ability to inhibit the oxidative stress-mediated inflammatory stress as shown in Table 1 [27, 29, 30]. Using lipopolysaccharide (LPS)-induced oxidative stress and damage Sprague Dawley rat model, Wang et al. [29] demonstrated that biochanin A (25, 50 mg/kg; intraperitoneal) significantly increased the reduced SOD, GPx activities in the midbrain of rat. Moreover, it also reduced the increased MDA level. Using HE staining, it significantly alleviated the nuclear condensation and acidophilia degeneration in neurons of the SNpc. It considerably decreased the quantity of apoptotic cells in the SNpc by using Hoechst 33258. It considerably reduced the quantity of activated microglia with an amoeboid morphology, according to immunohistochemical labeling. Additionally, it decreased the elevated Iba-1 level in the SNpc. In addition, it boosted the SNpc’s decreased TH-positive neurons. Furthermore, it greatly reduced the expression of p47phox, p67phox, and gp91phox in the SNpc, according to immunohistochemistry. It also decreased the expression of gp91phox, p47phox, and p67phox in the plasma lemma portion of SNpc, according to Western blot research. It also demonstrated that it also improved motor dysfunction in rats. Moreover, immunofluorescence analysis revealed that it also reduced the increased Iba level in the LPS-induced microglial activation rat model [29].
Numerous in vivo and in vitro studies that delineate the anti-Partkinson’s activity of biochanin A
| Model | Dose/concentration | Observed molecular modulations | Reference |
|---|---|---|---|
| LPS-induced oxidative stress and damage rat model | (25, 50 mg/kg; i.p., n = 8) | Increased SOD, GPx activities in the midbrain.Reduced MDA level.Reduced number of apoptotic cells, Iba-1 level in the SNpc.Increased TH-positive neurons.Suppressed the expression of p47phox, p67phox, and gp91phox in the SNpc. | [29] |
| LPS-induced damage to dopaminergic neurons rat model | (25, 50 mg/kg; i.p., n = 16) | Decreased microglia activation.Reduced the Iba-1 expression level.Increased the TH-positive neurons in the SNpc.Reduced the IL-1β, IL-6, and TNF-α levels in the serum.Reduced the IL-1β and TNF-α in the brain.Inhibited the phosphorylation of ERK, JNK, and p38. | [27] |
| Iron and rotenone-induced Parkinson’s disease rat model | (3 and 30 mg/kg; i.p., n = 9) | Increased the dopamine level and substantia nigra TH expression in the brain.Reduced the MDA level in the SNpc.Increased the level of GSH in the SNpc. | [30] |
| LPS-induced inflammatory responses in the primary microglia cell model | (1.25, 2.5, 5 µM) | Reduced the ROS, NO, IL-1β, and TNF-α levels. | [27] |
| LPS-induced inflammatory responses in the primary microglia cell model | (15, 20, 25, 30 µM) | Reduced the activity of microglia cells. | [27] |
| LPS-induced pro-inflammatory response in the BV2 microglial cell model | (1.25–5 µM) | Decreased the cell viability and increased pro-inflammatory cytokines.Reduced the activation of microglia in BV2 cells.Reduced the increased TNF-α and IL-1β levels.Decreased the mRNA expression of NO and iNOS. Reduced the JNK, ERK, p38, ROS, and MAPK levels. | [34] |
| LPS-induced damage in the microglial cell model | (0.25, 1, 2.5 µM) | Inhibited the production of pro-inflammatory cytokines such as TNF-α, NO, and SOD.Increased dopaminergic neurons.Reduced the TH-IR neurons. | [14] |
LPS: lipopolysaccharide; SOD: superoxide dismutase; GPx: glutathione peroxidase; MDA: malondialdehyde; Iba-1: indole-3-butyric acid; SNpc: substantia nigra pars compacta; TH: tyrosine hydroxylase; IL-1β: interleukin-1 beta; IL-6: interleukin-6; TNF-α: tumor necrosis factor alpha; ERK: extracellular signal-regulated kinase; JNK: c-Jun N-terminal kinase; BV2: mouse microglial cell line; iNOS: inducible nitric oxide synthase; ROS: reactive oxygen species; MAPK: mitogen-activated protein kinase
Oxidative stress plays a crucial role in inflammatory processes by disrupting cellular homeostasis and triggering immune responses. When ROS accumulate beyond the body’s antioxidant defenses, they activate inflammatory pathways such as NF-κB, leading to the production of cytokines and chemokines such as IL-1β, IL-6, and TNF-α [31, 32]. This oxidative imbalance contributes to tissue damage, exacerbating conditions like Parkinson’s and Alzheimer’s. Additionally, oxidative stress influences inflammasome activation, further amplifying inflammation [33]. Using LPS-induced damage of dopaminergic neurons in Sprague Dawley rats, biochanin A (25, 50 mg/kg) significantly suppressed microglia activation. Moreover, it also reduced the Iba-1 expression level. In addition to this, it also increased the reduced TH-positive neurons in the SNpc part of rat’s brain. Furthermore, ELISA revealed that it also reduced the increased IL-1β, IL-6, and TNF-α levels in the serum. Western blot analysis revealed that it reduced the increased expression of IL-1β and TNF-α in the rat’s brain. Furthermore, biochanin A (1.25, 2.5, 5 µM) also reduced the increased ROS, NO, IL-1β, and TNF-α levels [27]. Wu et al. [34] demonstrated that biochanin A (1.25–5 µM) significantly decreased the increased cell viability and increased pro-inflammatory cytokines. It also significantly reduced the increased TNF-α and IL-1β levels in LPS-stimulated microglial cells. Moreover, western blot analysis and RT-PCR revealed that it also significantly decreased the increased mRNA expression of NO and iNOS in LPS-induced pro-inflammatory response in the BV2 microglial cell line model, as shown in Figure 2 [34]. Using LPS-induced damage in microglial cell, Chen et al. [14] documented that biochanin A (0.25, 1, 2.5 µM) significantly inhibited the production of pro-inflammatory cytokines such as TNF-α, NO, and SOD. It also increased the reduced dopaminergic neurons. Moreover, it significantly increased the reduced dopamine uptake. Additionally, it also reduced the increased TH-IR neurons [14]. Iron and rotenone-induced PD Sprague Dawley rat model, Yu et al. [30] nstrated that biochanin A (3 and 30 mg/kg; i.p.) significantly increased the reduced dopamine level and substantia nigra TH expression in the brain of rat. Moreover, it also significantly reduced the increased MDA level in the SNpc. It also increased the reduced level of GSH in the SNpc. It also significantly increased the reduced latency time of rat [30]. By inhibiting the oxidative stress-mediated inflammatory stress cascade, biochanin may potentiate the anti-Parkinson’s activity.
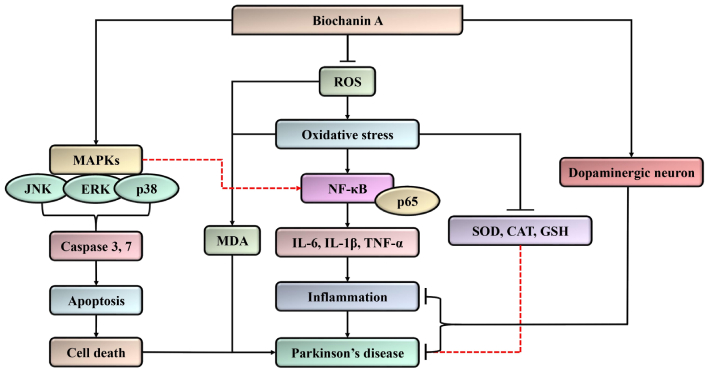
Mechanistic interplay of biochanin A in Parkinson’s disease by targeting the oxidative stress-mediated inflammatory stress and MAPK signaling pathway to inhibit apoptosis. It may protect the neuron by increasing the dopaminergic neurons to potentiate anti-Parkinson’s activity. MAPK: mitogen-activated protein kinase; ROS: reactive oxygen species; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; JNK: c-Jun N-terminal kinase; ERK: extracellular signal-regulated kinase; MDA: malondialdehyde; IL-6: interleukin-6; TNF-α: tumor necrosis factor alpha; IL-1β: interleukin-1 beta; SOD: superoxide dismutase; CAT: catalase; GSH: glutathione
The MAPK pathway plays a significant role in PD by influencing neurodegeneration, oxidative stress, and inflammation [35, 36]. Research suggests that improper protein phosphorylation due to dysfunction in kinases and phosphatases contributes to PD progression. Specific MAPKs such as JNKs, ERK1/2, and p38. MAPKs have been implicated in PD pathology [37]. Moreover, biochanin A (25, 50 mg/kg) inhibited the phosphorylation of ERK, JNK, and p38. MTT assay revealed that biochanin A (15, 20, 25, 30 µM) reduced the activity of microglial cells [27]. It also significantly reduced the increased JNK, ERK, p38, ROS, and MAPK levels in BV2 cells. Moreover, it also reduced the activation of microglial in BV2 cells [34]. The p38 MAPK and JNK pathways are often associated with stress-induced apoptosis, where they activate caspase cascades leading to cell death [38]. Biochanin A (10–60 mg/kg) showed significant improvement in motor functions, decreased T-turn and T-LA, and enhanced swim score. It increased striatal dopamine content in iron and MPTP-induced mice. Biochanin A (1–10 µM) significantly decreased superoxide anion generation in the cultures. Additionally, it significantly decreased the MDA level in the SNpc of mice. It also reduced p38 MAPK phosphorylation in the cultures. Furthermore, it also reduced the increased apoptosis in the substantia nigra parts of body [39]. Modulation of MAPK-mediated apoptosis potentiates the anti-Parkinson’s activity.
Mitochondrial dysfunction is a significant contributor to numerous degenerative diseases, and bochanin A has been found to support mitochondrial biogenesis by activating peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α). As a key regulator of energy metabolism, PGC-1α controls the expression of genes involved in oxidative phosphorylation. Through this activation, biochanin A enhances cellular energy production, which may strengthen neuronal and muscle function, offering potential therapeutic benefits for conditions such as Parkinson’s and Alzheimer’s disease [40–44].
Biochanin A plays a vital role in regulating apoptosis by enhancing the expression of Bcl-2, an anti-apoptotic protein, while simultaneously reducing levels of pro-apoptotic Bax and caspase-3. Apoptosis, or programmed cell death, is essential for cellular balance, but its excessive activation can lead to neurodegeneration and tissue damage, as seen in conditions like stroke and multiple sclerosis. By modulating apoptotic pathways, biochanin A may provide protective benefits against cellular stress and inflammatory damage [41].
Biochanin A demonstrates a strong affinity for Erβ receptors in the brain, effectively replicating the neuroprotective benefits of estrogen while avoiding activation of Erα, which is linked to adverse effects such as an increased risk of cancer. Estrogen signaling plays a vital role in cognitive function, synaptic plasticity, and neuronal health; however, conventional hormone therapies come with associated risks. By selectively targeting Erβ, biochanin A presents a promising therapeutic option for addressing cognitive decline, menopausal symptoms, and neurodegenerative disorders [42, 43].
Biochanin A has been associated with the upregulation of brain-derived neurotrophic factor (BDNF), a critical protein involved in synaptic plasticity, neuronal survival, and cognitive processes. Elevated BDNF levels may contribute to improvements in learning, memory retention, and neuroregeneration. Since decreased BDNF expression is commonly observed in neurodegenerative disorders and depression, biochanin A’s potential to boost its production highlights its promise as a therapeutic agent for cognitive enhancement [42].
Biochanin A functions as a regulator of histone deacetylases (HDACs), enzymes responsible for modifying chromatin structure to influence gene expression. Research has highlighted the potential of HDAC inhibition in neuroprotection, cancer treatment, and immune system regulation. Through its ability to adjust HDAC activity, bochanin A may facilitate favorable gene expression changes that help mitigate oxidative stress, reduce inflammation, and slow disease progression [42].
The accumulation of misfolded proteins is a defining characteristic of neurodegenerative conditions like Alzheimer’s and PD. Biochanin A has demonstrated potential in minimizing protein aggregation, which may help lessen the harmful effects associated with these dysfunctional proteins. By targeting this process, bochanin A could contribute to slowing disease progression or alleviating symptoms in individuals affected by neurodegenerative disorders [42].
The pharmaceutical potential of biochanin A, a naturally occurring isoflavone present in red clover and other legumes, is substantial because of its anti-inflammatory, anti-cancer, and antioxidant qualities. Nevertheless, a number of pharmacokinetic and metabolic issues restrict its practical use. Its low water solubility, which significantly impairs its absorption in the gastrointestinal system when taken orally, is one of its main disadvantages. It is difficult to attain therapeutic plasma concentrations because of its limited oral bioavailability, which is usually less than 10%. Biochanin A also undergoes a lot of fast metabolism, including phase I processes like demethylation to genistein and phase II reactions like glucuronidation and sulfation. These metabolic activities change it into more polar metabolites that are rapidly eliminated from the body and are frequently less active or inert. Its duration of effect is limited by the short plasma half-life caused by the fast clearance. Furthermore, biochanin A’s systemic distribution and effectiveness are further limited by its weak permeability across cellular membranes. These restrictions show that in order to improve its pharmacokinetic profile and therapeutic potential, sophisticated formulation techniques or structural alterations are required.
Future research should concentrate on methods to get over biochanin A’s pharmacokinetic constraints, which include poor solubility, limited oral bioavailability, quick metabolism, and short half-life, in order to maximize its therapeutic potential. The creation of innovative drug delivery vehicles, such as solid lipid nanoparticles, liposomes, micelles, and nanoparticles, is one encouraging avenue. These systems can increase absorption, defend against premature metabolism, and improve solubility. The production of prodrugs or analogs with better pharmacokinetic characteristics by structural alteration of biochanin A is another crucial strategy. Co-administration with enzyme inhibitors (such as cytochrome P450 or glucuronidation inhibitors) may also prevent metabolic breakdown and extend its systemic availability. Bypassing first-pass metabolism and boosting bioavailability, alternate administration methods, including transdermal, intranasal, or sublingual delivery, might be investigated. Lastly, in order to optimize dosage schedules and assess safety and effectiveness in people, pharmacokinetic-pharmacodynamic modeling and clinical translation studies are crucial. The goal of these future directions is to fully realize the therapeutic potential of biochanin A and optimize its clinical value.
Biochanin A is a phytoestrogen and naturally occurring isoflavone that has demonstrated encouraging neuroprotective potential in PD. In neurodegenerative diseases, it reduces oxidative stress and neuroinflammation, two major factors that cause neuronal damage. By triggering the PI3K/Akt signaling pathway and blocking the MAPK cascade, biochanin A reduces pro-apoptotic factors and increases anti-apoptotic proteins. Furthermore, it increases antioxidant defenses while suppressing inflammatory mediators such as TNF-α, IL-1β, NF-κB, and iNOS. Since these processes work together to prevent neuronal death and support cellular integrity, biochanin A is a promising therapeutic target for PD. Poor oral bioavailability is the main limitation of biochanin A, which is mostly caused by its high first-pass metabolism and poor water solubility. Developing biochanin A as a medicinal drug is hampered by the absence of clinical research and pharmacokinetic characterization of its metabolites. Future research might expand their potential as therapeutic agents for neurodegenerative diseases by validating their efficacy via preclinical and clinical investigations.
BDNF: brain-derived neurotrophic factor
GPx: glutathione peroxidase
GSH: glutathione
HDACs: histone deacetylases
IL-1β: interleukin-1 beta
IL-6: interleukin-6
LPS: lipopolysaccharide
MAPK: mitogen-activated protein kinase
NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells
PD: Parkinson’s disease
PGC-1α: peroxisome proliferator-activated receptor gamma coactivator 1-alpha
ROS: reactive oxygen species
SNpc: substantia nigra pars compacta
SOD: superoxide dismutase
TNF-α: tumor necrosis factor alpha
AK and S Kaur: Writing—original draft, Investigation. S Kumar and AM: Writing—review & editing, Investigation. AS and DD: Conceptualization, Writing—original draft, Writing—review & editing, Investigation.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1795
Download: 31
Times Cited: 0
