Affiliation:
1Department of Biochemistry, School of Life Sciences, Federal University of Technology, Akure 340001, Nigeria
Email: agunloye.odunayo9@gmail.com
ORCID: http://orcid.org/0000-0002-8835-0472
Affiliation:
1Department of Biochemistry, School of Life Sciences, Federal University of Technology, Akure 340001, Nigeria
Affiliation:
1Department of Biochemistry, School of Life Sciences, Federal University of Technology, Akure 340001, Nigeria
2Department of Biomedical Technology, School of Basic Medical Sciences, Federal University of Technology, Akure 340001, Nigeria
ORCID: http://orcid.org/0000-0002-1352-3252
Affiliation:
1Department of Biochemistry, School of Life Sciences, Federal University of Technology, Akure 340001, Nigeria
ORCID: http://orcid.org/0000-0001-5167-9779
Explor Neurosci. 2025;4:100690 DOI: https://doi.org/10.37349/en.2025.100690
Received: December 05, 2024 Accepted: April 07, 2025 Published: May 22, 2025
Academic Editor: Marcello Iriti, Milan State University, Italy
The article belongs to the special issue Medicinal Plants and Bioactive Phytochemicals in Neuroprotection
Aim: Natural products possess diverse pharmacological properties that are effective and safe for treating and managing amnesia; however, there is little or no scientific proof for most of their claims. This study evaluates the efficacy of Lecaniodiscus cupanioide-supplemented diets (LCSD) and Alchornea cordifolia-supplemented diets (ACSD) on scopolamine-induced amnesia in male rats. Roots of L. cupanioide and A. cordifolia were obtained and used to formulate 10% and 20% supplemented diets.
Methods: Experimental animals were orally pre-fed LCSD and ACSD for 14 days before the induction of amnesia via single i.p. (intraperitoneal) administration of scopolamine (2 mg/kg body weight). Experimental animals were subjected to a Y-maze test to evaluate cognitive performance before experiment termination. The activities of hippocampal key enzymes linked to cognitive function were determined.
Results: The result of the Y-maze showed that the induction of amnesia significantly (p < 0.001) reduced spatial memory function, which was protected against LCSD and ACSD pre-treated rats. Also, pre-treatment with supplemented diets inhibited the significant (p < 0.01) aggravation of monoamine oxidase, arginase, tumor necrosis factor-α, malonaldehyde, myeloperoxidase, acetylcholinesterase, and butyrylcholinesterase concentrations, and the significant (p < 0.05) depletion of dopamine, nitric oxide, interleukin-6, total thiol, and non-protein thiol concentrations, in comparison with that observed with amnesic-induced untreated rats. Comparatively, LCSD was more effective in preventing neuronal enzymatic imbalances, while ACSD was more effective in avoiding antioxidant status depletion.
Conclusions: Conclusively, this study established that the supplemented diets possess potent anti-amnesic and neuroprotective abilities. Furthermore, this study recommends supplemented diets as a dietary intervention for preventing and managing amnesic conditions.
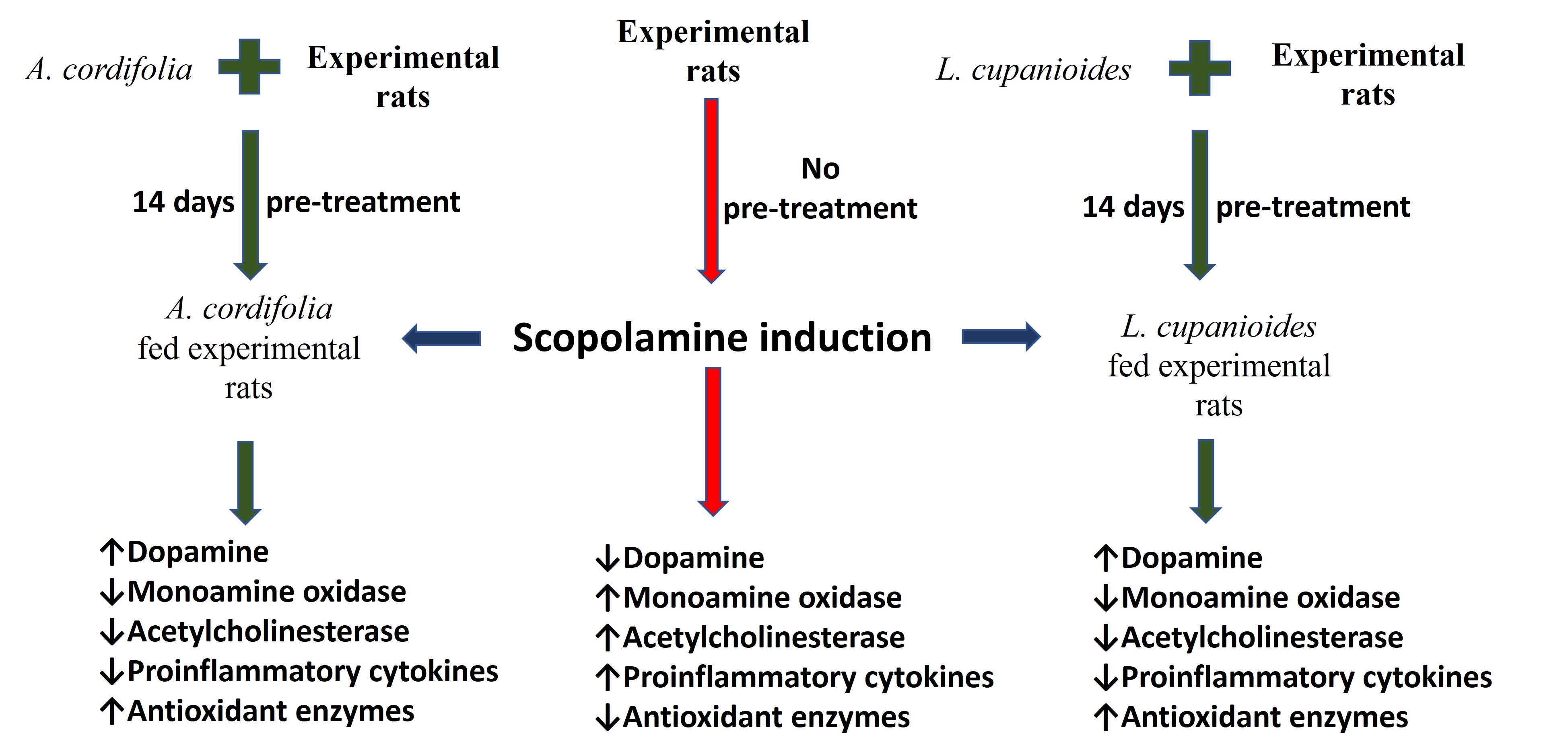
Learning refers to the acquisition of new knowledge, while the retention and retrieval of this knowledge at a later time is referred to as memory [1]. Memory is a vital cognitive process that consists of three fundamental stages: acquisition, consolidation, and retrieval. Research has demonstrated that reduced cholinergic activity, increased oxidative stress, and neuroinflammation in the brain are causally linked to amnesia-related memory impairments [2]. Amnesia is defined as the inability to create memories, or the partial/complete loss of memory caused by cerebral dysfunction resulting from degenerative diseases such as Alzheimer’s disease (AD), brain infections like herpes or encephalitis, traumatic injuries like strokes, alcohol or drug abuse, and emotional events like psychological trauma [3]. There are currently 55.2 million individuals worldwide who have this illness, and it is projected that this number will rise to 78 million by 2030 and 152.8 million by 2050. Unfortunately, this worldwide threat had an economic impact of US$604 billion in 2010, US$818 billion in 2015, and US$1.313 trillion in 2020 [4, 5]. This worldwide threat was distributed as follows: 49% in Asia, 25% in Europe, 18% in America, and 8% in Africa [6]. Nigeria is also affected by this condition, with a nationwide prevalence estimated at 4.9%, which represents a more than fourfold increase for 20 years [7].
Scopolamine, a substance that blocks muscarinic receptors in the brain, causes cognitive impairments by interfering with the acetylcholine neurotransmission systems in the central nervous system of both humans and rodents [8]. The use of a rat model with amnesia induced by scopolamine is commonly used to study and understand the treatment and underlying mechanisms of cognitive impairments [9, 10]. The cognitive losses caused by scopolamine are observable during the evaluation of behavioral paradigms, which validates the use of this model as a pharmacological test for assessing cognitive impairments [11]. Furthermore, research has shown that the administration of scopolamine leads to hippocampal amnesia [12]. Although there are already several advanced medications available for treating amnesia, the adverse effects they cause have made it necessary to create new therapeutic approaches that are both effective and safer [13].
Natural products and their bioactive components contain pharmacological qualities that are effective and safe in treating and managing various neurodegenerative illnesses [14–16]. Recently, certain plants grown in Nigeria for food and nutrition are becoming recognized for their potential to prevent and manage degenerative diseases [17, 18]. In Nigeria, it is common for over 80% of the population to depend on traditional and herbal medications for their healthcare needs [13]. Two plants that are now being studied for their potential to prevent and manage neurodegenerative illnesses are Lecaniodiscus cupanioide and Alchornea cordifolia [19, 20]. Although these plants are commonly used in folklore, their impact on neurotransmitter, neuroinflammatory, and antioxidant systems in animals with amnesia has not been investigated.
This study aimed to compare the effectiveness of diets supplemented with 10% and 20% of L. cupanioide and A. cordifolia in protecting memory in rats with scopolamine-induced amnesia. The Y-maze test was employed to assess spatial working memory in different treatment groups, followed by an evaluation of key enzyme activities associated with cognitive function in the hippocampus tissue homogenates. We assessed the levels of cytokines [tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6)] in the hippocampus to characterize neuroinflammation. Additionally, we examined the antioxidant status of neurons in different treatment groups. The results of this study are expected to be valuable in the advancement of nutraceuticals and dietary therapies for the management of cognitive dysfunction-related disorders, particularly amnesia.
Fresh L. cupanioide and A. cordifolia roots were sourced from local farm settlements in Ijare, Ifedore Local Government, Ondo State, Nigeria. The plants’ roots were authenticated and deposited at the University Herbarium of the Federal University of Technology, Akure, Nigeria. The roots were handpicked out of debris, rinsed with water, cut into sizes, and sun-dried. Afterward, the dried roots of the L. cupanioide and A. cordifolia were pulverized into a powdery form using an industrial blender and then sieved to obtain uniformity.
Reagents and chemicals used in this study were of analytical grades and obtained from Reagen (Colombo, Paraná, Brazil) [vanadium III chlorides (VCl3) (CAT No: 7718-98-10), bovine serum albumin (CAT No: 126605)]; and from BDH Chemicals Ltd., (Poole, England) [aluminum and ferric chloride, sodium dodecyl sulfate, potassium ferricyanide and acetate, ferric chloride, potassium ferricyanide, iron (II) sulfate, sodium carbonate (CAS No: 497-19-8), and dinitro salicylic acid (CAT No: 609-99-4)]; and Sigma-Aldrich, St. Louis, MO, USA (scopolamine CAS No: 6533-68-6).
Scopolamine hyoscine was dissolved in sterile saline (NaCl 0.9%) and administered at a concentration of 2 mg/kg; intraperitoneal (i.p.) in induced groups. This dose has been established to induce amnesia and produce cognitive decline [13, 17].
Donepezil powder was dissolved in 0.9% saline and administered at a concentration of 5 mg/kg orally for 14 days before scopolamine injection [17, 21].
The diets fed to experimental rats were freshly prepared (Table 1) according to the modified method of Oyeleye et al. [22]. The percentage inclusion (10 and 20%) used for this study was selected based on previous reports on the safety and efficacy of L. cupanioide and A. cordifolia [20, 23]. The diet formulations were named LCSD and ACSD to indicate L. cupanioide and A. cordifolia-supplemented diets, respectively.
Diet formulation for the control and treated groups
| Treatment/Group | Skimmed milk | Oil | Vitamin premix | Corn starch | L. cupanioide | A. cordifolia | Total |
|---|---|---|---|---|---|---|---|
| Normal control | 39.4 | 10.0 | 4.0 | 46.6 | - | - | 100 |
| Scopolamine | 39.4 | 10.0 | 4.0 | 46.6 | - | - | 100 |
| Scopolamine + donepezil | 39.4 | 10.0 | 4.0 | 46.6 | - | - | 100 |
| Scopolamine + L. cupanioide (10%) | 39.4 | 10.0 | 4.0 | 36.6 | 10 | - | 100 |
| Scopolamine + L. cupanioide (20%) | 39.4 | 10.0 | 4.0 | 26.6 | 20 | - | 100 |
| Scopolamine + A. cordifolia (10%) | 39.4 | 10.0 | 4.0 | 36.6 | - | 10 | 100 |
| Scopolamine + A. cordifolia (20%) | 39.4 | 10.0 | 4.0 | 26.6 | - | 20 | 100 |
Skimmed milk = 32% protein; vitamin premix composed of the following: 3,200 IU vitamin A, 600 IU vitamin D3, 2.8 mg vitamin E, 0.6 mg vitamin K3, 0.8 mg vitamin B1, 1 mg vitamin B2, 6 mg niacin, 2.2 mg pantothenic acid, 0.8 mg vitamin B6, 0.004 mg vitamin B12, 0.2 mg folic acid, 0.1 mg biotin H2, 70 mg choline chloride, 0.08 mg cobalt, 1.2 mg copper, 0.4 mg iodine, 8.4 mg iron, 16 mg manganese, 0.08 mg selenium, 12.4 mg zinc, 0.5 mg antioxidant and L. cupanioide and A. cordifolia were in equi-weight basis (Oyeleye et al. [22])
Forty-two (42) male albino Wistar rats, aged 8 weeks, were obtained from the Department of Veterinary Medicine at the University of Ibadan, Nigeria. The rats’ weights were assessed (ranging from 180 g to 200 g), and they were provided with a two-week timeframe to acclimate to their environment. Throughout this period, the rats were confined within enclosures maintained at a consistent ambient temperature of 25°–28°C and a relative humidity level of 60–70%. The rats were fed with pellet rat chow and provided with water. The exploitation of animals conforms to the ethical guidelines established by the US National Institute of Health (NIH). Permission for animal use was obtained from the Animal Ethical Committee, Centre for Research and Development (CERAD), Federal University of Technology, Akure, with the ethical number FUTA/ETH/2020/016. After two weeks of acclimatization, the experimental animals were randomly divided into several groups. A total of forty-two male rats were assigned at random to seven groups, with each group comprising six animals.
Group I: Rats were fed basal diets (normal control).
Group II: Induced rats were administered 2 mg/kg body weight (bwt) of scopolamine intraperitoneally on day 14 of the experiment.
Group III: Experimental rats were treated with donepezil 5 mg/kg bwt and administered with 2 mg/kg bwt of scopolamine intraperitoneally on day 14 of the experiment.
Group IV: Rats were fed with 10% LCSD and induced with 2 mg/kg bwt of scopolamine intraperitoneally on day 14 of the experiment.
Group V: Rats were fed with 20% LCSD and induced with 2 mg/kg bwt of scopolamine intraperitoneally on day 14 of the experiment.
Group VI: Rats were fed with 10% ACSD and induced with 2 mg/kg bwt of scopolamine intraperitoneally on day 14 of the experiment.
Group VII: Rats were fed with 20% ACSD and induced with 2 mg/kg bwt of scopolamine intraperitoneally on day 14 of the experiment.
The grouping consisted of seven groups with n = 6, and the dosage of donepezil was 5 mg/kg bwt, based on a previous study by Ademosun et al. [17]. The experimental animals were fed with LCSD and ACSD from day 1 of the experiment, and the administration lasted for 14 days. On day 14, scopolamine was induced intraperitoneally [13] (Figure 1).
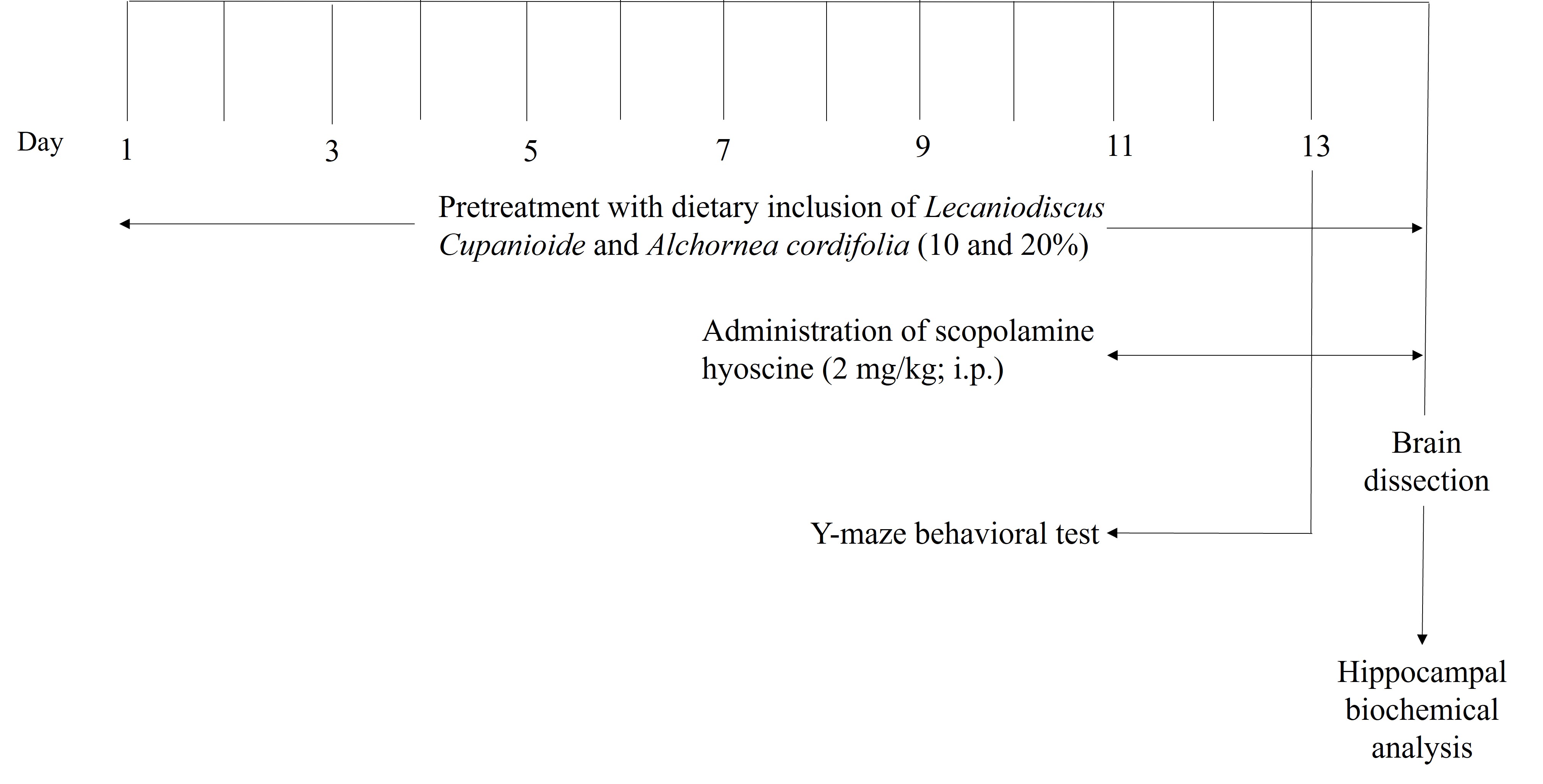
Experimental scheme for the effect of dietary inclusion of L. cupanioide and A. cordifolia (10 and 20%) on scopolamine-induced amnesic rats. i.p.: intraperitoneal. Adapted with permission from [36], © 2018 the authors (CC BY)
The behavioral study included a Y-maze test, which was conducted a week before drug administration to familiarize the experimental animals with the behavioral paradigm. Scopolamine (2 mg/kg bwt) was then administered on day 14 after drug administration to the experimental animals.
The Y-maze test was evaluated to assess the short-term memory of the experimental animals as described by Oyeleye et al. [24]. The percentage of correct alterations between Y-maze arms was recorded and analyzed to indicate the memory index of the experimental animals and to evaluate cognitive disorders.
After the behavioral study, the experimental animals were euthanasia using cervical dislocation, performed by a skilled person. The head was decapitated, the skull and brain were opened and excised respectively, while the hippocampus was carefully dissected [17]. The hippocampus was homogenized in ice-cold 0.1 M phosphate buffer (pH 7.4) and centrifuged using a high-speed cold centrifuge at 12,000 rpm for 10 minutes at 4°C. The clear supernatants obtained were used for biochemical analyses [25].
The approach provided by Fasakin et al. [26] was used to test the activity of monoamine oxidase (MAO), with some modifications. The reactant combination included a 0.025 mol/L phosphate buffer solution with a pH of 7.0, 0.0125 mol/L semicarbazides, 10 mmol/L benzylamine, and 100 µL of hippocampal homogenate. The mixture underwent a 30-minute reaction time. Subsequently, acetic acid was introduced into the mixture, which was then incubated for an additional 3 minutes utilizing a hot water bath. The resulting liquid (1 mL) was mixed with an equal volume of 2,4-dinitrophenylhydrazine. Subsequently, a volume of 1.25 mL of benzene was introduced, and the resulting combination was left to react at ambient temperature for 10 minutes. The benzene layer was isolated and mixed with an equal volume of 0.1 N NaOH. The alkaline layer was decanted and then incubated in an incubator for 10 minutes at a temperature of 80°C. The resultant color was quantified at a wavelength of 450 nm.
The dopamine concentration was assessed using the method reported by Guo et al. [27]. This phenomenon relies on the capacity of dopamine to catalyze the reduction of Fe3+ to Fe2+, followed by the reaction between Fe2+ and potassium ferricyanide to produce a soluble Prussian blue pigment. A brain sample of 1 mL was mixed with 1 mL of a solution containing 1.5 × 10–2 M ferric chloride. Another solution containing 1.5 × 10–2 M potassium ferricyanide was transferred to a tube and diluted with distilled water to a final volume of 25 mL. Next, well blended and left to rest for 35 minutes at ambient temperature. Ultimately, the solution’s absorbance was quantified at a wavelength of 735 nm relative to a blank sample.
Cholinesterase [acetylcholinesterase (AChE) and butyrylcholinesterase (BChE)] activities were determined using the spectrophotometric method developed by Ellman et al. [28], as reported by Fasakin et al. [29]. Acetylthiocholine and butyrylthiocholine iodide served as substrates for AChE and BChE assays, respectively. The enzyme activities were calculated in micromoles of substrate per hour per milligram of protein.
Arginase activity was determined using the spectrophotometric method of Bryan and Grisham [30]. L-arginine (50 mM/L) served as a substrate. The amount of urea produced using spectrophotometry at a wavelength of 450 nm was used to deduce arginase activity.
Nitric oxide (NO) concentration was determined using the spectrophotometric method of Green et al. [31].
The concentrations of hippocampal cytokines (TNF-α and IL-6) were determined using commercially available Enzyme-Linked Immunosorbent Assay (ELISA) kits (E-EL-R0064, E-EL R0016) as described by the manufacturer [25].
The evaluation of myeloperoxidase activity was conducted using the procedure established by Eiserich et al. [32]. For 10 minutes, 100 μL of the hippocampus homogenate was incubated with 100 μL of KCl and 25 μL of H2O2. Subsequently, 50 μL of ammonium molybdate was introduced into the solution and allowed to incubate for 5 minutes at a temperature of 25°C. The absorbance was measured at a wavelength of 405 nm.
The method of Ohkawa et al. [33] was used to determine lipid peroxidation as the formation of thiobarbituric acid-reactive substances.
The spectrophotometry approach, previously described by Ellman [34], was used to measure the non-protein thiol content. The brain homogenate was mixed with trichloroacetic acid (10%) in equal amounts. The solution was centrifuged at 10,000 rpm for 5 minutes at 4°C. Afterward, 50 μL of the supernatant was mixed with 450 μL of phosphate buffer and 1.5 mL of a solution containing 0.1 mM of 5,5'-dithiobis (2-nitrobenzoic acid). The solution obtained was incubated at 37°C for 10 minutes. The absorbance was measured at a wavelength of 412 nm. The non-protein thiol levels were measured in units of mol per milligram of protein.
The spectrophotometry method provided by Ellman [34] was used to quantify the total thiol content. The reaction mixture comprised 150 μL of potassium phosphate buffer (pH 7.4, 0.1 M), 40 μL of epididymal or testicular tissue homogenate, and 10 μL of 5,5'-dithiobis(2-nitrobenzoic acid) at a concentration of 10 mM. The solution was maintained at ambient temperature for 30 minutes, and the absorbance was quantified at a wavelength of 412 nm. A standard curve was generated using cysteine as a reference for each measurement, and the results were given in units of mol per milligram of protein.
Reverse-phase chromatographic analyses under gradient conditions [C18 column (4.6 × 150 mm) packed with 5 µm diameter particles] were employed for the characterization of the phenolic compounds in the A. cordifolia and L. cupanioide samples [13].
Power analysis and the Shapiro-Wilk test were employed to determine sample size and check data for normality patterns, respectively [35]. The results were pooled and expressed as the mean ± standard error of the mean (SEM) (n = 6). Results were analyzed using one-way analysis of variance (ANOVA), followed by the Tukey post hoc test. The level of significance was accepted at p < 0.05 after evaluation using GraphPad Prism (version 8.0.2) software. *: Mean values are significantly (p < 0.05) different compared with the scopolamine-induced amnesic group.
The current investigation utilized the Y-maze to assess the spatial working and short-term memory of experimental rats. The study found that the untreated amnesic rats had a significant (p < 0.001; F(16,85) = 508.1) decline in spatial memory function when compared to the rats in the control group (Figure 2). Nevertheless, experimental animals pre-treated with LCSD and ACSD had enhanced spatial memory performance compared with untreated amnesic rats. Memory impairment could not be fully restored in the pre-scopolamine treatment group in comparison to the normal control group levels. However, compared to the amnesia group, the post-treatment values in the extract treatment groups showed an improvement.
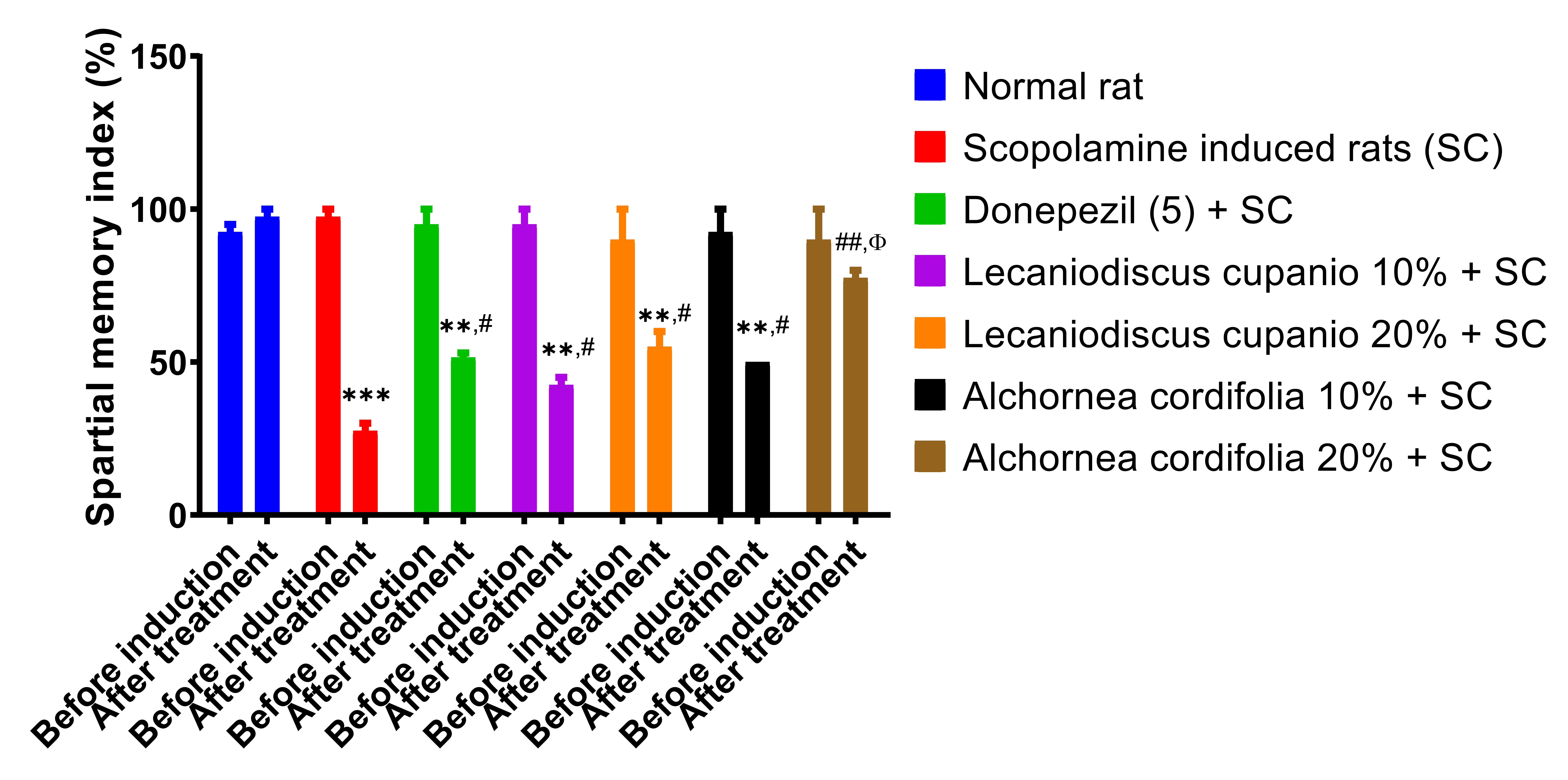
Effects of Lecaniodiscus cupanioide and Alchornea cordifolia supplemented diets on spatial memory index as expressed in a Y-maze test. Results are expressed as mean ± SEM (n = 6). *: p < 0.05, **: p < 0.01, ***: p < 0.001 compared with the normal control group. #: p < 0.05, ##: p < 0.01, ###: p < 0.001 compared with the scopolamine-induced amnesic experimental rats. Φ: p < 0.05, ΦΦ: p < 0.01, ΦΦΦ: p < 0.001 compared with the standard drug (donepezil) administered group. SC: scopolamine; SEM: standard error of the mean
Untreated amnesic rats showed a significant (p < 0.01; F(16,85) = 21.93) reduction in dopamine levels in their hippocampus compared to the dopamine levels of the rats in the control group (Figure 3). Rats that pre-feed with LCSD and ACSD before scopolamine injection demonstrate elevated dopamine levels in comparison to untreated rats with amnesia. Meanwhile, the activity of MAO was significantly (p < 0.001; F(16,85) = 49.2) higher compared to the normal group of rats. Animals who received the LCSD and ACSD enriched meal before the induction of amnesia showed a significantly (p < 0.001; F(16,85) = 49.2) reduced MAO activity in the hippocampus compared to the MAO activity of rats in the untreated group.
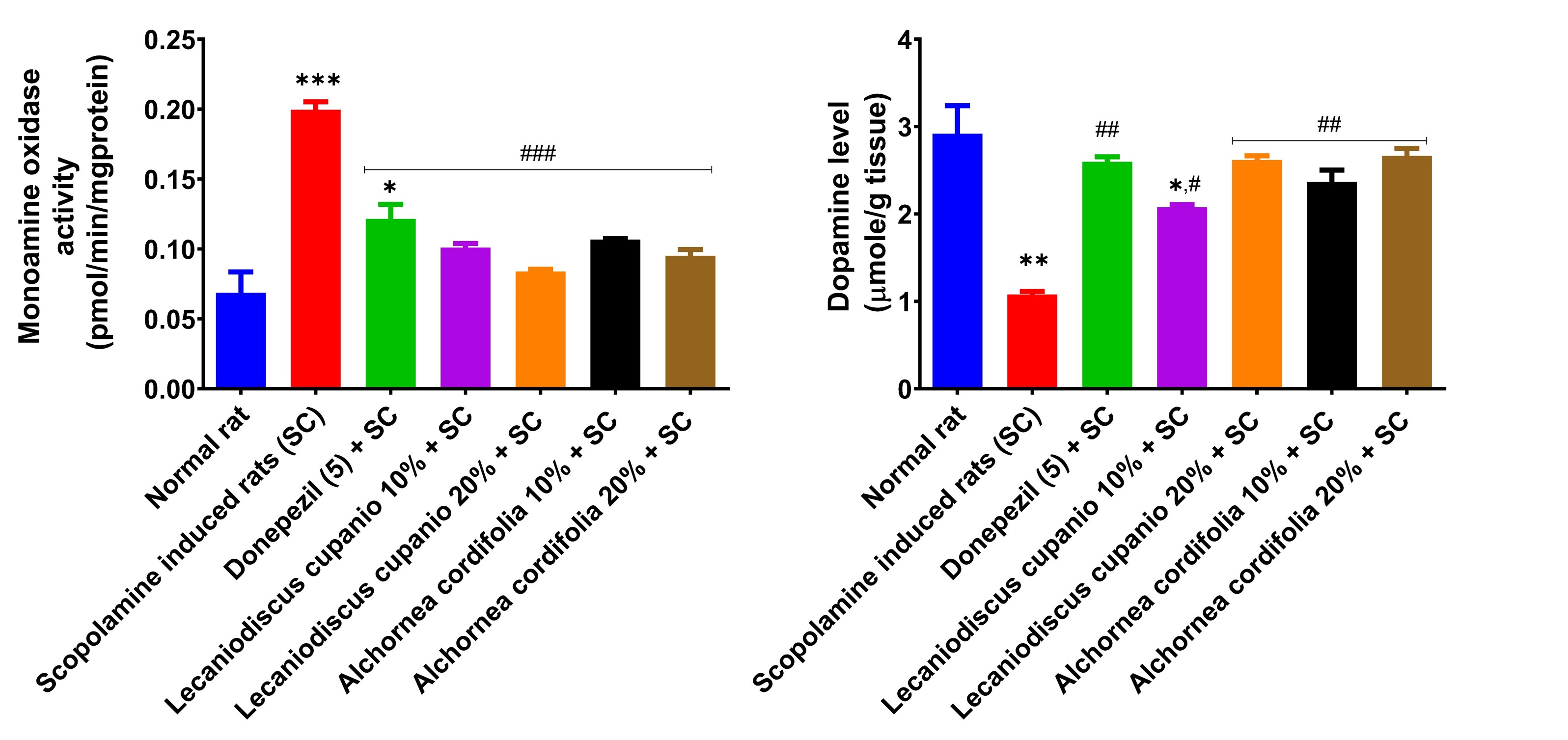
Effects of Lecaniodiscus cupanioide and Alchornea cordifolia supplemented diets on hippocampal monoaminergic neurotransmission system (monoamine oxidase and dopamine) of scopolamine-induced amnesic experimental rats. Results are expressed as mean ± SEM (n = 6). *: p < 0.05, **: p < 0.01, ***: p < 0.001 compared with the normal control group. #: p < 0.05, ##: p < 0.01, ###: p < 0.001 compared with the scopolamine-induced amnesic experimental rats. Φ: p < 0.05, ΦΦ: p < 0.01, ΦΦΦ: p < 0.001 compared with the standard drug (donepezil) administered group. SC: scopolamine; SEM: standard error of the mean
Figure 4 showed that the levels of AChE and BChE activities respectively in the hippocampus of the untreated amnesic rats were significantly (p < 0.001; F(16,85) = 64.91 and p < 0.001; F(16,85) = 41.99) increased respectively compared to the normal control group rats. Notably, administering meals supplemented with L. cupanioide and A. cordifolia to rats before inducing amnesia resulted in reduced activity of AChE and BChE in the hippocampus of the pre-treated rats, compared to untreated animals.
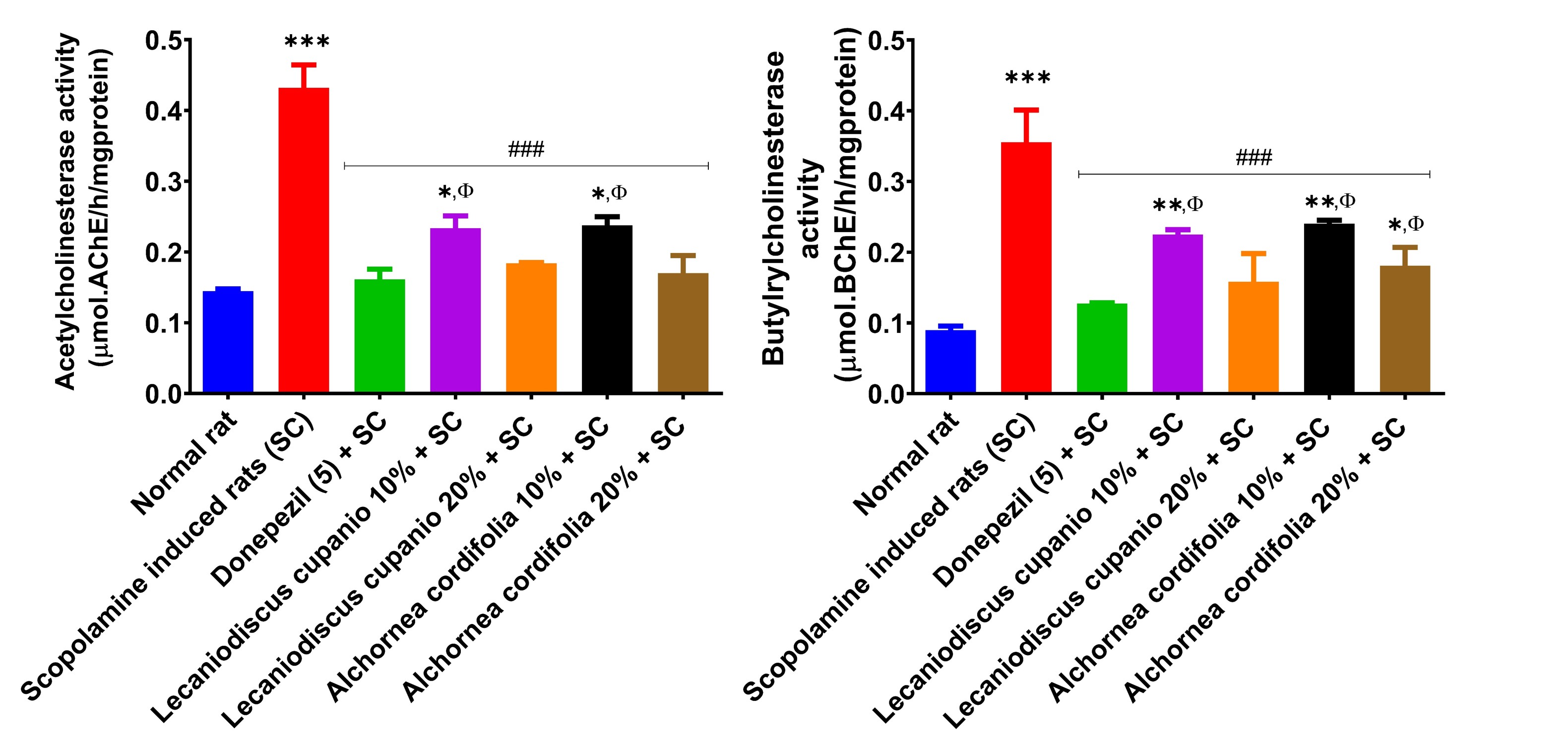
Effects of Lecaniodiscus cupanioide and Alchornea cordifolia supplemented diets on hippocampal cholinesterase activities of scopolamine-induced amnesic experimental rats. Results are expressed as mean ± SEM (n = 6). *: p < 0.05, **: p < 0.01, ***: p < 0.001 compared with the normal control group. #: p < 0.05, ##: p < 0.01, ###: p < 0.001 compared with the scopolamine-induced amnesic experimental rats. Φ: p < 0.05, ΦΦ: p < 0.01, ΦΦΦ: p < 0.001 compared with the standard drug (donepezil) administered group. SC: scopolamine; SEM: standard error of the mean
Figures 5 and 6 display the levels of arginase activity and NO concentration in the hippocampus of several groups of rats: normal rats, untreated amnesic rats, and rats fed with LCSD and ACSD before amnesia induction. As observed, the untreated amnesic rats exhibited a statistically significant (p < 0.001; F(16,85) = 99.51) increase in arginase activity and a significant (p < 0.01; F(16,85) = 125.89) decrease in NO levels compared to the normal rats. Nevertheless, administering LCSD and ACSD to the amnesic rats before treatment significantly mitigated the heightened activity of arginase and reduced levels of NO observed in the amnesic rats who did not receive any treatment.
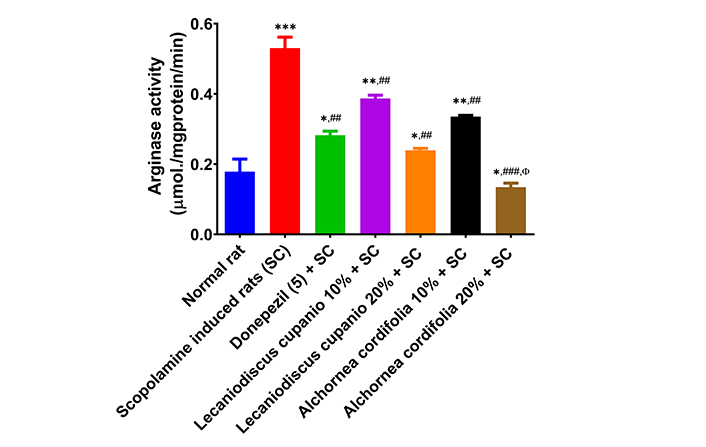
Effects of Lecaniodiscus cupanioide and Alchornea cordifolia supplemented diets on hippocampal arginase activity of scopolamine-induced amnesic experimental rats. Results are expressed as mean ± SEM (n = 6). *: p < 0.05, **: p < 0.01, ***: p < 0.001 compared with the normal control group. #: p < 0.05, ##: p < 0.01, ###: p < 0.001 compared with the scopolamine-induced amnesic experimental rats. Φ: p < 0.05, ΦΦ: p < 0.01, ΦΦΦ: p < 0.001 compared with the standard drug (donepezil) administered group. SC: scopolamine; SEM: standard error of the mean
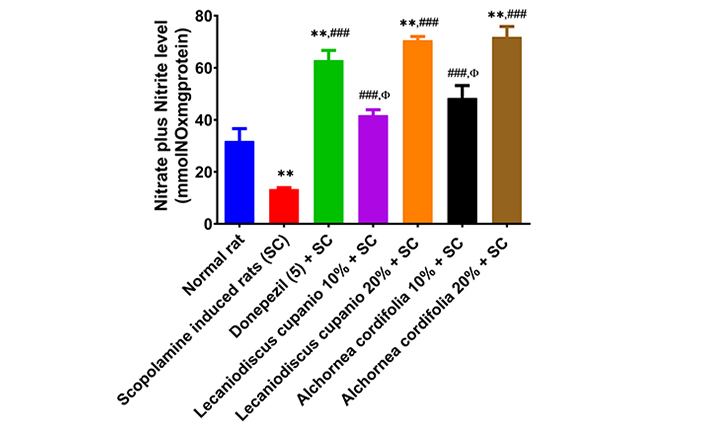
Effects of Lecaniodiscus cupanioide and Alchornea cordifolia supplemented diets on hippocampal nitric oxide concentration of scopolamine-induced amnesic experimental rats. Results are expressed as mean ± SEM (n = 6). *: p < 0.05, **: p < 0.01, ***: p < 0.001 compared with the normal control group. #: p < 0.05, ##: p < 0.01, ###: p < 0.001 compared with the scopolamine-induced amnesic experimental rats. Φ: p < 0.05, ΦΦ: p < 0.01, ΦΦΦ: p < 0.001 compared with the standard drug (donepezil) administered group. SC: scopolamine; SEM: standard error of the mean
Figure 7 depicts the concentration of inflammatory markers in the hippocampus of normal rats, untreated amnesic rats, and rats fed with LCSD and ACSD before amnesia induction. As presented, the untreated amnesic rats had significantly (p < 0.01; F(16,85) = 188.07) higher levels of TNF-α as well as significantly (p < 0.01; F(16,85) = 73.55) lower IL-6 concentrations when compared with normal rats. However, pre-treatment of amnesic rats with LCSD and ACSD significantly (p < 0.01; F(16,85) = 188.07) reduced the concentration of TNF-α while the concentration of IL-6 was significantly (p < 0.01; F(16,85) = 73.55) increased in comparison with untreated amnesic rats.
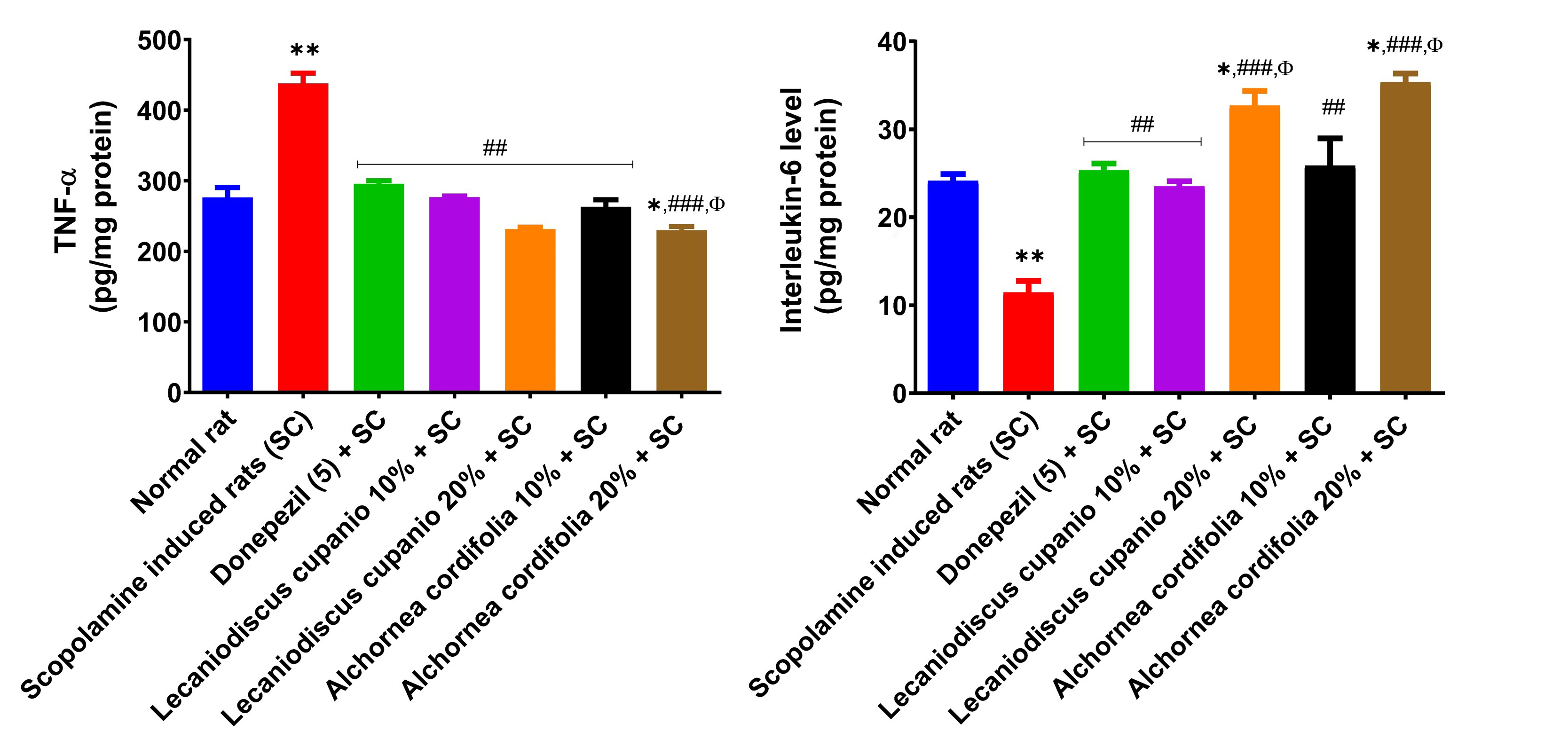
Effects of Lecaniodiscus cupanioide and Alchornea cordifolia supplemented diets on hippocampal inflammatory markers (TNF-α and interleukin-6) of scopolamine-induced amnesic experimental rats. Results are expressed as mean ± SEM (n = 6). *: p < 0.05, **: p < 0.01, ***: p < 0.001 compared with the normal control group. #: p < 0.05, ##: p < 0.01, ###: p < 0.001 compared with the scopolamine-induced amnesic experimental rats. Φ: p < 0.05, ΦΦ: p < 0.01, ΦΦΦ: p < 0.001 compared with the standard drug (donepezil) administered group. SC: scopolamine; SEM: standard error of the mean; TNF-α: tumour necrosis factor-α
Additionally, the untreated amnesic group’s hippocampal myeloperoxidase activity was significantly (p < 0.001; F(16,85) = 51.52) higher in comparison to the normal control group (Figure 8). Interestingly, treatment with LCSD and ACSD prior to the induction amnesia in rats caused a significant reduction (p < 0.01; F(16,85) = 51.52) in the myeloperoxidase activity when compared with the scopolamine induced rats (untreated). Meanwhile, we observed that pre-treated groups had higher significant higher myeloperoxidase activity when compared with normal rats but exhibited significantly (p < 0.01; F(16,85) = 51.52) lower myeloperoxidase activity when compared with scopolamine induced rats (untreated).
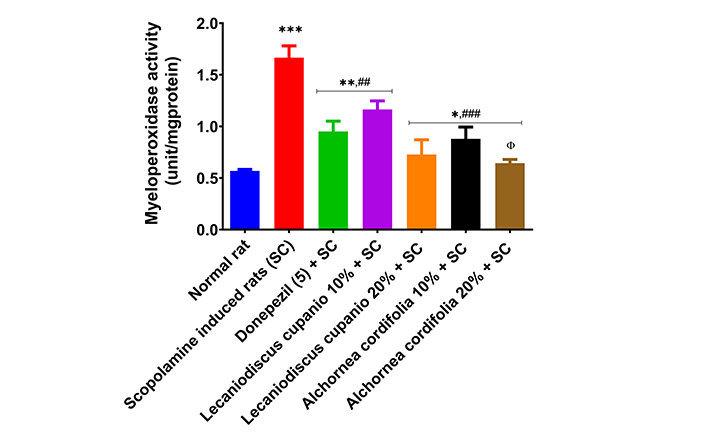
Effects of Lecaniodiscus cupanioide and Alchornea cordifolia supplemented diets on hippocampal myeloperoxidase activity of scopolamine-induced amnesic experimental rats. Results are expressed as mean ± SEM (n = 6). *: p < 0.05, **: p < 0.01, ***: p < 0.001 compared with the normal control group. #: p < 0.05, ##: p < 0.01, ###: p < 0.001 compared with the scopolamine-induced amnesic experimental rats. Φ: p < 0.05, ΦΦ: p < 0.01, ΦΦΦ: p < 0.001 compared with the standard drug (donepezil) administered group. SC: scopolamine; SEM: standard error of the mean
Furthermore, Figure 9 represents malonaldehyde (MDA) equivalent compounds in the hippocampus of normal rats, untreated amnesic rats, and rats fed with LCSD and ACSD before amnesia induction. The hippocampus of untreated amnesic rats had significantly (p < 0.001; F(16,85) = 239.34) higher MDA levels when compared to normal rats. Amnesic rats pre-treated with donepezil and those placed on LCSD and ACSD exhibited significantly (p < 0.01; F(16,85) = 239.34) lower MDA levels when compared to untreated amnesic rats. The hippocampus of the untreated amnesic rats had significantly (p < 0.01; F(16,85) = 108.59) lower total thiol and significantly (p < 0.01; F(16,85) = 86.57) lower non-protein thiol levels in comparison to the normal rats (Figure 10). Interestingly, amnesic rats pre-treated with LCSD and ACSD exhibited an elevated level of total thiol and non-protein thiol compared with the untreated amnesic rats. Furthermore, the total thiol levels of the amnesic rats pre-treated with LCSD and ACSD (except 10%) inclusion diets have an increasing trend when compared to the standard drug (donepezil) administered group, and non-protein thiol levels also have an increasing trend.
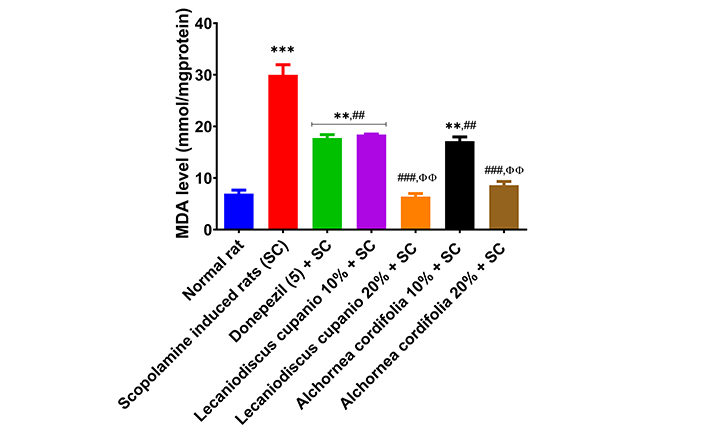
Effects of Lecaniodiscus cupanioide and Alchornea cordifolia supplemented diets on hippocampal lipid peroxidation of scopolamine-induced amnesic experimental rats. Results are expressed as mean ± SEM (n = 6). *: p < 0.05, **: p < 0.01, ***: p < 0.001 compared with the normal control group. #: p < 0.05, ##: p < 0.01, ###: p < 0.001 compared with the scopolamine-induced amnesic experimental rats. Φ: p < 0.05, ΦΦ: p < 0.01, ΦΦΦ: p < 0.001 compared with the standard drug (donepezil) administered group. MDA: malonaldehyde; SC: scopolamine; SEM: standard error of the mean
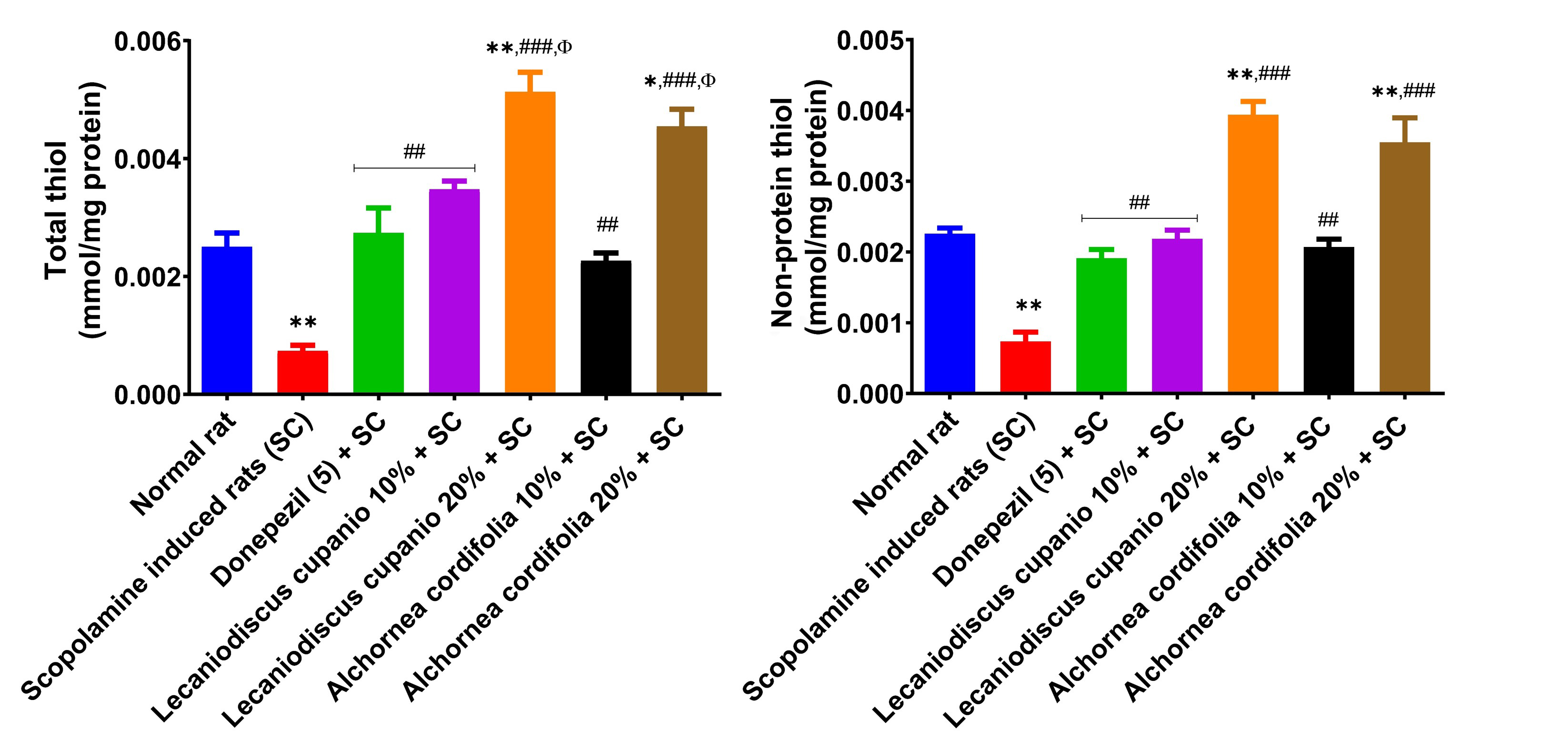
Effects of Lecaniodiscus cupanioide and Alchornea cordifolia supplemented diets on hippocampal total thiol and non-protein thiol concentrations of scopolamine-induced amnesic experimental rats. Results are expressed as mean ± SEM (n = 6). *: p < 0.05, **: p < 0.01, ***: p < 0.001 compared with the normal control group. #: p < 0.05, ##: p < 0.01, ###: p < 0.001 compared with the scopolamine-induced amnesic experimental rats. Φ: p < 0.05, ΦΦ: p < 0.01, ΦΦΦ: p < 0.001 compared with the standard drug (donepezil) administered group. SC: scopolamine; SEM: standard error of the mean
Tables 2 and 3 show the bioactive compound constituents of A. cordifolia and L. cupanioide roots. According to the obtained result, fourteen bioactive compounds such as benzoic acid, 4-hydroxy- (1.60 and 1.42 mg/g), gentisic acid (0.22 and 0.27 mg/g), quercetin (1.12 and 1.10 mg/g), catechol (1.37 and 1.66 mg/g) and others were quantified from the A. cordifolia and L. cupanioide roots.
Bioactive compound constituents of Alchornea cordifolia roots
| Peak # | Retention time (min) | Compound detected | Peak area (%) | Phenolic content (mg/g) |
|---|---|---|---|---|
| 1 | 5.76 | Benzoic acid, 4-hydroxy- | 12.84 | 1.60 |
| 2 | 7.50 | Gentisic acid | 4.37 | 0.22 |
| 3 | 8.41 | 1,2,3-benzenetriol | 7.38 | 0.63 |
| 4 | 11.00 | Quercetin | 10.70 | 1.12 |
| 5 | 15.50 | Catechol, 2TMS derivative | 13.50 | 1.37 |
| 6 | 17.00 | Tyrosol, 2TMS derivative | 4.28 | 0.18 |
| 7 | 17.50 | Benzoic acid, 3,4,5-trihydroxy- | 5.93 | 0.39 |
| 8 | 19.52 | Vanillic acid | 3.98 | 0.08 |
| 9 | 22.50 | p-coumaric acid | 5.27 | 0.26 |
| 10 | 22.61 | Cinnamic acid, TMS derivative | 15.15 | 2.02 |
| 11 | 24.50 | Syringic acid (2TMS) | 1.65 | 0.03 |
| 12 | 25.52 | Protocatechoic acid, 3TMS derivative | 9.06 | 1.13 |
| 13 | 28.50 | Kaempferol, TMS | 3.29 | 0.15 |
| 14 | 32.49 | 3-caffeoyl quinic acid, TMS | 2.63 | 0.32 |
#: number; TMS: trimethylsilyl
Bioactive compound constituents of Lecaniodiscus cupanioide roots
| Peak # | Retention time (min) | Compound detected | Peak area (%) | Phenolic content (mg/g) |
|---|---|---|---|---|
| 1 | 5.00 | Benzoic acid, 4-hydroxy- | 12.14 | 1.42 |
| 2 | 5.42 | Gentisic acid | 5.32 | 0.27 |
| 3 | 5.76 | 1,2,3-benzenetriol | 7.04 | 0.57 |
| 4 | 8.50 | Quercetin | 10.52 | 1.10 |
| 5 | 10.20 | Catechol, 2TMS derivative | 16.29 | 1.66 |
| 6 | 11.99 | Tyrosol, 2TMS derivative | 4.07 | 0.13 |
| 7 | 13.50 | Benzoic acid, 3,4,5-trihydroxy- | 6.11 | 0.42 |
| 8 | 18.18 | Vanillic acid | 3.39 | 0.07 |
| 9 | 21.50 | p-coumaric acid | 5.43 | 0.30 |
| 10 | 22.00 | Cinnamic acid, TMS derivative | 14.59 | 1.72 |
| 11 | 24.99 | Syringic acid (2TMS) | 1.36 | 0.01 |
| 12 | 25.52 | Protocatechoic acid, 3TMS derivative | 8.48 | 0.70 |
| 13 | 28.98 | Kaempferol, TMS | 3.22 | 0.13 |
| 14 | 36.00 | 3-caffeoyl quinic acid, TMS | 2.04 | 0.26 |
#: number; TMS: trimethylsilyl
Amnesia is a neurological disorder marked by memory deterioration and learning difficulties [13]. The current work utilized the Y-maze, a well-established behavioral test for short-term memory, to evaluate the impact of meals supplemented with L. cupanioide and A. cordifolia on amnesia-induced rats. The study detected a significant (p < 0.001) decrease in spontaneous modifications in experimental rats administered scopolamine, indicating the presence of scopolamine-induced amnesia. The capacity of scopolamine to cause amnesia is consistent with the findings of previous investigations conducted by Ogunsuyi et al. [36] and Memudu and Adanike [37]. LCSD and ACSD demonstrated anti-amnesic effects by safeguarding experimental animals from a drop in memory index in a manner that depended on the percentage of inclusion. In addition, ACSD showed a greater capacity for protection against scopolamine-induced amnesia compared to LCSD.
Research has shown that the dopaminergic neurotransmission system plays a major role in the retention of information and the acquisition of new knowledge [38]. Notably, the administration of scopolamine resulted in a significant (p < 0.01) reduction in dopamine levels compared to the control group. This study provides more evidence for the validity of the Y-maze test and confirms prior research that demonstrated memory impairment in experimental rats pre-exposed to i.p. injection of scopolamine [13]. However, the administration of LCSD and ACSD effectively prevented the dopamine concentration from decreasing when compared to the untreated rats with amnesia rats. This is consistent with the research conducted by Sinha et al. [39], which found a significant increase in dopamine levels following amnesia therapy.
The facilitation of dopamine concentration alteration in amnesic studies by an agent has been proven to occur through the cholinergic neurotransmission pathway. Dopaminergic receptor activation has also been linked to an elevated turnover rate of acetylcholine in the hippocampus [40]. The correlation between dopamine and acetylcholine to cognitive and memory processes has been confirmed [25]. Notably, the use of scopolamine greatly increased the activities of AChE and BChE, which have been shown to cause excessive acetylcholine and butyrylcholine hydrolysis [36]. This statement reinforces the findings of Shabani and Mirshekar [21], who identified cholinergic dysfunction as the primary cause of the cognitive decline observed in individuals with amnesic disorders. Nevertheless, prior administration of LCSD and ACSD provided significant (p < 0.001) protection against the aggravation of AChE and BChE activity. These findings indicated that LCSD and ACSD have protective functions in preventing the breakdown of choline and the transmission of cholinergic neurotransmitters [13].
Arginase activity was significantly higher (p < 0.001) in rats with induced amnesia from scopolamine compared to the normal control rats. However, pre-treatment with LCSD and ACSD reduced the increase in arginase activity. Arginase hydrolyses arginine into urea and ornithine. Excessive production of arginase decreases the required concentration of arginine for NO generation, whereas an excess of ornithine might cause neurotoxicity [41]. The suppression of arginase activity may have had a role in the enhanced levels of NO reported in experimental animals treated with LCSD and ACSD. Hence, the capacity of LCSD and ACSD to maintain NO levels, as seen in this investigation, may act as an additional neuroprotective mechanism utilized by the supplemented diets [42, 43].
Furthermore, there is a correlation between enhanced cholinergic neurotransmission and reduced inflammation, which is associated with a decline in NO neurotransmission [36]. Amnesia advancement has been linked to the stimulation of proinflammatory cytokines, such as TNF-α, and the suppression of anti-inflammatory cytokines, such as IL-6 [14]. The level of TNF-α in the hippocampus was dramatically increased (p < 0.01) in rats with induced amnesia caused by scopolamine, while the level of IL-6 was significantly decreased (p < 0.01). This is consistent with the research conducted by Mirshekar et al. [44], which demonstrated increased levels of TNF-α and decreased levels of IL-6 as a result of inflammation mediated by the nuclear kappa-light-chain-enhancer of activated B cells (NF-κB). Elevation of proinflammatory cytokines impacts memory performance by inhibiting the cholinergic neurotransmission pathway [37], a feature that was also found in this investigation. Pre-treatment with LCSD and ASCD was found to inhibit the increase of TNF-α and decrease of IL-6 levels following the injection of scopolamine, as compared to untreated rats with amnesia rats. The neuro-anti-inflammatory qualities of the supplemented diets are underscored by the LCSD and ASCD’s capacity to also inhibit the increase in myeloperoxidase activity [45].
Scopolamine, as a muscarinic receptor antagonist, directly hampers memory and the cholinergic system by inducing oxidative stress [37]. The oxidative stress and memory impairment patterns reported in amnesic individuals are comparable to those observed in scopolamine-induced amnesic experimental rats [46, 47]. Hence, the capacity of LCSD and ACSD to protect experimental rats from heightened hippocampus lipid peroxidation suggests an enhanced brain antioxidant state following pre-treatment with the supplemented diets. Significantly higher non-protein and total thiol levels were reported in the LCSD and ACSD pre-treated experimental rats compared to untreated rats with amnesia rats (p < 0.01). This may indicate the presence of a high phenolic content in the plants. These findings provide additional support to the research conducted by Odubanjo et al. [13], who proposed that the components found in medicinal plants are capable of effectively preventing the formation of lipid peroxidation.
The disparities in the inhibitory and protective abilities of LCSD and ACSD can be ascribed to the diverse bioactive ingredients and variations of the plants [48]. Flavonoids have been recognized as a primary component of L. cupanioide and A. cordifolia [49]. Flavonoids improve the ability to learn and remember in several animal models by reducing the activity of AChE and enhancing antioxidant capacity [47]. Studies have shown that alkaloids derived from plants can counteract cognitive impairments caused by their nitrogen-containing compounds [17]. The binding sites of AChE are involved in the interaction with positively charged nitrogen, which allows for inhibition by non-alkaloid chemicals, specifically terpenes, xanthones, and coumarins [50]. The results indicate that LCSD and ACSD have the potential to provide strong neuroprotection, making them promising candidates for the creation of functional foods to help control forgetfulness.
Comparatively, the findings indicate that LCSD was more successful in protecting against the imbalances in cholinergic, monoaminergic, purinergic, and inflammatory systems caused by scopolamine, as compared to ACSD. The HPLC analysis revealed that L. cupanioide exhibited larger concentrations of benzoic acid, 3,4,5-trihydroxy- (0.42 mg/g), catechol, 2TMS (trimethylsilyl) derivative (1.66 mg/g), and gentisic acid (0.27 mg/g) compared to ACSD. These bioactive chemicals have been found to have neuroprotective properties by preventing imbalances in monoaminergic and cholinergic activity, neuronal death, and the production of toxic amyloid plaques and tangles in the brain [17, 51, 52]. ACSD was shown to be more efficacious than LCSD in preventing the exacerbated oxidative stress and diminished antioxidant levels generated by scopolamine. The presence of higher levels of bioactive compounds in A. cordifolia compared to L. cupanioide has been linked to an improvement in antioxidant status and a reduction in oxidative stress [52, 53].
The neuronal antioxidant ability of benzoic acid has been established, as well as its ability to inhibit the enzymatic activities of the citric acid cycle and oxidative phosphorylation in the brain [54]. The natural polyphenolic derivative of benzoic acid, syringic acid (2TMS), has been shown to significantly inhibit hippocampal neuronal injury via p38 and JNK signaling pathways’ stimulation as well as diminish the hippocampal mRNA expressions of pro-inflammatory modulators such as IL-1β, IL-6, and TNF-α [55]. The ability of syringic acid to significantly upregulate brain mRNA expression of proliferator-activated receptor-γ coactivator 1 alpha (PGC-1α) and nuclear respiratory factor 1 (NRF-1), the key regulators of energy metabolism, oxidative phosphorylation, and mitochondrial biogenesis, has also been established in amnesic conditions [54]. Gentisic acid, a di-phenolic chemical derived from benzoic acid, has been shown to exhibit its neuroprotective potential by strongly scavenging free radicals in the brain [56]. 1,2,3-benzenetriol, an isomer of benzene-triol, has been shown to exhibit potent neuronal anti-inflammatory activities and act as a reducing agent in the brain due to its ready ability to donate electrons [57]. Quercetin improves memory and learning capabilities and confers strong neurovascular coupling protection, involving NVU (neurovascular unit) integrity maintenance, reduction of neurovascular oxidation, modulation of microvascular function, cholinergic system improvement, and regulation of neurovascular RAGE (receptor for advanced glycation end products) signaling and ERK/CREB/BDNF pathway [24, 58]. In the hippocampus of amnesic rats, pre-treatment with vanillic acid increased IL-10 levels while significantly lowering levels of IL-6, TNF-α, and TUNEL-positive cells [59]. Protocatechuic acid, as shown by Caruso et al. [60], counteracts mitochondrial membrane potential loss, reduces reactive oxygen species, depletes glutathione, activates caspase-3, and down-regulates Bcl-2 to attenuate mitochondrial malfunction and apoptotic cell death. Additionally, it alters the MAPK and NF-κB signaling pathways, which lowers pro-inflammatory indicators. By inhibiting the activation of TLR4, TRAF6, and NF-κB, cinnamic acid protects against dopaminergic cell death and autophagy (via p62 down-regulation). Additionally, in experimental animal brains, it counteracts high levels of endothelial-leukocyte adhesion molecule-1, TNF-α, IL-1β, and CCL2. Last but not least, kaempferol promotes neuronal survival, as seen by increased N-acetyl aspartate levels, preserves mitochondrial integrity, lowers acylcarnitine levels, and increases the flow of the pyruvate and TCA cycles [61].
This study reported the capacity of incorporating A. cordifolia and L. cupanioide into a diet to protect the cognitive ability of scopolamine-induced amnesia in rats. The study found that administering LCSD and ACSD at 10% and 20% concentrations before amnesia induction helped to preserve the levels of important enzymes (MAO, AChE, BChE, arginase, myeloperoxidase), molecules (dopamine and NO), inflammatory cytokines (TNF-α and IL-6), and antioxidants (non-protein thiol and total thiol) in the hippocampus of experimental rats. The therapeutic benefits of the plant’s roots were ascribed to the constituent phytochemicals. This study elucidates the potential processes underlying the neuroprotective effects of L. cupanioide and A. cordifolia while also giving empirical evidence to support the traditional use of these herbs in folklore. Inferentially, these roots have the potential to be used as sources of nutraceuticals and functional foods for the prevention and treatment of neurodegenerative illnesses, particularly amnesia. The limitations of this study include its reliance on a single animal model, scopolamine-induced amnesia, which may not fully replicate human cognitive dysfunctions. In addition, the effects of the diets were not compared across diverse biological systems or in clinical trials, limiting the generalizability of the findings. Thus, this study recommended assessing clinical trials, utilizing several experimental models, and examining histology, immunohistochemical analysis, and amyloid aggregation characteristics to further ascertain the neuroprotective capabilities of the plants.
AChE: acetylcholinesterase
ACSD: Alchornea cordifolia-supplemented diets
BChE: butyrylcholinesterase
bwt: body weight
i.p.: intraperitoneal
IL-6: interleukin-6
LCSD: Lecaniodiscus cupanioide-supplemented diets
MAO: monoamine oxidase
MDA: malonaldehyde
NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells
NO: nitric oxide
TMS: trimethylsilyl
TNF-α: tumor necrosis factor-α
OMA: Conceptualization, Supervision, Formal analysis. AEA: Investigation, Writing—original draft. OWF: Investigation, Writing—review & editing. GO: Supervision, Resources.
The authors declare that they have no conflicts of interest.
The exploitation of animals conforms to the ethical guidelines established by the US National Institute of Health (NIH). Permission for animal use was obtained from the Animal Ethical Committee, Centre for Research and Development (CERAD), Federal University of Technology, Akure, with the ethical number FUTA/ETH/2020/016.
Not applicable.
Not applicable.
All the data obtained in the present study are available on request from the authors.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2099
Download: 25
Times Cited: 0
Deepa A V, Dennis Thomas T
Neha Kamboj ... Rahul Kumar
Suvendu Ghosh ... Debosree Ghosh
Apoorva A. Bankar ... Nazma N. Inamdar
