





Background:
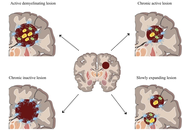
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disease affecting the central nervous system, the cause of which remains unknown. Environmental, genetic, and immunological factors are considered risk factors. MS has no cure; therefore, therapy focuses on reducing the number of outbreaks, controlling symptoms, and therapies aimed at modifying the course of the disease. Innovative strategies that promote remyelination and repair of damaged brain tissue are under investigation. This review aims to compile and systematize the available knowledge on the multifactorial nature of MS, highlighting the main risk factors. It also discusses the mechanisms underlying the pathogenesis of the disease, current therapies, and prospects, presenting a comprehensive overview of the effect of various drugs on remyelination and repair of central nervous system damage.
Methods:
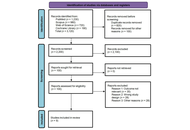
A comprehensive literature search, guided by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards, was conducted across PubMed, Cochrane Library, Web of Science, and ClinicalTrials.gov to identify relevant clinical trials. Of the studies retrieved, 13 were selected for this review. These trials specifically explored integrated therapeutic approaches, combining pharmacological and non-pharmacological interventions, for managing MS.
Results:
The results reflect the multifactorial nature of MS and the existence of several promising therapies to combat inflammation and demyelination, as well as to promote remyelination. Reducing inflammation remains the main target, but new approaches such as clemastine, liothyronine, interleukin (IL)-2, N-acetylglucosamine, and intracranial transplantation of fetal human neural precursor cells have shown promising results.
Discussion:
Currently, the therapies available for MS target the peripheral immune system. Therefore, more studies are needed on treatment therapies that combine immunomodulation of the peripheral and central nervous systems to reduce the neurological disability of patients. It is also concluded that the therapies were safe and were well tolerated, given the occurrence of a small number of adverse events.
Background:
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disease affecting the central nervous system, the cause of which remains unknown. Environmental, genetic, and immunological factors are considered risk factors. MS has no cure; therefore, therapy focuses on reducing the number of outbreaks, controlling symptoms, and therapies aimed at modifying the course of the disease. Innovative strategies that promote remyelination and repair of damaged brain tissue are under investigation. This review aims to compile and systematize the available knowledge on the multifactorial nature of MS, highlighting the main risk factors. It also discusses the mechanisms underlying the pathogenesis of the disease, current therapies, and prospects, presenting a comprehensive overview of the effect of various drugs on remyelination and repair of central nervous system damage.
Methods:
A comprehensive literature search, guided by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards, was conducted across PubMed, Cochrane Library, Web of Science, and ClinicalTrials.gov to identify relevant clinical trials. Of the studies retrieved, 13 were selected for this review. These trials specifically explored integrated therapeutic approaches, combining pharmacological and non-pharmacological interventions, for managing MS.
Results:
The results reflect the multifactorial nature of MS and the existence of several promising therapies to combat inflammation and demyelination, as well as to promote remyelination. Reducing inflammation remains the main target, but new approaches such as clemastine, liothyronine, interleukin (IL)-2, N-acetylglucosamine, and intracranial transplantation of fetal human neural precursor cells have shown promising results.
Discussion:
Currently, the therapies available for MS target the peripheral immune system. Therefore, more studies are needed on treatment therapies that combine immunomodulation of the peripheral and central nervous systems to reduce the neurological disability of patients. It is also concluded that the therapies were safe and were well tolerated, given the occurrence of a small number of adverse events.
DOI: https://doi.org/10.37349/ent.2026.1004138

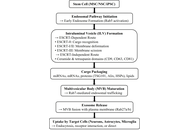
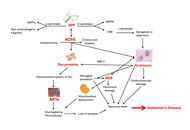
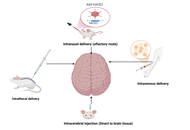
Neurodegenerative disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and ischemic stroke cause progressive and often irreversible neuronal loss, leading to major functional disability. Conventional pharmacological therapies primarily offer symptomatic relief and fail to promote neuro-restoration. Stem cell-derived exosomes have recently gained attention as acellular, regenerative biologics capable of modulating inflammation, enhancing synaptic repair, and facilitating neural recovery. These nanoscale vesicles carry bioactive molecules, including microRNAs (miRNAs) and growth factors, that replicate many of the paracrine benefits of stem cells without the associated risks of tumorigenicity or immune rejection. The objective of this review is to critically evaluate recent evidence on the neuroprotective, immunomodulatory, and translational mechanisms of stem cell-derived exosomes in major neurodegenerative and cerebrovascular disorders, highlighting their clinical relevance and therapeutic potential. Preclinical studies suggest that exosome administration may restore mitochondrial function, reduce oxidative stress, and support neuronal survival, with associated improvements in cognitive and motor outcomes in experimental models of AD, PD, and stroke. Exosomal miRNAs such as miR-21, miR-124, and miR-133b mediate neuroprotective effects through phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt), mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), and nuclear factor erythroid 2-related factor 2 (Nrf2) signaling, while miR-146a promotes immunomodulation by suppressing pro-inflammatory cytokines and facilitating microglial repair phenotypes. Early-phase clinical studies primarily demonstrate feasibility and short-term safety, with exploratory signals of neurological improvement that require confirmation in adequately powered trials. Despite challenges in standardization and regulation, exosome-based therapy represents a scalable, safe, and clinically translatable strategy for neuro-regeneration, with significant promise for future management of brain network disorders.
Neurodegenerative disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and ischemic stroke cause progressive and often irreversible neuronal loss, leading to major functional disability. Conventional pharmacological therapies primarily offer symptomatic relief and fail to promote neuro-restoration. Stem cell-derived exosomes have recently gained attention as acellular, regenerative biologics capable of modulating inflammation, enhancing synaptic repair, and facilitating neural recovery. These nanoscale vesicles carry bioactive molecules, including microRNAs (miRNAs) and growth factors, that replicate many of the paracrine benefits of stem cells without the associated risks of tumorigenicity or immune rejection. The objective of this review is to critically evaluate recent evidence on the neuroprotective, immunomodulatory, and translational mechanisms of stem cell-derived exosomes in major neurodegenerative and cerebrovascular disorders, highlighting their clinical relevance and therapeutic potential. Preclinical studies suggest that exosome administration may restore mitochondrial function, reduce oxidative stress, and support neuronal survival, with associated improvements in cognitive and motor outcomes in experimental models of AD, PD, and stroke. Exosomal miRNAs such as miR-21, miR-124, and miR-133b mediate neuroprotective effects through phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt), mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), and nuclear factor erythroid 2-related factor 2 (Nrf2) signaling, while miR-146a promotes immunomodulation by suppressing pro-inflammatory cytokines and facilitating microglial repair phenotypes. Early-phase clinical studies primarily demonstrate feasibility and short-term safety, with exploratory signals of neurological improvement that require confirmation in adequately powered trials. Despite challenges in standardization and regulation, exosome-based therapy represents a scalable, safe, and clinically translatable strategy for neuro-regeneration, with significant promise for future management of brain network disorders.
DOI: https://doi.org/10.37349/ent.2026.1004137
This article belongs to the special issue Breakthroughs in Mechanisms and Treatments for Neurodegenerative Diseases

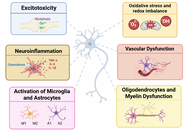
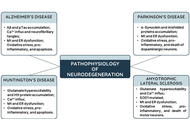
Neurodegenerative diseases, including Alzheimer’s, Parkinson’s, Huntington’s, and Amyotrophic Lateral Sclerosis, are characterized by multifactorial pathologies that extend beyond neuronal loss to include neuroinflammation, oxidative stress, mitochondrial dysfunction, and glial dysregulation. Despite extensive research, disease-modifying therapies remain elusive, hindered by late diagnosis, limited availability of specific biomarkers, and the persistent dominance of reductionist, single-target strategies. This comprehensive and informative review provides a critical synthesis of integrated neuroprotective strategies, with particular focus on glial mechanisms and biomarker-guided interventions. Therapeutic emphasis is placed on coordinated mechanisms targeting both neurons and non-neuronal cells, such as astrocytes, microglia, and oligodendrocytes. Emerging strategies are reported to include modulation of synaptic plasticity and neurotransmission, delivery of neurotrophic factors, activation of intrinsic cytoprotective pathways (e.g., Nrf2 signaling), restoration of proteostasis, and induction of regeneration via cellular reprogramming. Glial cells are discussed as therapeutic targets involved in inflammation, metabolism, myelination, and neuronal survival. Advances in predictive, preventive, personalized, and participatory (P4) medicine, supported by genomics, multi-omics, imaging, and real-world data, are presented as accelerating biomarker discovery and enabling earlier and more precise stage-specific interventions. Future success in combating neurodegeneration will depend on integrated approaches that combine protective, supportive, and regenerative strategies, appropriate for disease stage and patient profile. By reframing neuroprotection as a systemic, multicellular endeavor, this review highlights the potential to not only extend life expectancy, but also preserve meaningful quality of life in individuals affected by neurodegenerative diseases.
Neurodegenerative diseases, including Alzheimer’s, Parkinson’s, Huntington’s, and Amyotrophic Lateral Sclerosis, are characterized by multifactorial pathologies that extend beyond neuronal loss to include neuroinflammation, oxidative stress, mitochondrial dysfunction, and glial dysregulation. Despite extensive research, disease-modifying therapies remain elusive, hindered by late diagnosis, limited availability of specific biomarkers, and the persistent dominance of reductionist, single-target strategies. This comprehensive and informative review provides a critical synthesis of integrated neuroprotective strategies, with particular focus on glial mechanisms and biomarker-guided interventions. Therapeutic emphasis is placed on coordinated mechanisms targeting both neurons and non-neuronal cells, such as astrocytes, microglia, and oligodendrocytes. Emerging strategies are reported to include modulation of synaptic plasticity and neurotransmission, delivery of neurotrophic factors, activation of intrinsic cytoprotective pathways (e.g., Nrf2 signaling), restoration of proteostasis, and induction of regeneration via cellular reprogramming. Glial cells are discussed as therapeutic targets involved in inflammation, metabolism, myelination, and neuronal survival. Advances in predictive, preventive, personalized, and participatory (P4) medicine, supported by genomics, multi-omics, imaging, and real-world data, are presented as accelerating biomarker discovery and enabling earlier and more precise stage-specific interventions. Future success in combating neurodegeneration will depend on integrated approaches that combine protective, supportive, and regenerative strategies, appropriate for disease stage and patient profile. By reframing neuroprotection as a systemic, multicellular endeavor, this review highlights the potential to not only extend life expectancy, but also preserve meaningful quality of life in individuals affected by neurodegenerative diseases.
DOI: https://doi.org/10.37349/ent.2026.1004136

Aim:
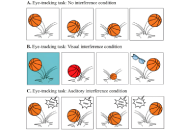
This study examined differences in attentional control and awareness of interference among children with attention-deficit/hyperactivity disorder (ADHD), children with subthreshold ADHD (children showing some but not all symptoms required for diagnosis), and children with typical development. Specifically, we investigated how visual and auditory distractions affect behavioral performance and eye movements, to clarify the degree and nature of attentional control impairments associated with subthreshold versus clinically diagnosed ADHD.
Methods:
One hundred and two children (mean age = 7.23 years, SD = 1.23; 34 per group) participated in three eye-tracking tasks involving a bouncing ball under no, visual, and auditory interference. Behavioral accuracy (number of correctly counted bounces), fixation duration on the target, gaze reorientation latency, and distractor awareness were analyzed using mixed-design analyses of variance (ANOVAs) and chi-square tests.
Results:
Significant group differences were found in counting accuracy, F(2, 99) = 16.42, p = 0.00069, η2p = 0.245, with typically developing children performing best, followed by those with subthreshold and full ADHD. Eye-tracking indices showed a similar gradient: fixation duration decreased with symptom severity, F(4, 198) = 7.65, p = 0.00094, η2p = 0.134, while gaze reorientation latency increased, F(2, 99) = 12.18, p = 0.00093, η2p = 0.197 (typical development ≈ 480 ms; subthreshold ≈ 621 ms; ADHD ≈ 721 ms). Awareness of distractors also varied significantly across groups, χ2(2, n = 102) = 38.12, p < 0.001, Cramer’s V = 0.61, with detection rates of approximately 80% (typical development), 50% (subthreshold), and 25% (ADHD).
Conclusions:
Both children with ADHD and children with subthreshold ADHD show measurable deficits in attentional control and awareness of interference, particularly under visual and auditory distraction. Children with subthreshold ADHD exhibited an intermediate profile, supporting a continuum rather than a categorical distinction in cognitive control impairments. These findings highlight the importance of early identification and interventions targeting attentional regulation and metacognitive monitoring across the ADHD spectrum.
Aim:
This study examined differences in attentional control and awareness of interference among children with attention-deficit/hyperactivity disorder (ADHD), children with subthreshold ADHD (children showing some but not all symptoms required for diagnosis), and children with typical development. Specifically, we investigated how visual and auditory distractions affect behavioral performance and eye movements, to clarify the degree and nature of attentional control impairments associated with subthreshold versus clinically diagnosed ADHD.
Methods:
One hundred and two children (mean age = 7.23 years, SD = 1.23; 34 per group) participated in three eye-tracking tasks involving a bouncing ball under no, visual, and auditory interference. Behavioral accuracy (number of correctly counted bounces), fixation duration on the target, gaze reorientation latency, and distractor awareness were analyzed using mixed-design analyses of variance (ANOVAs) and chi-square tests.
Results:
Significant group differences were found in counting accuracy, F(2, 99) = 16.42, p = 0.00069, η2p = 0.245, with typically developing children performing best, followed by those with subthreshold and full ADHD. Eye-tracking indices showed a similar gradient: fixation duration decreased with symptom severity, F(4, 198) = 7.65, p = 0.00094, η2p = 0.134, while gaze reorientation latency increased, F(2, 99) = 12.18, p = 0.00093, η2p = 0.197 (typical development ≈ 480 ms; subthreshold ≈ 621 ms; ADHD ≈ 721 ms). Awareness of distractors also varied significantly across groups, χ2(2, n = 102) = 38.12, p < 0.001, Cramer’s V = 0.61, with detection rates of approximately 80% (typical development), 50% (subthreshold), and 25% (ADHD).
Conclusions:
Both children with ADHD and children with subthreshold ADHD show measurable deficits in attentional control and awareness of interference, particularly under visual and auditory distraction. Children with subthreshold ADHD exhibited an intermediate profile, supporting a continuum rather than a categorical distinction in cognitive control impairments. These findings highlight the importance of early identification and interventions targeting attentional regulation and metacognitive monitoring across the ADHD spectrum.
DOI: https://doi.org/10.37349/ent.2025.1004134
This article belongs to the special issue Advances in the Pathogenesis, Diagnosis and Treatment of Attention Deficit Hyperactivity Disorder

Aim:
Neurodegenerative diseases, such as Alzheimer’s, are strongly associated with amyloid-β aggregation. This study aimed to explore bioactive metabolites from endophytic bacteria as potential anti-aggregation agents with relevance to neuroprotection, focusing on isolate D11 obtained from a geothermal fern at Gedong Songo hot springs.
Methods:
Isolate D11 was characterized by Gram staining and 16S rRNA sequencing. Growth curve analysis was conducted to determine metabolite production phases. Phytochemical screening, bovine serum albumin (BSA) aggregation inhibition assays, liquid chromatography mass spectroscopy (LCMS) profiling, and molecular docking against amyloid-β were employed to evaluate bioactivity and metabolite composition.
Results:
D11 was identified as a Gram-negative rod with 97.94% similarity to Stutzerimonas stutzeri. Metabolite production peaked during the stationary and death phases. Phytochemical tests revealed alkaloids and tannins in aqueous fractions. BSA aggregation inhibition assays demonstrated potent inhibitory activity, with IC50 values (2.40–3.29 µg/mL) significantly lower than quercetin. LCMS profiling identified diverse metabolites, dominated by flavonoid glycosides such as kaempferol-7-O-deoxyhexosyl-3-O-acetylhexoside, along with alkaloids, peptides, and diterpenoids. Molecular docking confirmed strong binding affinities of flavonoid glycosides to amyloid β (–7.6 kcal/mol), outperforming quercetin (–6.0 kcal/mol).
Conclusions:
These findings suggest that isolate D11 Stutzerimonas produces bioactive metabolites with anti-aggregation activity and potential relevance to neuroprotection. However, since Stutzerimonas-derived metabolites remain poorly explored and the docking results are tentative, further in-depth characterization and in vivo validation are required to confirm their therapeutic relevance, and further validation using amyloid-β or α-synuclein models is required to confirm therapeutic implications.
Aim:
Neurodegenerative diseases, such as Alzheimer’s, are strongly associated with amyloid-β aggregation. This study aimed to explore bioactive metabolites from endophytic bacteria as potential anti-aggregation agents with relevance to neuroprotection, focusing on isolate D11 obtained from a geothermal fern at Gedong Songo hot springs.
Methods:
Isolate D11 was characterized by Gram staining and 16S rRNA sequencing. Growth curve analysis was conducted to determine metabolite production phases. Phytochemical screening, bovine serum albumin (BSA) aggregation inhibition assays, liquid chromatography mass spectroscopy (LCMS) profiling, and molecular docking against amyloid-β were employed to evaluate bioactivity and metabolite composition.
Results:
D11 was identified as a Gram-negative rod with 97.94% similarity to Stutzerimonas stutzeri. Metabolite production peaked during the stationary and death phases. Phytochemical tests revealed alkaloids and tannins in aqueous fractions. BSA aggregation inhibition assays demonstrated potent inhibitory activity, with IC50 values (2.40–3.29 µg/mL) significantly lower than quercetin. LCMS profiling identified diverse metabolites, dominated by flavonoid glycosides such as kaempferol-7-O-deoxyhexosyl-3-O-acetylhexoside, along with alkaloids, peptides, and diterpenoids. Molecular docking confirmed strong binding affinities of flavonoid glycosides to amyloid β (–7.6 kcal/mol), outperforming quercetin (–6.0 kcal/mol).
Conclusions:
These findings suggest that isolate D11 Stutzerimonas produces bioactive metabolites with anti-aggregation activity and potential relevance to neuroprotection. However, since Stutzerimonas-derived metabolites remain poorly explored and the docking results are tentative, further in-depth characterization and in vivo validation are required to confirm their therapeutic relevance, and further validation using amyloid-β or α-synuclein models is required to confirm therapeutic implications.
DOI: https://doi.org/10.37349/ent.2025.1004135
This article belongs to the special issue Natural Products in Neurotherapeutic Applications

Age-related neurological disorders such as ALS (Lou Gehrig’s disease), Parkinson’s disease, and Alzheimer’s disease have few truly effective treatment options. At best, these may slow the inexorable disease progression without providing a cure. Part of the problem with therapeutic approaches may arise due to the stage at which these diseases are detected, particularly the sporadic forms. In most cases, early signs and symptoms may be insidious, thus hiding the significant damage done to the areas of the nervous system impacted prior to any firm clinical diagnosis. This situation appears to necessitate the development of earlier detection methods for “biomarkers” that might allow for much earlier phase disease state treatments that might serve to significantly slow or even halt disease progression. Currently, most biomarkers in use serve primarily as aids to disease diagnosis, at which point there are no successful treatment options. In contrast, a search for more effective early treatment options would need to identify characteristic and specific molecular signatures of disease onset and progression using methods that are simple, such as blood-based analytical assays, relatively cheap, and crucially minimally invasive.
Age-related neurological disorders such as ALS (Lou Gehrig’s disease), Parkinson’s disease, and Alzheimer’s disease have few truly effective treatment options. At best, these may slow the inexorable disease progression without providing a cure. Part of the problem with therapeutic approaches may arise due to the stage at which these diseases are detected, particularly the sporadic forms. In most cases, early signs and symptoms may be insidious, thus hiding the significant damage done to the areas of the nervous system impacted prior to any firm clinical diagnosis. This situation appears to necessitate the development of earlier detection methods for “biomarkers” that might allow for much earlier phase disease state treatments that might serve to significantly slow or even halt disease progression. Currently, most biomarkers in use serve primarily as aids to disease diagnosis, at which point there are no successful treatment options. In contrast, a search for more effective early treatment options would need to identify characteristic and specific molecular signatures of disease onset and progression using methods that are simple, such as blood-based analytical assays, relatively cheap, and crucially minimally invasive.
DOI: https://doi.org/10.37349/ent.2025.1004133

Aim:
Parkinson’s disease (PD) and Alzheimer’s disease (AD) represent critical neurological disorders that have emerged as significant health concerns in the 21st century. The pharmacological interventions currently employed to manage these diseases demonstrate limited efficacy and some adverse side effects. Historically, natural products have been used to develop therapeutic agents targeting neurodegenerative disorders. This study aimed to apply in silico techniques to investigate the pharmacological mechanisms of capsaicin as a possible alternative treatment or coadjutant phytotherapy for PD and AD.
Methods:
We obtained target genes for capsaicin, PD, and AD from the HERB database, the Swiss Target Prediction database, the Comparative Toxicogenomics Database, and the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform, and matched them. Subsequently, we constructed a protein-protein interaction network and performed enrichment analysis of the common targets. Then, the interactions of capsaicin with the proteins with the highest degree were tested using molecular docking. The stability of the complexes was verified using molecular dynamics techniques.
Results:
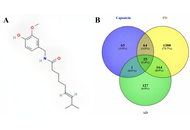
A total of 25 targets were found in common from the databases for capsaicin, AD, and PD. The enrichment analysis revealed that proteins from these targets influenced integrin activity in the IGF1-IGF1R complex, cholinesterase activity, and dopamine neurotransmitter receptor activity, all of which are coupled via protein Gi/Go, among other cellular processes. From the protein-protein interaction network, we identified the hub proteins IL6, GSK3B, CASP, BCL2, ESR1, SIRT1, NGF, IGF1, and HMOX1. Furthermore, molecular docking studies between hub proteins and capsaicin showed strong binding affinity. Finally, molecular dynamics simulations support a stable interaction between capsaicin and SIRT1, ESR1, HMOX1, and NGF.
Conclusions:
This work contributes to understanding the neuroprotective activity of capsaicin in PD and AD. However, these bioinformatic predictions require further experimental validation.
Aim:
Parkinson’s disease (PD) and Alzheimer’s disease (AD) represent critical neurological disorders that have emerged as significant health concerns in the 21st century. The pharmacological interventions currently employed to manage these diseases demonstrate limited efficacy and some adverse side effects. Historically, natural products have been used to develop therapeutic agents targeting neurodegenerative disorders. This study aimed to apply in silico techniques to investigate the pharmacological mechanisms of capsaicin as a possible alternative treatment or coadjutant phytotherapy for PD and AD.
Methods:
We obtained target genes for capsaicin, PD, and AD from the HERB database, the Swiss Target Prediction database, the Comparative Toxicogenomics Database, and the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform, and matched them. Subsequently, we constructed a protein-protein interaction network and performed enrichment analysis of the common targets. Then, the interactions of capsaicin with the proteins with the highest degree were tested using molecular docking. The stability of the complexes was verified using molecular dynamics techniques.
Results:
A total of 25 targets were found in common from the databases for capsaicin, AD, and PD. The enrichment analysis revealed that proteins from these targets influenced integrin activity in the IGF1-IGF1R complex, cholinesterase activity, and dopamine neurotransmitter receptor activity, all of which are coupled via protein Gi/Go, among other cellular processes. From the protein-protein interaction network, we identified the hub proteins IL6, GSK3B, CASP, BCL2, ESR1, SIRT1, NGF, IGF1, and HMOX1. Furthermore, molecular docking studies between hub proteins and capsaicin showed strong binding affinity. Finally, molecular dynamics simulations support a stable interaction between capsaicin and SIRT1, ESR1, HMOX1, and NGF.
Conclusions:
This work contributes to understanding the neuroprotective activity of capsaicin in PD and AD. However, these bioinformatic predictions require further experimental validation.
DOI: https://doi.org/10.37349/ent.2025.1004132
This article belongs to the special issue Natural Products in Neurotherapeutic Applications

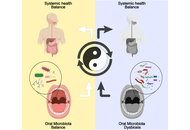
The oral microbiome has been increasingly implicated in the development and progression of neurological disorders. This narrative review synthesizes contemporary literature on alterations of oral microbial communities in Alzheimer’s disease, Parkinson’s disease, and migraine and evaluates their potential contribution to neuroinflammation and neurodegeneration. We first outline the core oral taxa that maintain microbial homeostasis and summarize evidence that patients with these neurological conditions exhibit dysbiosis characterized by reduced diversity and enrichment of periodontal pathogens. Proposed mechanisms include hematogenous or neural translocation of oral bacteria and their virulence factors, amplification of systemic inflammation, disruption of the blood-brain barrier, altered production of neuroactive metabolites, and bidirectional signaling along the ‘oral-gut-brain’ axis. On this mechanistic basis, microbiome-targeted strategies, particularly probiotics and fecal microbiota transplantation, have been explored as adjunctive approaches to restore microbial balance and potentially improve neurological outcomes, although available clinical data remain preliminary and heterogeneous. Current evidence is further limited by small samples, methodological variability in microbiome profiling, and a paucity of longitudinal and interventional studies, which hampers causal inference. Future research should adopt standardized sampling and multi-omic approaches and prioritize well-designed clinical trials to determine whether modulation of the oral microbiome can be translated into preventive or therapeutic strategies for neurological diseases.
The oral microbiome has been increasingly implicated in the development and progression of neurological disorders. This narrative review synthesizes contemporary literature on alterations of oral microbial communities in Alzheimer’s disease, Parkinson’s disease, and migraine and evaluates their potential contribution to neuroinflammation and neurodegeneration. We first outline the core oral taxa that maintain microbial homeostasis and summarize evidence that patients with these neurological conditions exhibit dysbiosis characterized by reduced diversity and enrichment of periodontal pathogens. Proposed mechanisms include hematogenous or neural translocation of oral bacteria and their virulence factors, amplification of systemic inflammation, disruption of the blood-brain barrier, altered production of neuroactive metabolites, and bidirectional signaling along the ‘oral-gut-brain’ axis. On this mechanistic basis, microbiome-targeted strategies, particularly probiotics and fecal microbiota transplantation, have been explored as adjunctive approaches to restore microbial balance and potentially improve neurological outcomes, although available clinical data remain preliminary and heterogeneous. Current evidence is further limited by small samples, methodological variability in microbiome profiling, and a paucity of longitudinal and interventional studies, which hampers causal inference. Future research should adopt standardized sampling and multi-omic approaches and prioritize well-designed clinical trials to determine whether modulation of the oral microbiome can be translated into preventive or therapeutic strategies for neurological diseases.
DOI: https://doi.org/10.37349/ent.2025.1004131
This article belongs to the special issue Role of Microbiota in Neurological Diseases

Apparent increases in autism and other forms of neurodivergence are often interpreted as a rise in incidence. Yet demographic expansion, diagnostic broadening, and growing cultural awareness all contribute to higher prevalence estimates. At the same time, contemporary sensory and digital environments have become increasingly overstimulating, characterized by persistent noise, visual saturation, hyperconnectivity, and unpredictable social rhythms. These conditions heighten sensory and cognitive load for many individuals, making neurodivergent traits more visible and increasing the urgency of diagnosis. Drawing on cognitive ecology, sensory neuroscience, and neuroaffirmative scholarship, this perspective proposes that neurodivergence can be understood as an adaptive response to environments that exceed nervous-system thresholds. Autistic regulatory behaviors—including withdrawal, shutdown, sensory avoidance, and monotropism-driven focus—may serve as mechanisms for maintaining coherence in overstimulating contexts. Interpreting neurodivergence as an ecological signal offers new pathways for public health, accessibility design, and social policy. It reframes autistic embodiment not as internal dysfunction but as meaningful information about the livability of contemporary environments.
Apparent increases in autism and other forms of neurodivergence are often interpreted as a rise in incidence. Yet demographic expansion, diagnostic broadening, and growing cultural awareness all contribute to higher prevalence estimates. At the same time, contemporary sensory and digital environments have become increasingly overstimulating, characterized by persistent noise, visual saturation, hyperconnectivity, and unpredictable social rhythms. These conditions heighten sensory and cognitive load for many individuals, making neurodivergent traits more visible and increasing the urgency of diagnosis. Drawing on cognitive ecology, sensory neuroscience, and neuroaffirmative scholarship, this perspective proposes that neurodivergence can be understood as an adaptive response to environments that exceed nervous-system thresholds. Autistic regulatory behaviors—including withdrawal, shutdown, sensory avoidance, and monotropism-driven focus—may serve as mechanisms for maintaining coherence in overstimulating contexts. Interpreting neurodivergence as an ecological signal offers new pathways for public health, accessibility design, and social policy. It reframes autistic embodiment not as internal dysfunction but as meaningful information about the livability of contemporary environments.
DOI: https://doi.org/10.37349/ent.2025.1004130

Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by motor and non-motor symptoms, in which gut microbiota alterations have emerged as a potential pathogenic factor, causing disruption of the brain-gut-microbiota (BGM) axis. Recent evidence supports the role of BGM axis disruption in enhancing neuroinflammation, alpha-synuclein (α-syn) aggregation, and dopaminergic neurodegeneration. Emerging therapeutic strategies targeting dysbiosis, such as probiotics and fecal microbiota transplantation (FMT), have become a new focus of investigation for PD treatment. Proposed mechanisms include modulation of immune responses, enhancement of intestinal barrier integrity, production of neuroactive metabolites such as short-chain fatty acids, and reduction of oxidative stress. This narrative review summarizes current evidence on probiotics as a therapeutic strategy in PD. By analyzing data from randomized controlled trials and preclinical studies, we highlight the beneficial effects of probiotics in improving motor and non-motor symptoms of PD, including constipation, depression, and anxiety. Strains such as Lactobacillus plantarum PS128 and Bifidobacterium animalis Probio-M8 show particular promise. Although probiotics have demonstrated a favorable safety profile and potential as an adjunctive therapy for PD, future research should focus on standardized protocols, biomarker identification, and exploration of combined microbiota-targeted strategies.
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by motor and non-motor symptoms, in which gut microbiota alterations have emerged as a potential pathogenic factor, causing disruption of the brain-gut-microbiota (BGM) axis. Recent evidence supports the role of BGM axis disruption in enhancing neuroinflammation, alpha-synuclein (α-syn) aggregation, and dopaminergic neurodegeneration. Emerging therapeutic strategies targeting dysbiosis, such as probiotics and fecal microbiota transplantation (FMT), have become a new focus of investigation for PD treatment. Proposed mechanisms include modulation of immune responses, enhancement of intestinal barrier integrity, production of neuroactive metabolites such as short-chain fatty acids, and reduction of oxidative stress. This narrative review summarizes current evidence on probiotics as a therapeutic strategy in PD. By analyzing data from randomized controlled trials and preclinical studies, we highlight the beneficial effects of probiotics in improving motor and non-motor symptoms of PD, including constipation, depression, and anxiety. Strains such as Lactobacillus plantarum PS128 and Bifidobacterium animalis Probio-M8 show particular promise. Although probiotics have demonstrated a favorable safety profile and potential as an adjunctive therapy for PD, future research should focus on standardized protocols, biomarker identification, and exploration of combined microbiota-targeted strategies.
DOI: https://doi.org/10.37349/ent.2025.1004129
This article belongs to the special issue Role of Microbiota in Neurological Diseases

Background:
Ischemic stroke is a leading cause of disability, with calcium (Ca2+) dysregulation contributing to neuronal injury and impaired recovery. While early clinical trials targeting calcium signaling showed limited success, growing preclinical evidence supports the potential of calcium modulation for long-term neuroprotection. This systematic review evaluates the long-term effects of calcium modulation in animal models of ischemic stroke.
Methods:
A comprehensive search across PubMed, Scopus, Web of Science, and the Cochrane Library up to June 2025 identified studies investigating calcium-targeted interventions (e.g., calcium channel blockers, chelators, antioxidants) with ≥ 30 days of follow-up. Risk of bias was assessed using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I).
Results:
Nine studies met the inclusion criteria. Interventions like L-type calcium channel blockers, magnesium sulfate, and ischemic preconditioning consistently reduced infarct volume (e.g., 22.4 ± 0.5% with preconditioning vs. 51.6 ± 2.1% with knockout) and improved neurobehavioral outcomes [e.g., epigallocatechin gallate (EGCG)-treated rats scored 2.17 ± 0.05 vs. 3.63 ± 0.06 in controls]. Molecular pathways involved included phosphoinositide 3-kinase (PI3K)/AKT, stromal interaction molecule 1 (STIM1)/ORAI1, and calcium-sensor proteins such as NCKX2.
Discussion:
Calcium modulation holds strong promise for neuroprotection in ischemic stroke models. Although clinical gaps remain, these findings support the development of calcium-targeted therapies for stroke recovery, especially when combined with multimodal strategies.
Background:
Ischemic stroke is a leading cause of disability, with calcium (Ca2+) dysregulation contributing to neuronal injury and impaired recovery. While early clinical trials targeting calcium signaling showed limited success, growing preclinical evidence supports the potential of calcium modulation for long-term neuroprotection. This systematic review evaluates the long-term effects of calcium modulation in animal models of ischemic stroke.
Methods:
A comprehensive search across PubMed, Scopus, Web of Science, and the Cochrane Library up to June 2025 identified studies investigating calcium-targeted interventions (e.g., calcium channel blockers, chelators, antioxidants) with ≥ 30 days of follow-up. Risk of bias was assessed using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I).
Results:
Nine studies met the inclusion criteria. Interventions like L-type calcium channel blockers, magnesium sulfate, and ischemic preconditioning consistently reduced infarct volume (e.g., 22.4 ± 0.5% with preconditioning vs. 51.6 ± 2.1% with knockout) and improved neurobehavioral outcomes [e.g., epigallocatechin gallate (EGCG)-treated rats scored 2.17 ± 0.05 vs. 3.63 ± 0.06 in controls]. Molecular pathways involved included phosphoinositide 3-kinase (PI3K)/AKT, stromal interaction molecule 1 (STIM1)/ORAI1, and calcium-sensor proteins such as NCKX2.
Discussion:
Calcium modulation holds strong promise for neuroprotection in ischemic stroke models. Although clinical gaps remain, these findings support the development of calcium-targeted therapies for stroke recovery, especially when combined with multimodal strategies.
DOI: https://doi.org/10.37349/ent.2025.1004127
This article belongs to the special issue Therapeutic Targets for Neuroprotection in Ischemic Stroke

Aim:
Alzheimer’s disease (AD) devastates learning and memory, the defining faculties of the human species. Extracellular amyloid beta (Aβ) deposits and intracellular hyperphosphorylated tau are hallmarks of AD pathology. The exact cause of the disease remains unknown, but a lot of data support AD to be a multifactorial disease. Given the central roles of oxidative stress and neuroinflammation in AD pathogenesis, apocynin, a potent antioxidant and anti-inflammatory agent, was selected for investigation. Apocynin is an aromatic ketone, a naturally occurring methoxy-substituted catechol known to possess numerous biological activities, namely anti-oxidant, anti-inflammatory, etc. The present study assessed apocynin’s potential against an Aβ1–42-induced sporadic AD rat model.
Methods:
In the present study, Wistar rats were subjected to intrahippocampal administration of 200 µmol/L of Aβ1–42 peptide in right hemisphere. Further were treated with apocynin 50, 150, and 300 mg/kg per orally for 28 days. The study examined the neurobehavioral aspects using the Barnes Maze test (BMT). Hippocampus was examined for the antioxidant (SOD, GSH, catalase, and LPO), inflammatory (TNF-α) parameters, RAGE, caspase-3, PGC-1α expression, and IHC analysis for Aβ load, adult hippocampal neurogenesis markers (BDNF, Ki67, DCX, NeuN), at the end of 28 days.
Results:
Apocynin administration demonstrated significant improvement in cognitive functions, diminished oxidative stress and inflammatory response triggered by Aβ administration. Apocynin additionally instigated adult hippocampal neurogenesis and triggered mitochondrial biogenesis.
Conclusions:
These primary results strongly advocate apocynin’s nootropic, neurotrophic and neuroprotective potential in an Aβ induced neurotoxicity in rats.
Aim:
Alzheimer’s disease (AD) devastates learning and memory, the defining faculties of the human species. Extracellular amyloid beta (Aβ) deposits and intracellular hyperphosphorylated tau are hallmarks of AD pathology. The exact cause of the disease remains unknown, but a lot of data support AD to be a multifactorial disease. Given the central roles of oxidative stress and neuroinflammation in AD pathogenesis, apocynin, a potent antioxidant and anti-inflammatory agent, was selected for investigation. Apocynin is an aromatic ketone, a naturally occurring methoxy-substituted catechol known to possess numerous biological activities, namely anti-oxidant, anti-inflammatory, etc. The present study assessed apocynin’s potential against an Aβ1–42-induced sporadic AD rat model.
Methods:
In the present study, Wistar rats were subjected to intrahippocampal administration of 200 µmol/L of Aβ1–42 peptide in right hemisphere. Further were treated with apocynin 50, 150, and 300 mg/kg per orally for 28 days. The study examined the neurobehavioral aspects using the Barnes Maze test (BMT). Hippocampus was examined for the antioxidant (SOD, GSH, catalase, and LPO), inflammatory (TNF-α) parameters, RAGE, caspase-3, PGC-1α expression, and IHC analysis for Aβ load, adult hippocampal neurogenesis markers (BDNF, Ki67, DCX, NeuN), at the end of 28 days.
Results:
Apocynin administration demonstrated significant improvement in cognitive functions, diminished oxidative stress and inflammatory response triggered by Aβ administration. Apocynin additionally instigated adult hippocampal neurogenesis and triggered mitochondrial biogenesis.
Conclusions:
These primary results strongly advocate apocynin’s nootropic, neurotrophic and neuroprotective potential in an Aβ induced neurotoxicity in rats.
DOI: https://doi.org/10.37349/ent.2025.1004128
This article belongs to the special issue Natural Products in Neurotherapeutic Applications

Non-traumatic arterial dissection exhibits a propensity for the Asian population and predominantly affects the posterior circulation. Regrettably, during the initial fortnight prior to diagnosis, approximately one in thirty cervicocephalic artery dissections (CAD) are misdiagnosed. Overlooked CAD, more prevalent in the younger demographic, can result in severe complications such as ischemic stroke, subarachnoid hemorrhage, and possibly death. Comprehensive investigations are necessary to prevent overlooking such a diagnosis. Digital subtraction angiography (DSA) is universally acknowledged as the most efficacious technique for assessing luminal morphology and hemodynamics, but may miss the vessel wall characteristics, an important component for diagnosing dissection. Magnetic resonance angiography (MRA), conversely, is less invasive and can assess vessel wall properties. A high-resolution MRA with vascular wall imaging can efficiently identify conditions such as intimal flaps, double-lumen signs, intramural hematomas, mural thrombi, and pseudoaneurysms, aiding in the evaluation of suspected CAD. MRA with vascular wall imaging and DSA complement each other in the identification and characterization of cerebral artery dissections, both contributing to treatment. In cases of undefined stroke etiology, particularly among the young demographic, utilizing both tests (when one yields no significant findings) may assist in detecting overlooked instances of CAD. The prompt identification and treatment of CAD are essential, particularly for surgical intervention and to avert recurrence in predisposed patients. Identifying the etiology of a stroke or transient ischemic attack is important for providing precise therapy and preventing recurrence.
Non-traumatic arterial dissection exhibits a propensity for the Asian population and predominantly affects the posterior circulation. Regrettably, during the initial fortnight prior to diagnosis, approximately one in thirty cervicocephalic artery dissections (CAD) are misdiagnosed. Overlooked CAD, more prevalent in the younger demographic, can result in severe complications such as ischemic stroke, subarachnoid hemorrhage, and possibly death. Comprehensive investigations are necessary to prevent overlooking such a diagnosis. Digital subtraction angiography (DSA) is universally acknowledged as the most efficacious technique for assessing luminal morphology and hemodynamics, but may miss the vessel wall characteristics, an important component for diagnosing dissection. Magnetic resonance angiography (MRA), conversely, is less invasive and can assess vessel wall properties. A high-resolution MRA with vascular wall imaging can efficiently identify conditions such as intimal flaps, double-lumen signs, intramural hematomas, mural thrombi, and pseudoaneurysms, aiding in the evaluation of suspected CAD. MRA with vascular wall imaging and DSA complement each other in the identification and characterization of cerebral artery dissections, both contributing to treatment. In cases of undefined stroke etiology, particularly among the young demographic, utilizing both tests (when one yields no significant findings) may assist in detecting overlooked instances of CAD. The prompt identification and treatment of CAD are essential, particularly for surgical intervention and to avert recurrence in predisposed patients. Identifying the etiology of a stroke or transient ischemic attack is important for providing precise therapy and preventing recurrence.
DOI: https://doi.org/10.37349/ent.2025.1004126

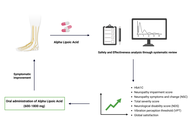
Background:
This study aims to assess oral alpha lipoic acid’s (ALA’s) safety and effectiveness in managing diabetic neuropathy.
Methods:
A thorough search of the literature was conducted using PubMed, Google Scholar, and Embase databases, and the study was performed as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results:
Based on inclusion and exclusion criteria, nine randomized controlled trials (RCTs) comprising 1,345 subjects were selected. The results showed that ALA has shown significant reduction in glycated hemoglobin (HbA1C) [inverse variance (IV): –0.66, (–0.81 → –0.51) at 95% CI, p < 0.00001, I2 = 92%], neuropathy impairment score (NIS) [IV: –1.19, (–2.28 → –0.09) at 95% CI, p = 0.03, I2 = 58%], along with improving the neuropathy symptoms and change (NSC) number [IV: –0.18, (–0.35 → –0.01) at 95% CI, p = 0.04, I2 = 0%], NSC severity score [IV: –0.65, (–0.83 → –0.48) at 95% CI, p < 0.00001, I2 = 89%], total severity score (TSS) [IV: –0.43, (–0.59 → –0.27) at 95% CI, p < 0.00001, I2 = 98%], neurological disability score (NDS) [IV: –0.72, (–1.03 → –0.40) at 95% CI, p < 0.00001, I2 = 98%], vibration perception threshold (VPT) [IV: –0.35, (–0.50 → –0.19) at 95% CI, p < 0.0001, I2 = 96%] and global satisfaction [Mantel-Haenszel (M-H) odds ratio (OR): 3.51, (1.06 → 11.61) at 95% CI, p = 0.04, I2 = 72%] when compared to the control or placebo group. However, ALA has not shown significant changes in motor nerve conduction velocity (MNCV) score [IV: 0.26, (–0.44 → 0.96) at 95% CI, p = 0.47, I2 = 49%] and NIS-low limb (NIS-LL) [IV: –0.58, (–1.35 → 0.19) at 95% CI, p = 0.14, I2 = 0%] when compared to the placebo group.
Discussion:
Based on the available moderate to high-quality evidence, we can conclude that oral administration of ALA at doses of 600–1,800 mg may be beneficial in improving/alleviating the symptoms resulting from diabetic neuropathy.
Background:
This study aims to assess oral alpha lipoic acid’s (ALA’s) safety and effectiveness in managing diabetic neuropathy.
Methods:
A thorough search of the literature was conducted using PubMed, Google Scholar, and Embase databases, and the study was performed as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results:
Based on inclusion and exclusion criteria, nine randomized controlled trials (RCTs) comprising 1,345 subjects were selected. The results showed that ALA has shown significant reduction in glycated hemoglobin (HbA1C) [inverse variance (IV): –0.66, (–0.81 → –0.51) at 95% CI, p < 0.00001, I2 = 92%], neuropathy impairment score (NIS) [IV: –1.19, (–2.28 → –0.09) at 95% CI, p = 0.03, I2 = 58%], along with improving the neuropathy symptoms and change (NSC) number [IV: –0.18, (–0.35 → –0.01) at 95% CI, p = 0.04, I2 = 0%], NSC severity score [IV: –0.65, (–0.83 → –0.48) at 95% CI, p < 0.00001, I2 = 89%], total severity score (TSS) [IV: –0.43, (–0.59 → –0.27) at 95% CI, p < 0.00001, I2 = 98%], neurological disability score (NDS) [IV: –0.72, (–1.03 → –0.40) at 95% CI, p < 0.00001, I2 = 98%], vibration perception threshold (VPT) [IV: –0.35, (–0.50 → –0.19) at 95% CI, p < 0.0001, I2 = 96%] and global satisfaction [Mantel-Haenszel (M-H) odds ratio (OR): 3.51, (1.06 → 11.61) at 95% CI, p = 0.04, I2 = 72%] when compared to the control or placebo group. However, ALA has not shown significant changes in motor nerve conduction velocity (MNCV) score [IV: 0.26, (–0.44 → 0.96) at 95% CI, p = 0.47, I2 = 49%] and NIS-low limb (NIS-LL) [IV: –0.58, (–1.35 → 0.19) at 95% CI, p = 0.14, I2 = 0%] when compared to the placebo group.
Discussion:
Based on the available moderate to high-quality evidence, we can conclude that oral administration of ALA at doses of 600–1,800 mg may be beneficial in improving/alleviating the symptoms resulting from diabetic neuropathy.
DOI: https://doi.org/10.37349/ent.2025.1004125
This article belongs to the special issue Natural Products in Neurotherapeutic Applications

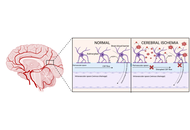
The glymphatic system (GS) consists of a paravascular fluid-exchange network that regulates cerebrospinal and interstitial fluid dynamics, clears metabolic waste, and modulates neuroinflammation. Aquaporin-4 (AQP-4), expressed in astrocytic end-feet, is central to GS function and blood-brain barrier integrity, but in cerebral ischemia (CI), GS disruption and AQP-4 mislocalization drive cytotoxic edema, inflammation, and vascular dysfunction, worsening outcomes. This review aimed to examine the role of the GS in CI, focusing on pathophysiology and potential therapeutic targets. A PubMed-based literature review was conducted, selecting 51 studies from 115 screened that addressed GS, AQP-4, and ischemic stroke. Evidence suggests that modulating GS flow, through strategies such as enhancing arterial pulsations or regulating AQP-4, may reduce edema and neuroinflammation, although selectively targeting AQP-4 without impairing waste clearance remains a key challenge. The GS represents a promising therapeutic target in ischemic stroke, and a deeper understanding of its physiology may guide the development of neuroprotective interventions; future research should refine pharmacological strategies to optimize glymphatic function and improve recovery in CI patients.
The glymphatic system (GS) consists of a paravascular fluid-exchange network that regulates cerebrospinal and interstitial fluid dynamics, clears metabolic waste, and modulates neuroinflammation. Aquaporin-4 (AQP-4), expressed in astrocytic end-feet, is central to GS function and blood-brain barrier integrity, but in cerebral ischemia (CI), GS disruption and AQP-4 mislocalization drive cytotoxic edema, inflammation, and vascular dysfunction, worsening outcomes. This review aimed to examine the role of the GS in CI, focusing on pathophysiology and potential therapeutic targets. A PubMed-based literature review was conducted, selecting 51 studies from 115 screened that addressed GS, AQP-4, and ischemic stroke. Evidence suggests that modulating GS flow, through strategies such as enhancing arterial pulsations or regulating AQP-4, may reduce edema and neuroinflammation, although selectively targeting AQP-4 without impairing waste clearance remains a key challenge. The GS represents a promising therapeutic target in ischemic stroke, and a deeper understanding of its physiology may guide the development of neuroprotective interventions; future research should refine pharmacological strategies to optimize glymphatic function and improve recovery in CI patients.
DOI: https://doi.org/10.37349/ent.2025.1004123
This article belongs to the special issue Therapeutic Targets for Neuroprotection in Ischemic Stroke

The increasing prevalence of neurodegenerative diseases (NDs), such as Alzheimer’s, Parkinson’s, Huntington’s, multiple sclerosis, and amyotrophic lateral sclerosis, represents a serious global public health issue. Consequently, the search for compounds with neuroprotective potential has intensified. In this context, resveratrol (RSV), a stilbene polyphenol found mainly in red grapes, exhibits important pharmacological properties, such as antioxidant and anti-inflammatory, and has been widely investigated in neuroscience due to its potential in the prevention and treatment of NDs. This narrative review was conducted using the PubMed® database, with the keywords “resveratrol”, “molecular mechanisms”, “mechanisms of action”, “neuroinflammation”, “oxidative stress”, “autophagy”, “gene regulation”, and “clinical studies”. This study discusses the molecular mechanisms of RSV on NDs, focusing on signaling pathways involved in neuroinflammation, oxidative stress, gene regulation, autophagy, and cell death. Intracellular pathways such as NF-κB, JAK/STAT, MAPK/ERK, PI3K/Akt, and Nrf2/Keap1 are associated with immune modulation mediated by RSV, leading to a decrease in oxidative stress, induction of autophagy, and inhibition of apoptosis. RSV has pharmacokinetic limitations, such as low bioavailability and stability, although RSV can cross the blood-brain barrier. Thus, researches involving nonencapsulated formulations aim to enhance their delivery to the central nervous system. Current in vitro and in vivo studies are promising, although further clinical trials are needed, as few have been conducted and available data remain preliminary. In conclusion, RSV presents multiple benefits to neurological health and shows therapeutic potential in NDs; however, additional clinical studies and translational research are essential to validate and optimize its application.
The increasing prevalence of neurodegenerative diseases (NDs), such as Alzheimer’s, Parkinson’s, Huntington’s, multiple sclerosis, and amyotrophic lateral sclerosis, represents a serious global public health issue. Consequently, the search for compounds with neuroprotective potential has intensified. In this context, resveratrol (RSV), a stilbene polyphenol found mainly in red grapes, exhibits important pharmacological properties, such as antioxidant and anti-inflammatory, and has been widely investigated in neuroscience due to its potential in the prevention and treatment of NDs. This narrative review was conducted using the PubMed® database, with the keywords “resveratrol”, “molecular mechanisms”, “mechanisms of action”, “neuroinflammation”, “oxidative stress”, “autophagy”, “gene regulation”, and “clinical studies”. This study discusses the molecular mechanisms of RSV on NDs, focusing on signaling pathways involved in neuroinflammation, oxidative stress, gene regulation, autophagy, and cell death. Intracellular pathways such as NF-κB, JAK/STAT, MAPK/ERK, PI3K/Akt, and Nrf2/Keap1 are associated with immune modulation mediated by RSV, leading to a decrease in oxidative stress, induction of autophagy, and inhibition of apoptosis. RSV has pharmacokinetic limitations, such as low bioavailability and stability, although RSV can cross the blood-brain barrier. Thus, researches involving nonencapsulated formulations aim to enhance their delivery to the central nervous system. Current in vitro and in vivo studies are promising, although further clinical trials are needed, as few have been conducted and available data remain preliminary. In conclusion, RSV presents multiple benefits to neurological health and shows therapeutic potential in NDs; however, additional clinical studies and translational research are essential to validate and optimize its application.
DOI: https://doi.org/10.37349/ent.2025.1004124
This article belongs to the special issue Neuro-Inflammation as a Target in the Design of Multifunctional Drug Candidates for Neurodegenerative Diseases

Aim:
Systemic inflammation is a key factor in cognitive decline and neurodegenerative diseases. Polyphenols, such as curcumin, resveratrol, and salidroside, exhibit neuroprotective effects, but their low bioavailability raises questions about their mechanism of action. The gut-brain axis, mediated by microbiome modulation, may play a critical role in their cognitive benefits. This study investigated whether polyphenols (curcumin, resveratrol, and salidroside) improve cognitive function in mice with lipopolysaccharide (LPS)-induced gut inflammation by modulating the gut microbiome and reducing neuroinflammation.
Methods:
C57BL/6 mice were divided into five groups: control, LPS, and LPS + polyphenol treatments (curcumin, resveratrol, or salidroside). LPS was administered intraperitoneally to induce inflammation, while polyphenols were given orally for three weeks. Cognitive performance was assessed using the Morris water maze. Gut microbiome composition (16S rRNA sequencing), mitochondrial DNA (mtDNA) damage, and gene expression in brain regions were analyzed.
Results:
LPS impaired spatial memory, but resveratrol and salidroside significantly mitigated these deficits. Polyphenols restored beneficial bacteria (e.g., Alloprevotella, Eubacterium) and suppressed pathogenic taxa (e.g., Peptostreptococcales). They also reduced pro-inflammatory markers in the cortex and hippocampus. Curcumin showed weaker effects. No significant mtDNA damage was detected.
Conclusions:
Polyphenols, particularly resveratrol and salidroside, improve cognition during systemic inflammation by remodeling the gut microbiome and attenuating neuroinflammation. These findings highlight the gut-brain axis as a therapeutic target for inflammation-driven cognitive disorders.
Aim:
Systemic inflammation is a key factor in cognitive decline and neurodegenerative diseases. Polyphenols, such as curcumin, resveratrol, and salidroside, exhibit neuroprotective effects, but their low bioavailability raises questions about their mechanism of action. The gut-brain axis, mediated by microbiome modulation, may play a critical role in their cognitive benefits. This study investigated whether polyphenols (curcumin, resveratrol, and salidroside) improve cognitive function in mice with lipopolysaccharide (LPS)-induced gut inflammation by modulating the gut microbiome and reducing neuroinflammation.
Methods:
C57BL/6 mice were divided into five groups: control, LPS, and LPS + polyphenol treatments (curcumin, resveratrol, or salidroside). LPS was administered intraperitoneally to induce inflammation, while polyphenols were given orally for three weeks. Cognitive performance was assessed using the Morris water maze. Gut microbiome composition (16S rRNA sequencing), mitochondrial DNA (mtDNA) damage, and gene expression in brain regions were analyzed.
Results:
LPS impaired spatial memory, but resveratrol and salidroside significantly mitigated these deficits. Polyphenols restored beneficial bacteria (e.g., Alloprevotella, Eubacterium) and suppressed pathogenic taxa (e.g., Peptostreptococcales). They also reduced pro-inflammatory markers in the cortex and hippocampus. Curcumin showed weaker effects. No significant mtDNA damage was detected.
Conclusions:
Polyphenols, particularly resveratrol and salidroside, improve cognition during systemic inflammation by remodeling the gut microbiome and attenuating neuroinflammation. These findings highlight the gut-brain axis as a therapeutic target for inflammation-driven cognitive disorders.
DOI: https://doi.org/10.37349/ent.2025.1004122
This article belongs to the special issue Natural Products in Neurotherapeutic Applications

The investigations pertaining to the effectiveness of natural products in novel drug development for the prevention of a myriad of neurodegenerative diseases are offering encouraging prospects in novel drug development. This review endeavors to offer a comprehensive insight into the neuroprotective effects of baicalein (BE) and baicalin (BI), bioactive flavonoids found in Scutellaria baicalensis, primarily from the perspective of Alzheimer’s disease (AD). It systematically encompasses the scientifically pertinent investigations on BE’s prospective benefits in AD models, highlighting its mechanistic approaches and impending therapeutic applications in the amelioration of AD. The multifaceted pharmacological interventions offered by these bioactives, including antioxidant, anti-inflammatory, and immunomodulation effects, reinforce the scientific evidence supporting them as promising candidates for anti-AD agents and for preventing and managing other allied neurodegenerative disorders. These findings suggest that BE and BI, along with other nutraceuticals, may offer a valuable therapeutic strategy for improving symptoms and slowing disease progression in neurodegenerative disorders. Thus, the review intends to offer comprehensive illustrations warranting further investigation to corroborate the safety and efficacy of these bioactives in clinical settings. The researchers are progressively entrusting nature’s own compounds for the treatment of neurodegeneration. Conclusively, this manuscript could aptly serve as an insight to embark upon the remarkable pharmacological actions of these bioactives, which might be harnessed to prevent and manage AD. Nevertheless, the findings so far are promising; still, further investigations are incumbent to establish their safety and efficacy in humans, as BE and BI may offer novel modalities to circumvent this devastating disease.
The investigations pertaining to the effectiveness of natural products in novel drug development for the prevention of a myriad of neurodegenerative diseases are offering encouraging prospects in novel drug development. This review endeavors to offer a comprehensive insight into the neuroprotective effects of baicalein (BE) and baicalin (BI), bioactive flavonoids found in Scutellaria baicalensis, primarily from the perspective of Alzheimer’s disease (AD). It systematically encompasses the scientifically pertinent investigations on BE’s prospective benefits in AD models, highlighting its mechanistic approaches and impending therapeutic applications in the amelioration of AD. The multifaceted pharmacological interventions offered by these bioactives, including antioxidant, anti-inflammatory, and immunomodulation effects, reinforce the scientific evidence supporting them as promising candidates for anti-AD agents and for preventing and managing other allied neurodegenerative disorders. These findings suggest that BE and BI, along with other nutraceuticals, may offer a valuable therapeutic strategy for improving symptoms and slowing disease progression in neurodegenerative disorders. Thus, the review intends to offer comprehensive illustrations warranting further investigation to corroborate the safety and efficacy of these bioactives in clinical settings. The researchers are progressively entrusting nature’s own compounds for the treatment of neurodegeneration. Conclusively, this manuscript could aptly serve as an insight to embark upon the remarkable pharmacological actions of these bioactives, which might be harnessed to prevent and manage AD. Nevertheless, the findings so far are promising; still, further investigations are incumbent to establish their safety and efficacy in humans, as BE and BI may offer novel modalities to circumvent this devastating disease.
DOI: https://doi.org/10.37349/ent.2025.1004121
This article belongs to the special issue Natural Products in Neurotherapeutic Applications

Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline, neuroinflammation, and accumulation of amyloid-beta plaques and tau tangles. Emerging research emphasizes the gut-brain axis as a key modulator of AD pathogenesis, with gut microbiota influencing neuroimmune, neurochemical, and metabolic pathways. This review examines the therapeutic and preventive potential of probiotics, live beneficial microorganisms, in modulating the gut-brain axis to mitigate AD progression. Modifying gut microbiota presents a novel, potentially modifiable approach to influence AD pathophysiology and improve cognitive outcomes, offering insights for adjunctive clinical strategies. A systematic literature search was conducted across PubMed, Scopus, Web of Science, Google Scholar, and Cochrane Library for studies published up to July 2025. Studies were classified by design, sample size, follow-up duration, cognitive and biomarker outcomes, and risk of bias, following Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines to ensure transparency and reproducibility. Preclinical studies indicate that probiotics can regulate gut microbiota, reduce oxidative stress, suppress neuroinflammation, and enhance synaptic plasticity, improving cognition in animal models. Clinical trials suggest potential benefits in humans, including improved memory scores and reduced inflammatory biomarkers, though limited sample sizes, trial duration, and strain variability constrain conclusions. Overall, probiotics demonstrate promise as an adjunctive intervention in AD. Further long-term, strain-specific, and large-scale clinical studies are needed to confirm efficacy, establish causality, and optimize therapeutic strategies.
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline, neuroinflammation, and accumulation of amyloid-beta plaques and tau tangles. Emerging research emphasizes the gut-brain axis as a key modulator of AD pathogenesis, with gut microbiota influencing neuroimmune, neurochemical, and metabolic pathways. This review examines the therapeutic and preventive potential of probiotics, live beneficial microorganisms, in modulating the gut-brain axis to mitigate AD progression. Modifying gut microbiota presents a novel, potentially modifiable approach to influence AD pathophysiology and improve cognitive outcomes, offering insights for adjunctive clinical strategies. A systematic literature search was conducted across PubMed, Scopus, Web of Science, Google Scholar, and Cochrane Library for studies published up to July 2025. Studies were classified by design, sample size, follow-up duration, cognitive and biomarker outcomes, and risk of bias, following Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines to ensure transparency and reproducibility. Preclinical studies indicate that probiotics can regulate gut microbiota, reduce oxidative stress, suppress neuroinflammation, and enhance synaptic plasticity, improving cognition in animal models. Clinical trials suggest potential benefits in humans, including improved memory scores and reduced inflammatory biomarkers, though limited sample sizes, trial duration, and strain variability constrain conclusions. Overall, probiotics demonstrate promise as an adjunctive intervention in AD. Further long-term, strain-specific, and large-scale clinical studies are needed to confirm efficacy, establish causality, and optimize therapeutic strategies.
DOI: https://doi.org/10.37349/ent.2025.1004120
This article belongs to the special issue Role of Microbiota in Neurological Diseases

Parkinson’s disease (PD) is a devastating neurodegenerative condition characterized primarily by the degeneration of the dopaminergic neurons in the substantia nigra, causing motor dysfunction and many non-motor symptoms. Available pharmacological treatments and therapies provide symptomatic relief but do not halt the progression of PD. Gene therapy has been recognized as a valuable therapeutic frontier, providing the possibility of disease modification by targeting the underlying molecular and cellular mechanisms of PD. The parts of the methodology used for gene therapy entail the delivery of genetic material into particular regions of the brain with the aid of viral vectors to improve the synthesis of dopamine, maintain the integrity of neurons, or control pathological pathways. Recent clinical trials have shown promising efficacy and safety profiles for many gene therapy methods, consisting of those targeting enzymes in the biosynthesis of dopamine [e.g., L-amino acid decarboxylase (AADC)], synuclein alpha pathology, and neurotrophic factors [e.g., growth-derived neurotrophic factor (GDNF)]. However, in spite of these developments, there are limitations in vector delivery and prolonged expression of genes, as well as patient-specific responses. This review highlights the present landscape of gene therapy in PD, discussing the latest successes, ongoing clinical trials, and future perspectives that could shape therapeutic paradigms for PD.
Parkinson’s disease (PD) is a devastating neurodegenerative condition characterized primarily by the degeneration of the dopaminergic neurons in the substantia nigra, causing motor dysfunction and many non-motor symptoms. Available pharmacological treatments and therapies provide symptomatic relief but do not halt the progression of PD. Gene therapy has been recognized as a valuable therapeutic frontier, providing the possibility of disease modification by targeting the underlying molecular and cellular mechanisms of PD. The parts of the methodology used for gene therapy entail the delivery of genetic material into particular regions of the brain with the aid of viral vectors to improve the synthesis of dopamine, maintain the integrity of neurons, or control pathological pathways. Recent clinical trials have shown promising efficacy and safety profiles for many gene therapy methods, consisting of those targeting enzymes in the biosynthesis of dopamine [e.g., L-amino acid decarboxylase (AADC)], synuclein alpha pathology, and neurotrophic factors [e.g., growth-derived neurotrophic factor (GDNF)]. However, in spite of these developments, there are limitations in vector delivery and prolonged expression of genes, as well as patient-specific responses. This review highlights the present landscape of gene therapy in PD, discussing the latest successes, ongoing clinical trials, and future perspectives that could shape therapeutic paradigms for PD.
DOI: https://doi.org/10.37349/ent.2025.1004119
 Previous
Previous