Affiliation:
1Department of Pharmacognosy, University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh 160014, India
ORCID: https://orcid.org/0000-0001-9890-8428
Affiliation:
1Department of Pharmacognosy, University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh 160014, India
ORCID: https://orcid.org/0000-0001-8908-5255
Affiliation:
2Independent Researcher, Chandigarh 160047, India
Email: pharmsoniapahuja2020@gmail.com
ORCID: https://orcid.org/0000-0001-7346-0938
Explor Neuroprot Ther. 2025;5:1004121 DOI: https://doi.org/10.37349/ent.2025.1004121
Received: July 25, 2025 Accepted: September 28, 2025 Published: November 12, 2025
Academic Editor: Marcello Iriti, Università degli Studi di Milano, Italy
The article belongs to the special issue Natural Products in Neurotherapeutic Applications
The investigations pertaining to the effectiveness of natural products in novel drug development for the prevention of a myriad of neurodegenerative diseases are offering encouraging prospects in novel drug development. This review endeavors to offer a comprehensive insight into the neuroprotective effects of baicalein (BE) and baicalin (BI), bioactive flavonoids found in Scutellaria baicalensis, primarily from the perspective of Alzheimer’s disease (AD). It systematically encompasses the scientifically pertinent investigations on BE’s prospective benefits in AD models, highlighting its mechanistic approaches and impending therapeutic applications in the amelioration of AD. The multifaceted pharmacological interventions offered by these bioactives, including antioxidant, anti-inflammatory, and immunomodulation effects, reinforce the scientific evidence supporting them as promising candidates for anti-AD agents and for preventing and managing other allied neurodegenerative disorders. These findings suggest that BE and BI, along with other nutraceuticals, may offer a valuable therapeutic strategy for improving symptoms and slowing disease progression in neurodegenerative disorders. Thus, the review intends to offer comprehensive illustrations warranting further investigation to corroborate the safety and efficacy of these bioactives in clinical settings. The researchers are progressively entrusting nature’s own compounds for the treatment of neurodegeneration. Conclusively, this manuscript could aptly serve as an insight to embark upon the remarkable pharmacological actions of these bioactives, which might be harnessed to prevent and manage AD. Nevertheless, the findings so far are promising; still, further investigations are incumbent to establish their safety and efficacy in humans, as BE and BI may offer novel modalities to circumvent this devastating disease.
Dementia and other neurodegenerative diseases represent a rapidly escalating global health crisis. The number of people with dementia is projected to soar from 57 million in 2021 to 81.1 million by 2040 and ultimately to 139 million by 2050. Dementia is a leading cause of disability and death, disproportionately affecting women. As the most prevalent form of dementia, Alzheimer’s disease (AD) cases are expected to nearly double in the U.S. by 2060. This crisis carries an immense economic burden, with global costs projected to reach $2.8 trillion by 2030. Despite the scale, persistent stigma leads many to view dementia as a normal part of aging, hindering crucial early diagnosis and support [1–5].
AD is a complex brain disorder first documented in 1906. While decades of research have led to eight FDA-approved drugs, with the efficacy of recent antibody-based drugs still debated [6, 7], a cure remains elusive. Current treatments have limited efficacy, often due to the difficulty of drugs to cross the blood-brain barrier (BBB) and also due to their significant side effects [8, 9]. The disease’s pathology is multifaceted, with several leading hypotheses (Figure 1). The amyloid hypothesis suggests that plaques formed by amyloid-beta (Aβ) proteins are a hallmark cause [10, 11]. The tau hyperphosphorylation hypothesis indicates that neurofibrillary tangles (NFTs) disrupt brain cell function [12]. The cholinergic hypothesis links AD to a deficiency in acetylcholine, a neurotransmitter crucial for memory [13]. Additionally, factors like neuroinflammation [14], mitochondrial dysfunction [15], oxidative stress [16], vascular dysfunction [17], insulin signaling abnormalities [18], cholesterol [19], cell cycle deregulation [20], and gut microbiota dysbiosis [21, 22] are also being explored. Genetically, mutations in the PSEN genes are also known to cause the disease [23], which is clinically defined by a progressive decline in memory, cognitive function, emotional changes, and even psychiatric symptoms [24].
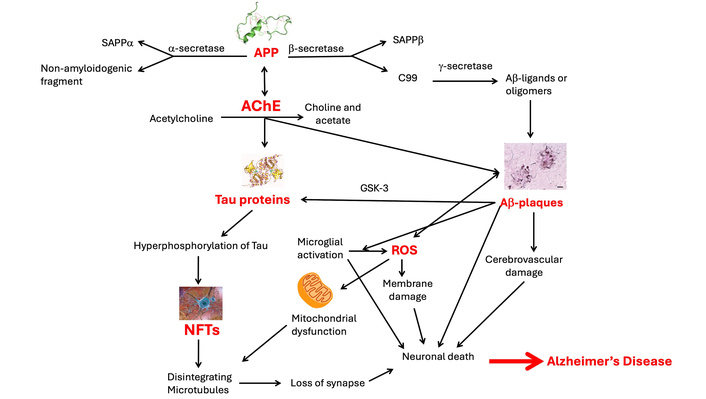
Pathological pathways of Alzheimer’s disease. APP: amyloid precursor protein; AChE: acetylcholinesterase; Aβ: amyloid-beta; ROS: reactive oxygen species; NFTs: neurofibrillary tangles. Adapted with permission from [142]. © 2025 Springer Nature. APP icon reprinted from https://en.wikipedia.org/wiki/Amyloid_beta. Accessed July 22, 2025. CC BY-SA 3.0. Tau protein icon reprinted from https://en.wikipedia.org/wiki/Tau-protein_kinase. Accessed July 22, 2025. CC BY-SA 4.0. Aβ-plaques icon reprinted from https://en.wikipedia.org/wiki/File:Neuritic_Abeta_plaques_stained_with_NF-PAS;_Bar%3D20_microns.jpg. Accessed July 22, 2025. CC BY-SA 4.0.
Current research seeks safe, effective therapies for AD. Oxidative stress is a key factor linking various pathogenic mechanisms and thus leading to the exploration of natural flavonoids with antioxidant properties, a focus of study. These compounds offer promising outcomes by inhibiting Aβ aggregation, tau hyperphosphorylation, and promoting mitochondrial autophagy [25]. Complementary and alternative medicine (CAM) is also gaining attention for preventing neurodegenerative diseases. However, more research, including advances in genomics and proteomics, is needed to validate their quality, safety, and efficacy, and to understand their mechanisms and potential drug interactions [26, 27].
This narrative review is an attempt to provide an insight into the neuroprotective effects of baicalein (BE) and baicalin (BI), bioflavones obtained from Scutellaria baicalensis. It compiles current research on their neuroprotective mechanisms offered by both bioactive components. The search strategy for this review encompassed a systematic approach wherein databases such as PubMed, Web of Science, Scopus, Science Direct, Embase, Medline, Cochrane Library, and ClinicalTrials.gov were searched from inception to June 2025. The keywords and terms used included “baicalein,” “baicalin,” “Scutellaria,” “Scutellaria baicalensis,” “Alzheimer’s disease,” “neuroprotection,” “amyloid-beta,” “tau pathology,” “oxidative stress,” and “neuroinflammation”. The eligible studies included peer-reviewed research, review articles, book chapters, and books, including in vitro, in vivo, and clinical research in English focusing on BI/BE in AD or related mechanisms, while case reports, conference abstracts, and studies with drug combinations were mainly excluded, except for a few remarkable ones. Initially, 256 articles were selected, and finally, 170 were used in the present article. The authors have interpreted the scientific evidence, which supports the establishment of both components as a potential and effective therapy for AD, and advocate for further clinical investigations to confirm their safety and efficacy.
BI (7.98%) and BE (0.1–1.5%) are flavonoids found in the roots of the Chinese herb Scutellaria baicalensis Georgi (Huangqin or Ogon), a plant grown in several European countries and native to several East Asian countries, including the Russian Federation [28–31]. These compounds are also present in other Scutellaria species, including S. lateriflora, S. galericulata, and S. rivularia Wall, Oroxylum indicum (L.), the fungus Trametes versicolor, Astragalus membranaceus, and Taxus chinensis fruit (TCF) [28, 32–42].
BI (BE-7-O-β-D-glucuronic acid) must first be hydrolyzed by gut microbiota’s β-glucuronidase enzymes to yield BE for absorption. This conversion is a crucial, rate-limiting step [43, 44]. After absorption, BE can be reconverted to BI in the liver, leading to enterohepatic circulation that prolongs its effects [45]. BE (C15H10O5), the aglycone form derived from chrysin, has a unique molecular structure that gives it diverse biological activities [43] (Figure 2). While highly permeable, its poor aqueous solubility and rapid, extensive metabolism through glucuronidation limit its oral bioavailability (13–23% in monkeys). It is quickly converted to BI and other conjugated metabolites in the liver and intestines, meaning that oral BE administration results in low concentrations of the parent compound in the blood [46]. It has shown no toxic effects in human clinical trials at doses up to 2,800 mg [47]. Thus, key differences in the chemical structure of both moieties and the way they are processed by the body impart them distinct pharmacological profiles, which have significant implications for human therapy [48]. The distinct metabolic pathways of these two compounds have significant clinical implications. BI’s effectiveness depends heavily on an individual’s gut microbiome composition for its conversion to BE [49]. Conversely, BE’s poor bioavailability makes it less ideal for oral delivery, prompting research into advanced delivery systems to improve its absorption and therapeutic potential. The interplay between these two forms and their metabolism is vital to comprehend their neuroprotective and other pharmacological roles. Thus, for a detailed PK/PD overview of both moieties, a comparative table of PK/PD is provided in Table S1.
Experimental models for AD provide a comprehensive framework for drug development and are crucial for understanding its mechanisms. These are categorized into three types (Figure 3).
In vitro models use cell lines like neuroblastoma (SHSY-5Y) [50–53], neuroblast (N2a), microglia (SIM-A9) [54], along with yeast cells [55, 56]. These in vitro models, such as cell lines and yeast, are cost-effective for high-throughput screening [56] and identifying therapeutic targets [50, 51]. However, they lack a full physiological context, failing to replicate the brain’s complex environment.
In vivo models, primarily transgenic mice (e.g., APP/PS1, 3Tg-AD) [57–61] and zebra fish [62], are used to replicate core AD pathologies like Aβ plaques and tau tangles and cognitive decline [57, 58], and allow for investigation of systemic effects like neuroinflammation [59–61]. Their limitations include biological differences from humans and the fact that they often require genetic engineering to show pathology, potentially leading to translational failures [59].
In silico models utilize computational tools to simulate molecular interactions and predict drug efficacy [63–68]. These models tend to provide versatile and cost-effective simulations of AD pathology and can accelerate drug discovery by screening compounds and predicting disease progression [64, 67], and simulate processes like Aβ aggregation [63, 65] and whole-brain dynamics [68]. However, their accuracy depends on the quality of existing data and requires experimental validation [63].
Since no single model can fully capture the complexity of AD, a multi-model approach is essential. Combining in vitro, in vivo, and in silico methods allows researchers to overcome individual limitations, creating a more comprehensive understanding of the disease and increasing the potential for successful treatment development.
BE and BI, prominent bioflavonoids primarily derived from Scutellaria baicalensis (commonly known as Chinese skullcap or Huang-Qin), exhibit a wide array of pharmacological properties. These compounds are promising candidates for treating and preventing various chronic ailments. Their extensively investigated properties include anti-cancer activities, liver protection (hepatoprotection), broad-spectrum antibacterial and antiviral effects, powerful antioxidant capabilities, and significant anticonvulsant and neuroprotective benefits [29, 49, 69–73]. They have been extensively utilized in traditional medicine, particularly in China and South Korea, for anti-inflammatory and cancer disorders [74, 75].
Both BE and BI exhibit similar pharmacological effects, but with differences in potency and specific mechanisms [76]. BE is often considered more potent in certain contexts, such as inhibiting inflammatory mediators like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) [77]. In terms of anticancer activity, both compounds induce apoptosis and inhibit proliferation, but their specific efficacy can vary depending on the cancer type and the signaling pathways involved. Some research suggests BE has a stronger antiproliferative effect on cancer cells [78]. These differences have significant implications for human therapy. BE’s higher potency could make it a better choice for conditions requiring a rapid, strong anti-inflammatory response, provided its low bioavailability is addressed. Conversely, the enterohepatic circulation of BI and its metabolites might be more suitable for therapeutic applications requiring sustained, long-term effects. The choice between the two, or using a combination, must be tailored to the specific disease and desired biological outcome [79].
It is interesting to note that BE and BI achieve their dual neuroprotective and anticancer effects by modulating a common set of critical signaling pathways (PI3K/AkT, NF-κB, Nrf2, MAPK) as given in Figure 4. Their distinct outcomes are determined by the cellular context; they can be anti-inflammatory and cytoprotective in normal and neuronal cells, but pro-apoptotic and antiproliferative in cancer cells. The difference in their chemical structure and metabolism also impacts their potency, with BE often showing more potent effects in direct cellular assays due to its higher bioavailability.
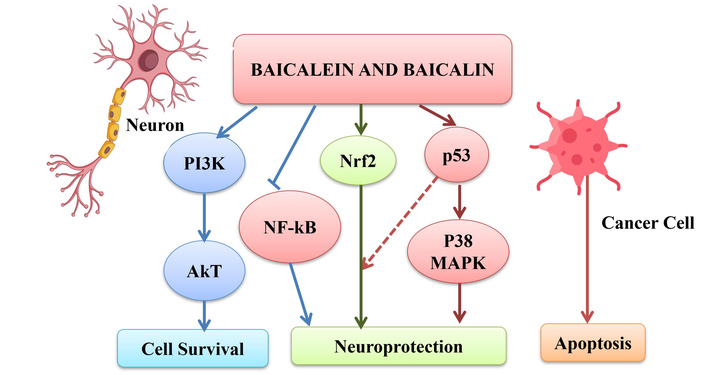
Dual role of baicalein and baicalin in neuroprotection and cancer. The following subsections provide selected literature reports mentioning various other actions of baicalein and baicalin. Icons are designed by Freepik (http://www.freepik.com/).
BE and BI have shown efficacy in various inflammatory conditions, including respiratory ailments like pulmonary fibrosis and pulmonary artery hypertension, by influencing pathways such as TGF-β/smad, ERK1/2, P38 MAPK, and NF-κB. Their anti-inflammatory action also extends to arthritis (reducing joint swelling and inhibiting inflammatory cascades), type-2 diabetes and obesity (reducing body weight, fatty acids, and cholesterol through AMPK activation), neurodegenerative diseases (protecting against neurotoxicity and reducing apoptosis), and microbial infections (suppressing inflammatory markers) [80].
Both flavonoids BE and BI induce programmed cell death (apoptosis) in various tumor types via both extrinsic and intrinsic pathways, triggering apoptosis by influencing calcium influx, reactive oxygen species (ROS) production, and the activation of caspases. They also suppress cancer metastasis by inhibiting epithelial-mesenchymal transition (EMT), down-regulating matrix metalloproteinases (MMPs), and interfering with angiogenesis. Additionally, they can induce autophagy and cause cell cycle arrest at different checkpoints, further contributing to their anticancer properties [80].
According to [81], BI can inhibit Aβ-induced microglial activation by regulating the JAK2/STAT3 signaling pathway. Furthermore, Ding et al. (2019) [82] studied BI’s effects in an AD rat model, where they found that it has anti-apoptotic effects by regulating mitochondrial membrane potential, the Bax/Bcl-2 ratio, cytochrome-c release, and caspase-9/-3 activation. The researchers also reported that BI enhances antioxidant capacity by restoring the activity and gene expression of key antioxidant enzymes, an effect associated with Nrf2 activation [73]. These findings, along with recent research on mitochondria, highlight BI’s potential in treating Aβ toxicity [83].
A team of researchers investigated BE’s protective effects on PC12 cells exposed to Aβ25–35, a toxic amyloid peptide associated with AD. It inhibited Aβ aggregation, reduced apoptosis, and restored mitochondrial function by improving membrane potential, ATP levels, and mitochondrial complex I activity. It also decreased intracellular ROS and NO levels, indicating strong antioxidant activity. LC-MS metabolomics identified BE-induced regulation of five metabolites linked to arginine/proline and nicotinate/nicotinamide pathways. These metabolic corrections underline BE’s ability to modulate energy metabolism and oxidative balance. The study confirmed BE’s multifaceted neuroprotection through anti-apoptotic and metabolic regulatory mechanisms [84].
A study explored the role of BE in protecting rat cortical neurons from Aβ25–35-induced apoptosis. BE, a 12-lipoxygenase inhibitor, significantly reduced neuronal cell death and suppressed c-jun protein overexpression, a key regulator in apoptosis pathways. Interestingly, other lipoxygenase inhibitors, including nordihydroguaiaretic acid and caffeic acid, showed no protective effect. These results suggested that 12-lipoxygenase plays a critical role in Aβ-induced neurotoxicity via the c-jun-dependent pathway, and selective inhibition by BE may offer a therapeutic strategy in AD. This work highlighted the significance of lipid-mediated oxidative pathways in Aβ-induced neuronal damage [85].
A study revealed for the first time that BI lowers blood pressure by relaxing blood vessels through its ability to regulate intracellular calcium (Ca2+) and was found to be partially dependent on the activation of KATP channels. These findings provide new scientific support for BI’s potential as a therapeutic agent for hypertension, validating its long-standing use in traditional Chinese medicine (TCM) [86].
Both BE and its metabolite BI demonstrate promising anti-SARS-CoV-2 and anti-inflammatory effects. They show antiviral action, primarily by inhibiting crucial SARS-CoV-2 enzymes like the 3C-like protease (3CLpro/Mpro) and RNA-dependent RNA polymerase (RdRp), which are essential for viral replication. They may also interfere with viral entry into host cells by affecting the spike protein-ACE2 receptor interaction. Regarding anti-inflammatory properties, these compounds suppress the NF-κB signaling pathway, a key regulator of inflammation, leading to reduced production of pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6) and mediators (e.g., NO, PGE2). They also exhibit antioxidant activity, modulate immune cell polarization towards a neuroprotective state (M2 phenotype), and can regulate the NLRP3 inflammasome, which triggers strong inflammatory responses. Overall, their multi-targeted mechanisms highlight BE and BI as valuable candidates for further research in treating viral infections and inflammatory conditions like COVID-19 [87].
A review explored recent advancements in understanding BE’s hepatoprotective action against different toxicities, both in vitro and in vivo models (acetaminophen, cisplatin, doxorubicin, CCL4, monocrotaline, and d-galactosamine), and concluded that it exhibited hepatoprotective potential due to its antioxidant, anti-inflammatory, and anti-apoptotic properties [88].
In another study, mice treated with BE with type-2 diabetes improved glucose tolerance and blood insulin levels. BE (5 mM) also advocated viability and enhanced glucose-stimulated insulin secretion in both insulin-secreting pancreatic cells and islets’ human cells [80].
BE showed promise for treating neurodegenerative disorders beyond AD. Studies demonstrated its ability to improve cognitive function by reducing oxidative stress and inflammation. It worked by influencing key cellular pathways, such as the PI3K/AkT/NF-κB signaling. BE also protected neurons and reduced levels of proteins associated with cell death and neurodegeneration. Its neuroprotective effects were independent of estrogenic activity, making it a strong candidate for new drug development [89–91].
BI’s neuroprotective mechanism is multifaceted, involving a range of biological effects. It acts as a powerful antioxidant and anti-inflammatory agent, which helps protect the brain from damage caused by oxidative stress and chronic inflammation. BI also exhibited antiapoptotic effects, preventing programmed cell death in neurons [92]. It further supported brain health by upregulating neurotrophic factors and providing mitochondrial protection, which enhanced cellular energy production and survival [92–94]. Additionally, BI promoted vasodilation and improved cerebral blood flow, contributing to its effectiveness in conditions like ischemic stroke [92]. Its interaction with the gut-brain axis also played a role in its broad therapeutic benefits for neurological well-being [95]. These combined actions underscore its potential as a therapeutic agent for various neurological and neuropsychiatric disorders [83, 94].
Several clinical trials have demonstrated the potential of BE and BI in different conditions, with the following key findings. Pang and co-workers [96] conducted two Phase I clinical trials on BE, which showed that oral BE tablets were safe and well-tolerated in healthy Chinese adults. A study by Hang et al. [97] found that BI reduced blood lipids and inflammation in patients with coronary heart disease and rheumatoid arthritis. Yet another finding by scientists indicated that BI affects innate immunity and apoptosis in children with acute lymphocytic leukemia [98] while Yu et al. [99] demonstrated that BI can balance immune function and ease inflammation in patients with ulcerative colitis. Additionally, BI has been studied for its use in patients with photodamaged skin [100] and in non-surgical periodontal therapy and post-surgical tooth removal [101, 102]. While these studies highlight the therapeutic promise of BE and BI, there is a notable absence of targeted clinical research specifically investigating their effects on neurodegenerative disorders.
To advance their potential, future efforts should concentrate on:
Clinical trials for neurodegenerative diseases: Conducting trials to assess the efficacy of BI and BE specifically in patients with neurodegenerative diseases.
Mechanism of action: Further research is needed to identify their precise molecular targets or receptors, which is crucial for understanding how they work, improving drug design, and advancing their development.
Delivery systems: Due to the limited solubility of BI and BE, innovative delivery methods like nanoparticles will be essential to enhance their bioactivity, improve their ability to cross the BBB, and ultimately increase their effectiveness [71].
Our understanding of AD evolved to recognize it as a complex, multifactorial disorder, and the need for therapeutic agents that could simultaneously target multiple pathological pathways became paramount. BE and BI emerged as promising candidates in this regard, exhibiting broad neuroprotective effects against AD through a wide array of mechanisms (Figure 5). Preclinical studies consistently showed that these compounds ameliorated memory deficits, reduced amyloid plaques, and modulated tau phosphorylation in various AD models (Table 1). Both BE and BI improved mitochondrial function, synaptic plasticity, and neuronal survival, ultimately preventing cell death and enhancing cognition [103–112].
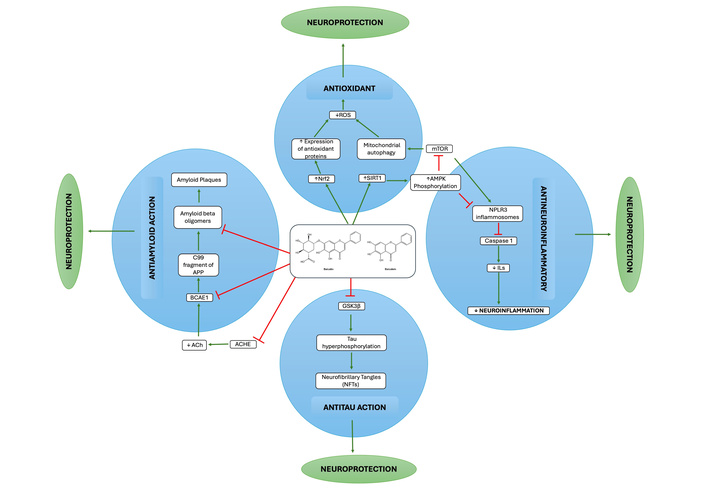
Neuroprotective mechanism of baicalein and baicalin in Alzheimer’s disease. ROS: reactive oxygen species; APP: amyloid precursor protein; ACHE: acetylcholinesterase; ILs: interleukins.
Recent research leveraging advanced modalities such as network pharmacology, bioinformatics, molecular simulations, and metabolomics further elucidated the multifaceted mechanisms through which these flavonoids exert their neuroprotective effects. The following subsections present a comprehensive detail of the various investigations that illuminated the multifaceted neuroprotective mechanisms and effects of BE and BI, incorporating the key findings from literature reports.
Aβ accumulation is a central event in AD progression. BE and BI demonstrated significant potential in inhibiting Aβ aggregation and mitigating its toxicity. A study utilizing molecular dynamics simulations provided valuable insights into how BE disrupts the early-stage, beta-sheet-rich structure of Aβ42 protofibrils, a key therapeutic strategy for preventing the formation of toxic oligomers [113]. Furthermore, BE showed protection against membrane damage induced by Aβ42 oligomers, a critical step in the neurodegenerative cascade [114, 115].
A study also supported BI’s therapeutic potential to mitigate Aβ-induced neuronal cytotoxicity. BI, identified as a novel Aβ aggregation inhibitor, protected SH-SY5Y cells by both inhibiting Aβ1–42 aggregation and reducing hydrogen peroxide (H2O2)-mediated oxidative stress. Recognizing that metal ions (e.g., copper) contributed to Aβ aggregation and ROS production in AD, the study found BI directly interacts with copper and inhibits Aβ1–42 aggregation, both with and without copper [51].
Beyond its direct effects on Aβ structure, BE is also a potent dual-target inhibitor. It can inhibit both Aβ1–42 aggregation and acetylcholinesterase (AChE) activity, a mechanism that addresses two major aspects of AD pathology [111]. This dual-action approach has inspired the development of novel compounds, such as a hybrid of BE and donepezil, which demonstrated superior inhibitory effects on AChE and better BBB penetration than either parent compound [109]. Another study highlighted BE’s dual action against protein aggregation, specifically involving both Aβ42 and alpha-synuclein (α-syn). It not only prevented the formation of new α-syn fibrils but could also disassemble existing mature α-syn fibrils in a dose-dependent manner. This could be partly attributed to a covalent modification of α-syn by BE quinone, a derivative of BE, which formed a Schiff base with a lysine side chain [51, 116].
The presence of α-syn pathology has been reported in a significant portion of AD cases, highlighting a potential overlap between AD and other neurodegenerative diseases [117–121]. Therefore, BE’s ability to inhibit both Aβ and α-syn aggregation positioned it as a promising therapeutic agent for addressing the complex proteinopathy often observed in AD patients [122].
Tau protein hyperphosphorylation and subsequent aggregation into NFTs is another hallmark of AD. In vitro techniques showed that BE exhibited significant inhibitory properties against repeat tau aggregation by dissolving preformed tau oligomers and mature fibrils [123]. Similarly, BI was found to inhibit paired helical filaments (PHFs) and in vitro tau aggregation by promoting the formation of non-toxic tau oligomers, thus acting as a lead molecule against tau pathology [124].
A study on AD model rats revealed that flavonoids from Scutellaria baicalensis stems and leaves (SSFs) had a protective effect against AD by directly regulating and reducing the hyperphosphorylation of tau protein at crucial sites in the hippocampus and cerebral cortex. This mechanism could prevent the formation of NFTs, reduce neuronal toxicity, and thereby ameliorate the cognitive deficits associated with AD [108]. Furthermore, long-term oral administration of BE in mice inhibited the activity of glycogen synthase kinase 3β (GSK-3β), an enzyme responsible for tau hyperphosphorylation, further contributing to its anti-tau effects [125].
Oxidative stress, an imbalance between ROS production and antioxidant defenses, is a key contributor to neuronal damage in AD. BE and BI are potent antioxidants that directly scavenge free radicals at rates comparable to vitamin C [126]. A study on H2O2-induced cell death demonstrated that BE effectively blocked pro-apoptotic events by preventing the activation of the JNK/ERK pathways and maintaining healthy levels of glutathione (GSH), a crucial antioxidant [127].
BE also addresses mitochondrial dysfunction, a common feature in AD. A study on J20 AD transgenic mice demonstrated that chronic administration of BE restored cerebral blood flow, normalized metabolic pathways, and alleviated mitochondrial dysfunction, thereby improving cognitive function and reducing hyperactivity [107]. Another investigation showed that BE protects against endoplasmic reticulum (ER) stress-induced apoptotic death in neuronal cells by inhibiting ROS production and mitochondrial membrane potential reduction [128].
An investigation by Ding et al. (2015) [86] demonstrated that BI significantly mitigated cognitive impairments induced by Aβ1–42 in a rat model of AD. It alleviated hippocampal damage, reduced malondialdehyde (MDA) levels, and restored antioxidant enzyme activities (SOD, CAT, GPx). Notably, its antioxidant effect was mediated through the activation of the Nrf2 signaling pathway.
Neuroinflammation, characterized by the activation of glial cells, is a major driver of AD pathology. BE and BI have been shown to be effective in mitigating this inflammation.
Inhibition of astrocytic gamma-aminobutyric acid (GABA) synthesis: An ethanolic extract of Scutellaria baicalensis and its constituent compounds, BI and BE, were found to inhibit monoamine oxidase B (MAO-B), a key enzyme for astrocytic GABA synthesis. This action reduced astrocyte reactivity and reversed aberrant neuronal tonic inhibition in a lipopolysaccharide (LPS)-induced neuroinflammation mouse model [129].
Shifting microglial polarization: BE can shift microglial polarization from the pro-inflammatory M1 phenotype to the neuroprotective M2 phenotype, thereby supporting neuronal survival and improving cognitive function [130]. Similar findings reported that this shift in microglial polarization, which alleviated neuronal injury and inflammation, was achieved through targeting HMOX1/PDE4D [131]. Another evaluation on the 3Tg-AD mice model revealed that BE promoted M2 polarization by inhibiting the CX3CR1/NF-κB signaling pathway, which ultimately reduced neuroinflammation and improved learning and memory [132].
Synaptic dysfunction is an early event in AD that correlates strongly with cognitive decline. BE has been shown to protect synaptic function and enhance neuroplasticity. An in vivo study demonstrated that BE prevented damage to long-term potentiation (LTP), a measure of synaptic strength, that was caused by Aβ42 oligomers. It achieved this by activating AkT phosphorylation and inhibiting key enzymes like 12/15-lipoxygenase and GSK-3β. Furthermore, BE restored normal dendritic spine density, a critical factor for synaptic connections, and reversed memory deficits in AD model mice [125].
A study investigating an Aβ oligomer (AβO)-induced model discerned that BI improved memory by enhancing synaptic plasticity (increasing synaptophysin, PSD95, and MAP-2), mitigating mitochondrial fragmentation, and rescuing mitochondrial dysfunction. This action is linked to PDE4 inhibition, leading to pDrp1S637 activation, and restored levels of key mitochondrial components (SDHB, COXI) [93].
The ability of BE to improve memory and cognitive function is also linked to its modulation of the cAMP/cGMP-pCREB-BDNF pathway, a crucial pathway for neuronal survival and plasticity [133]. A proteomic approach further revealed that BE treatment significantly ameliorated Aβ-induced cognitive dysfunction by influencing the expression levels of 24 proteins, many of which are involved in energy metabolism and neurotransmission [112]. Another study using a novel screening system found that BE not only inhibits Aβ-induced neuronal depolarization but also acts as an antagonist for both AMPA and NR2B/NMDA receptors, suggesting a new therapeutic avenue for AD treatment [110].
Recent advancements in computational and systems biology approaches have provided a more integrated view of the neuroprotective mechanisms of BE and BI.
An integrated approach involving network pharmacology, molecular docking, and experimental verification revealed that active ingredients of Scutellaria baicalensis protect against AD by inhibiting the PIK3R1/SRC/STAT3 pathway in N2a cells, providing strong evidence for its therapeutic potential [134]. Another study used a combined network pharmacology, bioinformatics, and animal model approach to identify AD-related target genes (e.g., APP, PIK3R1, CALM1) and confirm the involvement of the cAMP-PKA-CREB signaling pathway in SSF’s neuroprotective effects [135].
A study on AD transgenic mice explored the underlying mechanisms of BE’s efficacy through behavioral tests, metabolomics, and molecular docking. It demonstrated that BE restored cerebral blood flow, normalized metabolic pathways, and reduced oxidative stress and neuroinflammation, thereby restoring cognitive function [107]. Computational studies have also corroborated BE’s ability to inhibit key AD targets such as BACE1 and AChE, highlighting its potential as a multi-functional therapeutic candidate [111, 136]. Their findings showed that BE forms a stable, reversible competitive inhibitor complex with AChE through hydrogen bonding and hydrophobic interactions, exhibiting a strong binding affinity [107].
Recent findings have indicated that alterations in the gut microbiota and their by-products can play a role in the development of AD through the gut-brain axis. Research suggests that BE may enhance cognitive function by positively modulating these gut bacteria. It could restore gut microbiota balance, strengthen the gut barrier to prevent the leakage of inflammatory and neurotoxic substances, and modulate the production of beneficial microbial metabolites like short-chain fatty acids (SCFAs) [103].
Myelin sheath degeneration is increasingly recognized as a contributing factor to AD-related neurodegeneration. A study concluded that SSFs could effectively alleviate myelin sheath degeneration in AD model rats by increasing the expression of crucial myelin proteins (Claudin 11, MOG, MAG, MBP) and positively regulating enzymes involved in sphingomyelin metabolism (SMS1 and SMPD2) [137]. This suggests a novel approach to addressing AD pathology.
A study highlighted the potential of flavonoids like BE to modulate indoleamine dioxygenase 1 (IDO-1) activity, which is associated with immune suppression and neurodegenerative diseases. BE exhibited potent IDO-1 inhibitory activity and promoted neurite outgrowth in human neural stem cells (hNSCs), suggesting it could provide therapeutic options for managing neurodegenerative diseases like AD [138].
The combination of BE with other neuroprotective agents has shown promising results. An in vitro study found that a combination of trans-chalcone and BE synergistically reduced both ROS and Aβ42 levels in yeast cells, suggesting a multi-targeted therapeutic strategy [56]. Combining BE with daidzein enhanced both their estrogenic and neuroprotective effects by inhibiting Aβ aggregation and cytotoxicity [139]. Another combination of BE and wogonin protected neuronal cells from apoptosis and inflammation induced by Aβ25–35 [140]. Furthermore, a study showed that BE in combination with memantine provided significant improvement in behavioral and biochemical parameters in an AD rat model [141].
Thus BE and BI are not merely symptomatic treatments but are emerging as powerful, multi-target therapeutic agents against AD. Their ability to address the core pathological features of the disease namely Aβ aggregation, tau hyperphosphorylation, oxidative stress, and neuroinflammation positions them as a promising alternative to current FDA-approved drugs, which primarily offer temporary relief. The comprehensive evidences supported by advanced research modalities, underscores their potential to prevent and treat AD. The low toxicity and synergistic potential of these compounds further strengthen the case for their development as a proactive strategy for early intervention in neurodegenerative conditions, offering a path towards a more holistic and effective approach to AD therapy.
Neuroprotective role of baicalein and baicalin in Alzheimer’s disease.
| Mechanism/Effect | Major outcomes | Model/Method | Reference(s) |
|---|---|---|---|
| Baicalein | |||
| ER stress inhibition | Inhibited ROS, CHOP induction, mitochondrial depolarization | HT22 cells, thapsigargin & brefeldin A-induced stress | [128] |
| H2O2-induced oxidative stress | Blocked JNK/ERK pathways, restored GSH, reduced ROS | PC12 cells | [127] |
| Aβ toxicity & antioxidant action | Antioxidant activity > vitamin C, protected PC12 cells | PC12, Aβ-induced | [126] |
| Gut-brain axis modulation | Improved cognition via microbiota modulation | AD mouse model | [103] |
| Tau aggregation inhibition | Dissolved preformed tau fibrils | In vitro, MALDI-TOF | [123] |
| Paired helical filament inhibition | Promoted non-toxic tau oligomers | In vitro | [105] |
| Reversed Aβ-induced memory loss | Regulated cAMP/cGMP-pCREB-BDNF pathway | Aβ-injected mice | [133] |
| Synaptic protection & memory rescue | Restored spine density, inhibited Aβ & tau pathology | AD mouse model | [125] |
| Anxiety & memory deficits | Reduced AChE activity, improved anxiety in zebrafish | Scopolamine-induced model | [104] |
| Memory improvement | Enhanced ChAT neurons, reduced microglia | Ibotenic acid rat model | [105] |
| Aβ25–35-induced amnesia | Prevented & reversed memory loss | Passive avoidance test | [106] |
| Neuroplasticity regulation | Restored CaM-CamkIV-CREB signaling | Composite AD rat model | [107] |
| Tau hyperphosphorylation reduction | Reduced tau pathology in the hippocampus/cortex | AD rat model | [108] |
| Myelin sheath degeneration reversal | Upregulated myelin proteins, modulated sphingomyelin metabolism | AD rat model | [137] |
| Inhibits astrocytic GABA synthesis | Inhibited MAO-B, reversed tonic inhibition | LPS mouse model | [129] |
| Microglial M2 polarization | Reduced neuroinflammation, improved cognition | 3Tg-AD mice | [130–132] |
| Dual inhibition of Aβ & AChE | Aryl-coumarin derivative is more potent than donepezil | Zebrafish AD model | [109] |
| α-syn & Aβ aggregation inhibition | Prevented/Disaggregated α-syn & AβOs | In vitro, cell lines | [51, 116, 122] |
| Inhibits Aβ42 membrane permeabilization | The flavone scaffold is effective in membrane protection | Liposome assay | [114, 115] |
| IDO-1 inhibition | Inhibited IDO-1, promoted neurite outgrowth in hNSCs | In vitro | [138] |
| Aβ/AMPA/NMDA depolarization reversal | Inhibited receptor-induced depolarization | DiBAC4(3) dye, cortical neurons | [110] |
| BACE1 & AChE inhibition | Strong dual inhibition with good docking affinity | In vitro, in silico | [111] |
| Lipoxygenase & GSK3β inhibition | Lowered BACE1 & Aβ levels | Hippocampal slices | [125] |
| Proteomic alterations | Altered proteins linked to metabolism & signaling | AD rat model, proteomics | [112] |
| With trans-chalcone | Reduced ROS & Aβ42 more effectively | Yeast model | [56] |
| With daidzein | Synergistic estrogenic & neuroprotective activity | PC12 cells | [139] |
| With wogonin | Reduced TNF-α, NO, and apoptosis | PC12 cells, Aβ25–35 | [140] |
| With memantine | Decreased plaques, increased BDNF | Wistar rats, AD model | [141] |
| Baicalin | |||
| Aβ Aggregation Inhibition | Inhibits Aβ aggregation (± Cu2+), reduces oxidative stress, and H2O2-induced toxicity. | SH-SY5Y cell line | [51] |
| Anti-apoptotic effect | Inhibits NO, TNF-α, and PGE2 in PC12 cells | In vitro | [143] |
| Anti-apoptotic and antioxidant effect | Improves cognition, reduces oxidative stress markers, restores antioxidant enzymes, and prevents mitochondrial damage via the Nrf2 pathway. | Aβ1–42-induced rat model | [86] |
| Anti-neuroinflammatory | Reduces TNF-α, IL-6, and glial activation; improves memory | Aβ1–42 mouse model | [144] |
| Microglial modulation | Suppresses TLR4/NF-κB and NLRP3 inflammasome, reduces microglia-mediated inflammation, and improves cognition. | APP/PS1 mice, BV2 microglial cells | [143] |
| Mitochondrial plasticity | Improves synaptic proteins, inhibits PDE4 & mitochondrial fission | AβO-induced model | [83] |
| Neural regeneration | Enhances spatial learning, hippocampal neurogenesis, and regulates NPTX-1/2 and CRP levels. | AD rat model | [145] |
| Synaptic & mitochondrial protection | Increases synaptic proteins (PSD95, MAP-2), reduces mitochondrial fragmentation and dysfunction via PDE4 inhibition. | AβO-induced model | [83] |
ER: endoplasmic reticulum; ROS: reactive oxygen species; CHOP: C/EBP homologous protein; GSH: glutathione; Aβ: amyloid-beta; AD: Alzheimer’s disease; MALDI-TOF: matrix-assisted laser desorption/ionization-time of flight; AChE: acetylcholinesterase; ChAT: choline acetyltransferase; GABA: gamma-aminobutyric acid; MAO-B: monoamine oxidase B; LPS: lipopolysaccharide; α-syn: alpha-synuclein; IDO-1: indoleamine dioxygenase 1; hNSCs: human neural stem cells; TNF-α: tumor necrosis factor-alpha; IL-6: interleukin-6; AβO: Aβ oligomer; CRP: C-reactive protein.
This review lays a foundation for future research on the bioactive flavonoids BE and BI from Scutellaria baicalensis, exploring their applications and mechanisms for neuroprotection, particularly against AD. These compounds demonstrate vital antioxidant, anti-inflammatory, and neuroprotective properties, suggesting significant preclinical promise in mitigating AD pathology.
However, despite this potential, their therapeutic utility has been significantly challenged by limitations in solubility and brain delivery. To overcome these hurdles, innovative formulation strategies are crucial. These range from TCM decoctions, which leverage synergistic herbal interactions, to modern nanocarriers like liposomes and solid lipid nanoparticles. These advanced delivery systems are essential for enhancing the bioavailability, stability, and brain targeting of BE and BI, thereby maximizing their therapeutic potential for AD.
While preclinical studies consistently show promising results and a good safety profile, the full clinical relevance, especially regarding efficacy, safety, and appropriate dosages in humans, remains to be established. The clinical application of BE and BI is currently limited by insufficient human data. Therefore, comprehensive human clinical trials are imperative to fully understand their therapeutic utility and confirm their safety profile before they can be recommended for AD treatment. This review’s insights can serve as a guide for future research to define disease markers and optimize dosing, emphasizing therapeutic drug monitoring. Furthermore, studies on their interaction with various signaling pathways, including ADME processes, are crucial. Continued interdisciplinary research in pharmaceutical science and neuroscience, incorporating modern approaches like transcriptomics, systems biology, and metabolomics, should guide the design of novel formulations, enhance bioavailability via varied routes for maximum efficacy, investigate drug interactions, and establish long-term toxicity, pharmacokinetic, and pharmacodynamic profiles of these phytocompounds, both alone and in combination with other drugs.
AChE: acetylcholinesterase
AD: Alzheimer’s disease
Aβ: amyloid-beta
BBB: blood-brain barrier
BE: baicalein
BI: baicalin
GABA: gamma-aminobutyric acid
GSK-3β: glycogen synthase kinase 3β
H2O2: hydrogen peroxide
IDO-1: indoleamine dioxygenase 1
IL-6: interleukin-6
NFTs: neurofibrillary tangles
ROS: reactive oxygen species
SSFs: Scutellaria baicalensis stems and leaves
TCM: traditional Chinese medicine
TNF-α: tumor necrosis factor-alpha
α-syn: alpha-synuclein
The supplementary table for this article is available at: https://www.explorationpub.com/uploads/Article/file/1004121_sup_1.pdf.
PS: Validation, Writing—review & editing. JM: Validation, Supervision. SP: Conceptualization, Writing—original draft, Writing—review & editing. All authors have read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Leonel Pereira, Ana Valado
Sharon Smith ... David Heal
Ekaterina P. Krutskikh ... Artem P. Gureev
Susmita Das ... Shylaja Hanumanthappa
Sneha Bagle ... Sadhana Sathaye
Luis Antonio Ramirez-Contreras ... Andrés Frausto de Alba
Agustina Lulustyaningati Nurul Aminin ... Muhammad Ajmal Shah
