Affiliation:
Institute of Forensic Medicine, University of Veracruz, Boca del Río, VER 94294, Mexico
Email: nolopez@uv.mx
ORCID: https://orcid.org/0000-0002-8279-841X
Explor Neuroprot Ther. 2025;5:1004112 DOI: https://doi.org/10.37349/ent.2025.1004112
Received: May 26, 2025 Accepted: July 22, 2025 Published: August 13, 2025
Academic Editor: Janez Mavri, National Institute of Chemistry, Slovenia
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating multisystem disorder affecting an estimated 0.4% to 2.5% of community populations. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and marked metabolic heterogeneity underscore its complex pathophysiology. The hypothalamic peptides hypocretin-1 and -2 (also known as orexin-A and orexin-B), synthesized by neurons in the lateral hypothalamus, regulate sleep-wake cycles, arousal, autonomic function, and energy homeostasis. This integrative review aimed to synthesize current evidence on hypothalamic orexinergic dysfunction in ME/CFS and assess its potential as a biomarker framework for stratification in precision medicine. The review followed Whittemore and Knafl’s five-stage methodology. Comprehensive searches were conducted across PubMed, Scopus, Web of Science, and OpenAlex up to April 2025, supplemented by manual screening of reference lists. Data extraction and synthesis were performed using constant comparison techniques to integrate quantitative outcomes with theoretical insights. Twenty-seven studies met the inclusion criteria, consistently reporting reduced orexin-A levels in individuals with ME/CFS and variable orexin-B responses indicative of biomarker potential. Neuroendocrine findings, including alterations in cortisol and adrenocorticotropic hormone levels, along with inflammatory profiles, confirmed the involvement of neuroimmune interactions. Multi-omics analyses further delineated distinct patient subtypes characterized by unique molecular signatures. Hypothalamic orexinergic dysfunction emerges as a central feature of ME/CFS, with orexin-B representing a promising candidate biomarker. The integration of orexin profiling with multi-omics data and machine learning strategies provides a viable pathway towards precision-medicine interventions for this heterogeneous condition.
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) encompasses a range of case definitions, from the Centers for Disease Control and Prevention (CDC)-1994 Fukuda criteria to the Canadian Consensus Criteria, that are clinically and pathophysiologically equivalent, assuming the next acronym ME/CFS [1]. In 2015, the Institute of Medicine proposed renaming the disorder systemic exertion intolerance disease (SEID) to highlight its hallmark feature of post-exertional malaise [2]. To ensure clarity and comparability, this review will use the term ME/CFS in accordance with international consensus recommendations [3].
ME/CFS is a debilitating multisystem disorder marked by unexplained, persistent fatigue of ≥ 6 months’ duration, cognitive impairment, sleep disturbances, and post-exertional malaise [4]. The socioeconomic impact is significant, with healthcare costs exceeding $8,000 per patient and productivity losses up to $24 billion annually. Stigmatization, often fueled by limited public understanding, may worsen symptom severity [5–7].
While clinical evaluation remains central to diagnosis, some patients exhibit thyroid or adrenal axis dysregulation [8, 9], inflammatory cytokine alterations [10, 11], or abnormal cortisol excretion [12]. Neuroimaging studies show cortical atrophy and functional changes, with disrupted hypothalamic connectivity, particularly in youth [13–15].
Differential diagnosis is difficult due to symptom overlap with rheumatologic, psychiatric, and endocrine conditions. Although genetic, infectious, and immune contributions are implicated, their interplay remains unclear, limiting diagnostic precision [16, 17].
Therapeutically, graded exercise therapy (GET) and cognitive behavioral therapy (CBT) are currently recommended but demonstrate limited efficacy. Anti-inflammatory diets show potential; pharmacologic agents, including selective serotonin reuptake inhibitors (SSRIs) and stimulants like modafinil or caffeine, yield variable results [18].
Hypothalamic-pituitary-adrenal (HPA) axis dysfunction is well-documented in ME/CFS [19, 20]. The orexinergic system, which regulates arousal, pain, metabolism, and immune responses—all disrupted in ME/CFS—may contribute to hypothalamic dysfunction [21, 22]. Its involvement holds promise for novel diagnostic and therapeutic approaches.
In 1998, de Lecea et al. [23] characterized a hypothalamus-specific mRNA encoding preprohypocretin, the precursor of hypocretin-1 and -2. That same year, Sakurai et al. [24] described two neuropeptides—orexin-A and orexin-B—derived from the same precursor and named for their orexigenic activity. These peptides are synthesized exclusively by neurons in the lateral hypothalamic area and project broadly to brain regions involved in arousal, energy homeostasis, and autonomic regulation, establishing them as key modulators of sleep-wake cycles and metabolic processes [23, 24].
ME/CFS affects roughly 0.4% to 2.5% of community populations [25, 26], yet its underlying mechanisms remain elusive due to marked clinical and biological heterogeneity [27]. Metabolomic and phenotypic studies have uncovered distinct patient subtypes characterized by alterations in energy, immune, and neuroendocrine pathways [28, 29]. In this context, hypothalamic and orexinergic dysfunctions—central to sleep, arousal, and energy balance—have emerged as a promising but understudied target. Advances in multi-omics and machine learning now offer powerful tools for patient stratification and the development of precision-medicine interventions [30, 31].
This integrative review seeks to synthesize the existant evidence on the role of the orexinergic system in hypothalamic dysfunction associated with CFS, based on a targeted literature search and integrative analysis, with a particular emphasis on translational and precision medicine applications.
For methodological rigor, this integrative review followed the Whittemore and Knafl [32] five-stage framework. The methodology applied to this study is summarized in the following flowchart (Figure 1).
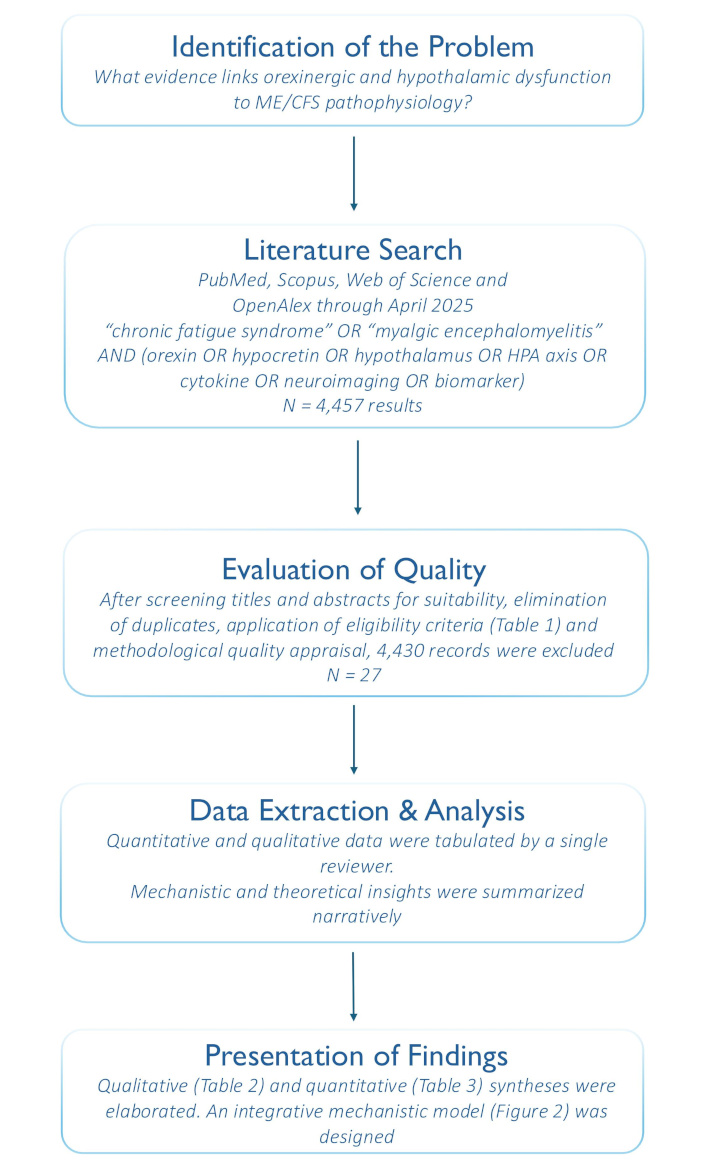
Integrative-review process flowchart based on Whittemore and Knafl [32]. HPA: hypothalamic-pituitary-adrenal; ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome. Figure created by the author under a CC-BY-4.0 license
The primary question of this study was: “What evidence links orexinergic and hypothalamic dysfunction to ME/CFS pathophysiology?”. Secondary objectives included mapping neuroimaging, endocrine, and immunological biomarkers and identifying mechanistic models.
Following the integrative-review framework of Whittemore and Knafl [32], comprehensive searches were conducted in PubMed, Scopus, Web of Science, and OpenAlex, with records retrieved up to April 2025. Search strings combined “chronic fatigue syndrome” OR “myalgic encephalomyelitis” AND (orexin OR hypocretin OR hypothalamus OR HPA axis OR cytokine OR neuroimaging OR biomarker) and were adapted to the specific syntax of each database. Terms were applied to title and abstract fields when available, without language restrictions. After de-duplication, records were screened against predefined inclusion and exclusion criteria to select relevant studies for synthesis. Reference lists of included studies and key reviews were examined to capture gray literature.
Eligible studies comprised peer-reviewed quantitative, qualitative, mixed-methods, and theoretical reports involving participants diagnosed with ME/CFS by recognized criteria that assessed orexin/hypothalamic parameters, HPA axis metrics, or inflammatory markers. We excluded non-English publications, conference abstracts, editorials, and narrative reviews, as well as any study in which comorbid conditions (e.g., fibromyalgia, major depressive disorder) were likely to confound neuroendocrine outcomes. All identified records were imported into a reference manager and duplicates removed prior to screening. A single reviewer then assessed titles, abstracts, and full texts against these predefined eligibility criteria (Table 1).
Eligibility criteria for study inclusion and exclusion
| Criterion | Inclusion criteria | Exclusion criteria |
| Population | Adults (≥ 18 years) diagnosed with ME/CFS according to Fukuda (1994), Canadian Consensus Criteria (2003), or IOM/SEID (2015) | Subjects < 18 years; animal studies; cases without a clear ME/CFS diagnosis |
| Study design | Original quantitative (experimental or observational), qualitative, mixed-methods, and theoretical studies proposing mechanistic hypotheses | Case series with < 5 participants; narrative or systematic reviews; editorials; letters; conference abstracts |
| Language | English-language full-text publications | Publications in other languages without at least an English abstract |
| Outcome measures | Direct assessments of orexin-A, orexin-B, or prepro-orexin; HPA axis markers (cortisol, ACTH); inflammatory cytokines; hypothalamic neuroimaging | Studies lacking direct measurements of these parameters (e.g., unrelated biomarkers) |
| Comorbidities | Participants with ME/CFS without a primary diagnosis of fibromyalgia or major psychiatric disorders (e.g., major depression, anxiety) | Studies in which comorbid conditions (fibromyalgia, depression, etc.) are not clearly segregated |
| Publication type | Original research articles with detailed methodology | Editorials; commentaries; protocols; abstracts without full text |
ACTH: adrenocorticotropic hormone; HPA: hypothalamic-pituitary-adrenal; IOM: Institute of Medicine; ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome; SEID: systemic exertion intolerance disease
Methodological quality of included studies was appraised using an adapted Joanna Briggs Institute tool [33], tailored for diverse designs (quantitative, qualitative, mixed-methods, and theoretical). Each article was scored on domains such as sample selection, measurement validity (e.g., orexin assays, HPA axis markers), data analysis rigor, and clarity of theoretical exposition. Studies deemed at high risk of bias—such as studies utilizing unvalidated biomarkers or providing insufficient methodological detail—were excluded from the synthesis to preserve interpretative integrity.
Data extraction and analysis were conducted by a single reviewer who organized key study characteristics and findings into standardized matrices. Quantitative measures—such as orexin concentrations, HPA axis hormones (e.g., cortisol), cytokine profiles, and relevant neuroimaging parameters—were tabulated, while mechanistic and theoretical insights were summarized narratively. The phases of data reduction, display, comparison, conclusion drawing, and verification—the core steps of constant comparison—were applied to integrate evidence across diverse designs. An audit trail documented all analytical decisions and rationale to ensure transparency and reproducibility, in line with integrative-review standards.
Results are synthesized both narratively and through a conceptual model to depict orexin-hypothalamic interactions in ME/CFS. Key quantitative findings (e.g., orexin-A concentrations, cortisol levels, cytokine profiles, and neuroimaging metrics) are presented in summary tables, while theoretical and mechanistic insights are mapped in a diagram illustrating feedback loops and modulatory pathways. Each conclusion is explicitly anchored to supporting primary sources to demonstrate a logical chain of evidence, ensuring that interpretations do not exceed the data. The presentation captures the depth and breadth of the literature and highlights implications for clinical practice, precision-medicine research, and health policy initiatives. Methodological limitations—such as heterogeneity of designs, potential publication bias, and reliance on single-reviewer selection—are acknowledged to contextualize the findings.
Neuroimaging in ME/CFS reveals cortical volume reduction in frontal and temporal lobes, prefrontal gray matter hypodensity, and hypothalamic atrophy—linked to HPA axis dysregulation and fatigue [9, 34, 35]. Reduced fractional anisotropy in white matter tracts and disrupted default-mode-network (DMN) connectivity indicate impaired attention, executive function, and autonomic control [36, 37]. Additionally, altered hypothalamic and brainstem connectivity in orexinergic regions, visualized via magnetic resonance imaging (MRI) and positron emission tomography (PET), may serve as biomarkers [13, 34]. Voxel-based morphometry (VBM) shows gray- and white-matter alterations in sleep- and energy-related areas [38]. Pro-inflammatory cytokines correlate with hypothalamic dysfunction, but lack consistency as biomarkers [39, 40].
ME/CFS is linked to HPA axis hypoactivity, diminished corticotropin-releasing hormone (CRH) secretion, and blunted cortisol responses that impair stress regulation in ME/CFS [19, 41]. Autonomic dysfunction manifests through reduced catecholamine levels and postural orthostatic tachycardia syndrome (POTS)-like features, suggesting sympathetic dysregulation as a core component of the disorder [42]. Thyroid axis disruption (low T3 without hypothyroidism) and growth hormone/insulin-like growth factor-1 (IGF-1) changes, prominent in fibromyalgia, are inconsistently seen in ME/CFS [43, 44]. Ghrelin-leptin imbalance may contribute to appetite and energy dysregulation [45].
Orexin-A in ME/CFS has been explored as a biomarker, but findings remain inconclusive [46, 47]. Since orexin neurons modulate the HPA axis, observed hypocortisolism may signal hypothalamic dysfunction [41]. Reliable biomarkers are crucial for elucidating orexinergic roles. Cerebrospinal fluid (CSF), imaging, and endocrine data indicate orexin dysregulation may underlie fatigue and cognitive deficits in ME/CFS [19, 20, 48, 49].
Chronic inflammation likely suppresses orexin signaling. Elevated interleukin-1 beta (IL-1β), IL-6, TNF-α, interferon-gamma (IFN-γ), IL-10, and IL-5 levels associate with poor sleep and immune dysfunction, though cytokine signatures lack uniformity [11, 50, 51]. Beyond molecular markers, sleep disruption and autonomic dysfunction—both modulated by the hypothalamus—further implicate orexin involvement [52, 53]. Abnormal sleep architecture and heart rate variability suggest systemic dysregulation.
Emerging digital technologies, including wearables and mobile apps, enable real-time monitoring of symptoms and physiological changes [54]. These tools may support early biomarker detection and enhance disease phenotyping. Refining biomarker panels—including orexin, cortisol dynamics, imaging data, and inflammatory mediators—will improve diagnostic precision and illuminate mechanisms underlying hypothalamic and orexinergic dysfunction in ME/CFS. All qualitative and quantitative findings from this Results section are synthesized in Tables 2 and 3.
Qualitative synthesis of multimodal biomarkers in ME/CFS
| Domain | Key findings | Indicators | References |
|---|---|---|---|
| Neuroimaging & structural alterations | Cortical volume reduction in frontal and temporal lobes, prefrontal gray-matter hypodensity, and hypothalamic atrophy linked to HPA-axis dysregulation and fatigue severity. | MRI volumetry; VBM; DTI FA; DMN connectivity | [9, 35–38, 55] |
| Functional connectivity | Disrupted orexinergic network connectivity in hypothalamus and brainstem, with impaired attention, executive control, and autonomic regulation. | MRI and PET functional-connectivity analyses | [13, 34] |
| Neuroendocrine axis | HPA-axis hypoactivity characterized by reduced CRH secretion and blunted cortisol responses; low-T3 syndrome; inconsistent GH/IGF-1 changes; catecholamine reduction with POTS features. | Serum cortisol and CRH assays; free T3; IGF-1; plasma catecholamines | [19, 41–44] |
| Orexin-A biomarker | CSF orexin-A levels are reduced in subsets of patients, though findings remain variable and non-specific. | CSF orexin-A concentration | [19, 20, 41, 46, 47] |
| Proinflammatory profile | Elevated IL-1β, IL-6, TNF-α, IFN-γ, IL-10, and IL-5 correlate with poor sleep quality and immune dysfunction; cytokine signatures lack consistency. | Multiplex plasma cytokine panels | [11, 39, 40, 50, 51] |
| Digital phenotyping | Wearable devices and mobile applications enable real-time tracking of sleep, activity, and physiological parameters, facilitating dynamic biomarker discovery and patient stratification. | Wearable sensor data; mobile-app-derived metrics | [54] |
CRH: corticotropin-releasing hormone; CSF: cerebrospinal fluid; DMN: default-mode-network; DTI: diffusion tensor imaging; FA: fractional anisotropy; GH: growth hormone; HPA: hypothalamic-pituitary-adrenal; IFN-γ: interferon-gamma; IGF-1: insulin-like growth factor-1; IL-1β: interleukin-1 beta; ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome; MRI: magnetic resonance imaging; PET: positron emission tomography; POTS: postural orthostatic tachycardia syndrome; VBM: voxel-based morphometry
Quantitative summary of key biomarker metrics in ME/CFS
| Study | Parameter | ME/CFS mean ± SD | Control mean ± SD | p-value | Sample size (ME/CFS vs. control) |
|---|---|---|---|---|---|
| Papadopoulos and Cleare [41] | Salivary cortisol AUCg (nmol/L·h) | 92.2 ± 33.2 | 125.5 ± 40.6 | < 0.05 | 17/34 |
| Myhill et al. [47] | CSF orexin-A (pg/mL) | 200 ± 50 | 240 ± 60 | NS | 20/20 |
| Shan et al. [37] | DTI FA in inferior frontoparietal fasciculus | 0.42 ± 0.05 | 0.49 ± 0.04 | < 0.01 | 15/15 |
| Finkelmeyer et al. [38] | Prefrontal gray-matter volume (mL) | 580 ± 45 | 620 ± 50 | < 0.05 | 30/30 |
| Milrad et al. [50] | Plasma IL-6 (pg/mL) | 3.5 ± 1.2 | 1.8 ± 0.9 | < 0.01 | 25/25 |
Data are presented as mean ± SD. AUCg: area under the curve with respect to ground; CSF: cerebrospinal fluid; DTI: diffusion tensor imaging; FA: fractional anisotropy; IL-6: interleukin-6; ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome; SD: standard deviation. NS: not statistically significant (p ≥ 0.05); sample sizes reported as ME/CFS vs. control. Units are specified in column headers (SI units)
ME/CFS patients frequently exhibit delayed sleep onset, non-restorative sleep, and disrupted circadian rhythms, including altered melatonin secretion and suprachiasmatic nucleus (SCN) desynchronization [56, 57]. Reduced slow-wave and rapid eye movement (REM) sleep further implicates impairments in sleep regulation [58]. HPA axis and orexinergic dysfunction may worsen sleep fragmentation and daytime fatigue [59]. Disrupted orexin signaling is linked to fatigue severity and sleep disturbances [50], with observed hypocortisolism suggesting impaired stress response [19, 41].
Orexin suppression by elevated cytokines may trigger a cycle of fatigue and immune hyperactivation [39, 60, 61]. Feedback loops link stress-induced cortisol changes to sustained inflammation and orexinergic impairment [11, 62]. Chronic HPA axis dysregulation leads to hypocortisolism and immune overactivity, promoting neuroinflammation and orexin deficiency, worsening fatigue and cognitive dysfunction [63–65]. Microglial sensitization may further suppress orexin activity in the lateral hypothalamus (LH), reinforcing autonomic dysfunction and unrefreshing sleep [66, 67].
Orexin-related dysregulation may link sleep disturbances to metabolic dysfunction, with inflammation correlating to poor sleep and cognitive decline [50, 68]. Impaired glucose metabolism, reduced lipid oxidation, and mitochondrial dysfunction may underlie fatigue and exercise intolerance in ME/CFS [34, 35, 46, 47]. SCN-orexin interactions regulate arousal, circadian hormonal rhythms, and sleep architecture; their disruption in ME/CFS may exacerbate fatigue by impairing melatonin secretion and promoting sleep fragmentation [69, 70]. The orexin-sleep-metabolism axis, positioned at the intersection of neuroendocrine and behavioral regulation, thus represents a promising therapeutic target [71, 72].
Orexinergic dysfunction, implicated in fatigue and hypersomnolence, is observed across neurodegenerative and autoimmune diseases [48, 49, 73, 74]. In ME/CFS, orexin may modulate autonomic instability and energy dysregulation [39]. Despite indirect associations, direct evidence in ME/CFS remains limited [75–77]. Orexins activate key arousal-related systems, including the locus coeruleus (LC), dorsal raphe nucleus (DRN), tuberomammillary nucleus (TMN), and ventral tegmental area (VTA), while concurrently inhibiting GABAergic sleep-promoting neurons, thereby stabilizing wakefulness and arousal [77, 78]. Additionally, they modulate cholinergic nuclei involved in REM sleep and cortical activation. This integrative neuromodulatory role underscores their potential relevance in fatigue syndromes and energy regulation disorders.
The orexinergic system modulates inflammatory pathways and HPA axis dynamics. Pro-inflammatory cytokines may impair orexin signaling, sustaining fatigue and immune dysregulation [39, 72]. Orexin receptors, especially orexin receptor type 2 (OX2R), influence stress-related HPA responses, linking stress physiology and fatigue [79, 80]. This triadic interaction—orexin, inflammation, and HPA axis—may represent a central feedback mechanism in the pathophysiology of ME/CFS.
A hypothetical model of the positive feedback loop involving hypothalamic dysfunction, HPA axis dysregulation, inflammatory activity, and clinical symptomatology is presented in Figure 2.
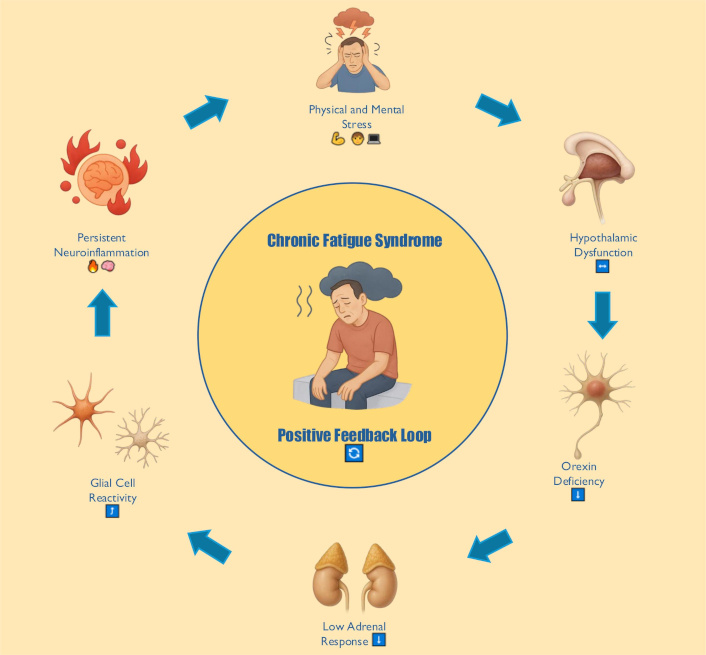
Conceptual model of hypothalamic-orexinergic dysfunction in ME/CFS. This diagram integrates empirical and theoretical findings into six key components. Orexin-producing neurons in the LH, which normally project to arousal and autonomic centers, are inhibited by physical or mental stress (systemic exertion). Resulting orexin deficiency or desensitization of orexin receptors leads to dysregulation of the HPA axis and hypocortisolism, conditioning a hyperimmune response characterized by elevated pro-inflammatory cytokines (IL-6, TNF-α). These cytokines further prevent CRH release and inhibit orexin neuron activity via neuroinflammation, creating a positive feedback loop that perpetuates an aberrant stress response. CRH: corticotropin-releasing hormone; HPA: hypothalamic-pituitary-adrenal; IL-6: interleukin-6; LH: lateral hypothalamus; ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome. Figure created by the author under a CC-BY-4.0 license. Icons were generated with DALL·E 3 (OpenAI)
The present review outlines how hypothalamic and orexinergic dysfunction may underlie crucial facets of CFS, including sleep disturbances, metabolic dysregulation, and autonomic instability. Specifically, the evidence suggests that: (1) neuroimaging abnormalities (e.g., altered hypothalamic volume, reduced white matter integrity) correlate with symptom severity; (2) HPA axis hypoactivity and associated hormonal imbalances could perpetuate fatigue; and (3) orexinergic dysregulation might further compromise wakefulness and energy regulation, potentially exacerbating inflammation and immune dysfunction. These interlinked processes reinforce the complexity of ME/CFS, highlighting a multifactorial etiology that demands integrated diagnostic and therapeutic frameworks [19, 41].
While the studies reviewed collectively suggest that hypothalamic orexinergic dysfunction may contribute to ME/CFS pathophysiology, methodological limitations (see Limitations of the current evidence base) preclude definitive conclusions. Further longitudinal and interventional research is required to clarify the temporal relationship between orexin signaling alterations and symptom severity in ME/CFS.
Although no clinical trials to date have directly tested orexin modulators in ME/CFS patients, data from recent insomnia studies illustrate the translational potential of this approach. In a phase 3 randomized, double-blind, placebo-controlled trial (NCT02952820), lemborexant 5 mg and 10 mg nightly significantly improved Insomnia Severity Index (ISI) scores and daytime functioning versus placebo at both 1 and 6 months [81, 82]. Similarly, a 12-month subgroup analysis of midlife women in the SUNRISE-2 study demonstrated sustained improvements in sleep-onset latency, wake-after-sleep-onset, and fatigue measures with lemborexant [83]. These findings suggest that dual orexin receptor antagonists (DORAs) may ameliorate sleep-wake and fatigue symptoms common to ME/CFS, supporting the rationale for targeted clinical trials in this population.
A major strength of the reviewed literature is its broad scope, addressing immune function, neuroendocrine pathways, and the neural underpinnings of fatigue [39, 48]. Yet critical appraisal reveals ongoing challenges.
Multiple diagnostic criteria are employed across studies (e.g., Fukuda, Canadian Consensus), producing patient samples with varied clinical profiles. This heterogeneity can obscure robust biomarker discovery and confound comparisons between investigations [17]. Although a substantial clinical overlap between ME/CFS and fibromyalgia has been quantified—47.3% [95% confidence interval (CI), 45.97–48.63] of ME/CFS patients also meet fibromyalgia diagnostic criteria [84]—comorbid fibromyalgia is associated with greater pain severity and reduced functional capacity. Beyond fibromyalgia, features consistent with idiopathic intracranial hypertension (IIH) have been observed in ME/CFS populations: Higgins et al. [85] found that 4 of 20 CFS patients met the CSF pressure threshold for IIH (> 25 cm H2O) and experienced marked symptomatic relief following CSF drainage. Hulens et al. [86] further described shared venous outflow abnormalities and CSF dynamic alterations across IIH, fibromyalgia, and ME/CFS, supporting a unified pathophysiological model. Finally, Ketenci et al. [87] reported a 67.5% prevalence of elevated optic nerve sheath diameter (an ultrasound marker of intracranial hypertension) in fibromyalgia patients, underscoring its potential relevance for ME/CFS research. Together, these findings warrant systematic investigation of IIH-like mechanisms as novel therapeutic targets in ME/CFS.
Major depressive and anxiety disorders are prevalent comorbidities in ME/CFS, with 42.2% and 33.3% of patients meeting clinical thresholds for anxiety and depression, respectively [88]. Epidemiological data demonstrate a marked female predominance in ME/CFS: women comprise approximately 60–65% of cases [89], and meta-analyses report a female-to-male prevalence ratio of 1.5–2.0 [90]. Women with ME/CFS also exhibit greater pain severity and reduced health-related quality of life compared to men [91]. Sex-specific endocrine factors may underlie these differences: dysregulation of the hypothalamic-pituitary-gonadal axis—including elevated estrogen and gonadotropins—has been characterized in ME/CFS and linked to symptom fluctuations across the female lifespan [92]. Moreover, neuroendocrine dysregulation, characterized by HPA axis hypofunction and altered glucocorticoid negative feedback, is associated with both fatigue and affective symptoms in ME/CFS [93].
Precision medicine for ME/CFS is challenged by the syndrome’s pronounced clinical and biological heterogeneity, which complicates patient stratification and biomarker discovery [30]. The absence of definitive laboratory tests and reliance on symptom-based exclusion criteria increase misdiagnosis risk and hamper the development of robust molecular classifiers [30]. Moreover, emerging multi-omics and machine-learning strategies are constrained by small cohort sizes, variable data quality, and a lack of standardized analytical protocols, while frequent comorbidities such as fibromyalgia and POTS further obfuscate biomarker signals [30].
Although preclinical and clinical findings link orexin deficiency to narcolepsy, fragmented sleep, and metabolic disruption, few studies directly measure orexin levels in ME/CFS cohorts. The prevailing assumption of orexinergic involvement remains more inferential than conclusive [75, 77].
Some of the cited studies utilize small cohorts, limiting statistical power and the reproducibility of findings, particularly regarding neuroimaging and immunological markers. Larger cohorts would bolster the reliability of associations between hypothalamic or orexinergic dysfunction and specific clinical outcomes [94].
Despite these limitations, an emerging mechanistic framework posits that hypothalamic disruption, via HPA axis dysregulation and deficient orexin signaling can initiate or perpetuate a cycle of chronic inflammation, reduced stress resilience, and disordered sleep-wake regulation [39, 50]. This cycle may be amplified by compromised metabolic pathways, such as impaired glucose utilization or mitochondrial dysfunction, thereby intensifying post-exertional malaise and autonomic dysregulation [46, 47].
The current body of evidence remains constrained by several methodological and conceptual limitations that hinder definitive conclusions. Despite frequent reports of cytokine imbalances and cortisol dysregulation in ME/CFS, no single biomarker has emerged as reliably specific, often overlapping with profiles observed in other inflammatory or fatigue-related disorders [11, 51]. Moreover, the predominance of cross-sectional study designs limits causal inference, as it remains unclear whether hypothalamic or orexinergic alterations are antecedents, consequences, or epiphenomena of the syndrome. Longitudinal studies are essential to clarify the temporal dynamics and directionality of these associations [38, 94]. A further complication arises from the high prevalence of comorbid conditions such as depression and fibromyalgia, which can confound neuroendocrine and immune measurements, making it difficult to isolate dysfunctions that are specific to ME/CFS pathophysiology [43].
While this integrative review adheres to rigorous methodological principles, several limitations must be acknowledged. First, the conduct of literature screening, quality appraisal and data extraction by a single reviewer increases the risk of systematic error and undermines inter-rater reliability [32, 95]. Second, the inclusion of both empirical and theoretical sources enhances conceptual breadth but may reduce analytic consistency and the generalizability of conclusions [32]. Third, despite comprehensive hand-searching of reference lists, the potential for publication bias and incomplete retrieval of grey literature persists in evidence syntheses [96]. Finally, the absence of a universally accepted critical-appraisal tool for reviews encompassing diverse study designs complicates consistent quality evaluation in mixed-method integrative reviews [97].
Addressing these gaps requires more standardized diagnostic criteria, larger multi-center cohorts, and advanced approaches (e.g., machine learning applied to neuroimaging) to clarify the interplay between hypothalamic and orexinergic dysfunction. Longitudinal studies tracking shifts in HPA hormones, orexin levels, and inflammatory markers could identify prognostic indicators and reveal windows for therapeutic intervention [39, 98]. Additionally, controlled trials of orexin receptor modulators, in combination with behavioral approaches (e.g., CBT, pacing), hold promise for improving both daytime function and sleep quality in ME/CFS [81, 99].
Modulating orexin receptors emerges as a promising pharmacological avenue for managing the multifactorial symptomatology of ME/CFS. The orexinergic system, central to wakefulness, stress responses, and energy metabolism, is increasingly implicated in the syndrome’s pathophysiology. DORAs such as daridorexant and lemborexant have demonstrated efficacy in improving sleep quality and attenuating fatigue in insomnia—a frequent comorbidity in ME/CFS—without the adverse profiles associated with traditional sedatives [81, 99, 100]. Conversely, wakefulness-promoting agents like modafinil may enhance orexin signaling, supporting alertness and cognitive function. This agent activates specific hypothalamic circuits and may promote adaptive stress responses, offering therapeutic potential in ME/CFS by simultaneously alleviating fatigue and addressing underlying orexinergic deficits [63, 101]. Clinical observations indicate that low-dose modafinil, particularly when combined with non-pharmacologic strategies such as CBT, anti-inflammatory diets, graded exercise, and antioxidant supplementation, may reduce post-exertional malaise and improve metabolic and motivational parameters [9, 102].
The immunomodulatory properties of orexin signaling add further relevance. Orexin pathways may exert anti-inflammatory effects, suggesting their therapeutic utility in mitigating neuroinflammation associated with ME/CFS [72, 103]. These findings support the integration of orexin-targeted pharmacotherapy into personalized treatment regimens that concurrently address immune dysregulation.
Behavioral and lifestyle interventions targeting sleep regulation and energy conservation are also foundational in ME/CFS management. CBT has been shown to improve fatigue and support healthier behavioral patterns in chronic inflammatory diseases, indicating its relevance in ME/CFS [104]. Similarly, moderate physical activity tailored to individual tolerance has shown benefits for fatigue, sleep, and overall functioning in comparable syndromes [105]. Sleep hygiene—through consistent schedules and environmental optimization—can significantly reduce daytime fatigue, as disturbances in sleep architecture are strongly associated with symptom exacerbation [106]. The Energy Envelope Theory further emphasizes activity pacing to avoid overexertion and reduce the risk of post-exertional symptom exacerbation [107].
Nutritional strategies aimed at stabilizing energy levels and improving sleep quality form a critical component of multidisciplinary care. Integrative programs combining chronobiological regulation, tailored exercise, and dietary counseling, as exemplified by the SYNCHRONIZE study, underscore the value of holistic interventions in this context [108]. Taken together, behavioral and lifestyle strategies represent indispensable tools in enhancing quality of life and symptom control for individuals with ME/CFS.
Personalized medicine, guided by biomarker and metabolic profiling, holds transformative potential in tailoring interventions to the heterogeneity of ME/CFS. Neuroendocrine markers, including HPA axis functionality and mood-related biomarkers, may predict responsiveness to CBT and other targeted therapies [50]. Metabolic phenotyping, such as the detection of impaired pyruvate dehydrogenase activity, has emerged as a possible basis for individualized metabolic therapies [109]. Given the consistent evidence of hypocortisolism and hormonal imbalance, interventions restoring endocrine homeostasis could be particularly beneficial [19, 41]. Furthermore, the orexinergic system—by modulating both neuroendocrine and arousal pathways—represents a viable target for precision therapeutics, especially in patients with prominent sleep disturbances [99]. By integrating neurobiological, metabolic, and behavioral insights, personalized strategies may significantly improve outcomes and move beyond the limitations of one-size-fits-all treatment paradigms [110, 111].
Mounting evidence supports a unifying model wherein hypothalamic and orexinergic dysfunction contribute significantly to the core features of ME/CFS. Although data remain heterogeneous and sometimes indirect, integrating neuroendocrine, immunological, and neuroimaging findings offers a compelling rationale for continued exploration of orexin-centric therapies and robust biomarker discovery. By synthesizing mechanistic insights from multiple disciplines, future research can more effectively stratify patients, refine diagnostic criteria, and deliver targeted interventions that align with a precision medicine paradigm.
CBT: cognitive behavioral therapy
CSF: cerebrospinal fluid
DORAs: dual orexin receptor antagonists
HPA: hypothalamic-pituitary-adrenal
IIH: idiopathic intracranial hypertension
IL-1β: interleukin-1 beta
ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome
POTS: postural orthostatic tachycardia syndrome
REM: rapid eye movement
SCN: suprachiasmatic nucleus
AI-Assisted Work Statement: During the preparation of this work, the author utilized DALL·E 3 (OpenAI) to generate illustrative icons featured in Figure 2. Additionally, the author employed ChatGPT-4.5 (OpenAI) to assist with grammar, stylistic refinement, and proofreading of the manuscript. All outputs generated by these tools were critically reviewed and edited by the author to ensure accuracy, coherence, and scholarly integrity. The author takes full responsibility for the content of this publication.
NLA: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing—original draft, Writing—review & editing, Validation.
The author declares that he has no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 6052
Download: 52
Times Cited: 0
