Affiliation:
1Postgraduate Program in Pharmaceutical Sciences, Federal University of Ceará, Fortaleza 60.430-370, Brazil
Email: macdionys@gmail.com
ORCID: https://orcid.org/0000-0002-3809-0377
Affiliation:
1Postgraduate Program in Pharmaceutical Sciences, Federal University of Ceará, Fortaleza 60.430-370, Brazil
ORCID: https://orcid.org/0000-0001-9396-659X
Affiliation:
1Postgraduate Program in Pharmaceutical Sciences, Federal University of Ceará, Fortaleza 60.430-370, Brazil
ORCID: https://orcid.org/0000-0002-2886-6450
Affiliation:
1Postgraduate Program in Pharmaceutical Sciences, Federal University of Ceará, Fortaleza 60.430-370, Brazil
ORCID: https://orcid.org/0009-0008-3254-0729
Affiliation:
2Department of Pharmacy, Federal University of Ceará, Fortaleza 60.430-370, Brazil
ORCID: https://orcid.org/0009-0002-8809-9203
Affiliation:
2Department of Pharmacy, Federal University of Ceará, Fortaleza 60.430-370, Brazil
ORCID: https://orcid.org/0009-0007-4171-1334
Affiliation:
2Department of Pharmacy, Federal University of Ceará, Fortaleza 60.430-370, Brazil
ORCID: https://orcid.org/0009-0001-6429-9369
Affiliation:
2Department of Pharmacy, Federal University of Ceará, Fortaleza 60.430-370, Brazil
ORCID: https://orcid.org/0009-0009-4867-4868
Affiliation:
3Department of Clinical and Toxicological Analyses, Federal University of Ceará, Fortaleza 60.430-370, Brazil
ORCID: https://orcid.org/0000-0002-5848-3020
Affiliation:
1Postgraduate Program in Pharmaceutical Sciences, Federal University of Ceará, Fortaleza 60.430-370, Brazil
ORCID: https://orcid.org/0000-0002-8041-7935
Affiliation:
3Department of Clinical and Toxicological Analyses, Federal University of Ceará, Fortaleza 60.430-370, Brazil
ORCID: https://orcid.org/0000-0003-3109-9683
Affiliation:
3Department of Clinical and Toxicological Analyses, Federal University of Ceará, Fortaleza 60.430-370, Brazil
ORCID: https://orcid.org/0000-0001-8160-2027
Affiliation:
1Postgraduate Program in Pharmaceutical Sciences, Federal University of Ceará, Fortaleza 60.430-370, Brazil
3Department of Clinical and Toxicological Analyses, Federal University of Ceará, Fortaleza 60.430-370, Brazil
ORCID: https://orcid.org/0009-0003-1278-4391
Explor Neuroprot Ther. 2025;5:1004124 DOI: https://doi.org/10.37349/ent.2025.1004124
Received: June 30, 2025 Accepted: November 10, 2025 Published: November 20, 2025
Academic Editor: Claudio Viegas-Junior, Federal University of Alfenas, Brazil; Rafael Franco, Universidad de Barcelona, Spain
The article belongs to the special issue Neuro-Inflammation as a Target in the Design of Multifunctional Drug Candidates for Neurodegenerative Diseases
The increasing prevalence of neurodegenerative diseases (NDs), such as Alzheimer’s, Parkinson’s, Huntington’s, multiple sclerosis, and amyotrophic lateral sclerosis, represents a serious global public health issue. Consequently, the search for compounds with neuroprotective potential has intensified. In this context, resveratrol (RSV), a stilbene polyphenol found mainly in red grapes, exhibits important pharmacological properties, such as antioxidant and anti-inflammatory, and has been widely investigated in neuroscience due to its potential in the prevention and treatment of NDs. This narrative review was conducted using the PubMed® database, with the keywords “resveratrol”, “molecular mechanisms”, “mechanisms of action”, “neuroinflammation”, “oxidative stress”, “autophagy”, “gene regulation”, and “clinical studies”. This study discusses the molecular mechanisms of RSV on NDs, focusing on signaling pathways involved in neuroinflammation, oxidative stress, gene regulation, autophagy, and cell death. Intracellular pathways such as NF-κB, JAK/STAT, MAPK/ERK, PI3K/Akt, and Nrf2/Keap1 are associated with immune modulation mediated by RSV, leading to a decrease in oxidative stress, induction of autophagy, and inhibition of apoptosis. RSV has pharmacokinetic limitations, such as low bioavailability and stability, although RSV can cross the blood-brain barrier. Thus, researches involving nonencapsulated formulations aim to enhance their delivery to the central nervous system. Current in vitro and in vivo studies are promising, although further clinical trials are needed, as few have been conducted and available data remain preliminary. In conclusion, RSV presents multiple benefits to neurological health and shows therapeutic potential in NDs; however, additional clinical studies and translational research are essential to validate and optimize its application.
Neurodegenerative diseases (NDs) have a negative impact on global health. The worldwide prevalence of NDs was 50 million people in 2019 and could reach 152 million people by 2060 [1]. An increase in the number of patients diagnosed with NDs has been observed over the last decade, which can be associated with scientific and technological advances responsible for improvement in early diagnosis. The most important examples of NDs are Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS). Studies have shown that NDs will double over in next 30 years; PD prevalence is estimated at 0.3% of the general population, with 8–18 individuals per 100,000 persons-years incidence. According to studies, about 2.71 per 100,000 person-years suffer from HD [2, 3].
Age is considered the main risk factor; thus, longer life expectancy represents a significant challenge for new research seeking treatment and prevention methods for NDs [4]. In this context, cellular senescence is characterized as a natural process of irreversible cellular damage caused by the loss of regulatory functions, which is accelerated with advancing age. Additionally, other factors may be associated with NDs, for example, genetic factors such as APOE allele variation, mitochondrial dysfunction, and environmental exposure to toxic compounds such as toxins and heavy metals [5].
Because these are challenging conditions with a limited therapeutic arsenal, several studies have been conducted to develop effective pharmacological tools. Among the research strategies, the search for natural substances stands out. RSV-IUPAC: 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol, a natural polyphenolic compound belonging to the stilbene class, can be extracted from several fruits, such as grapes, peanuts, blueberries, and blackberries, as well as red wine. Also, RSV content is used as a wine quality indicator, and its decrease is related to diminished beneficial effects [6–9].
Studies associate the use of RSV with antioxidant, anti-inflammatory, and neuroprotective properties. One of its main therapeutic functions is its ability to prevent and reduce inflammation caused by oxidative stress, contributing to cellular longevity. Given its antioxidant and anti-apoptotic effects, it is important to investigate pathways of prevention of NDs mediated by RSV [10]. Thus, considering the possible mechanisms involved in the pathogenesis of NDs, this review sought to describe and discuss the main molecular mechanisms involved in the action of RSV on the prevention and treatment of these diseases, considering its evident capacity for neuroprotection and reduction of oxidative damage.
This narrative review was conducted with the aim of discussing the RSV’s potential in the prevention and treatment of NDs. To this, the PubMed® database (https://pubmed.ncbi.nlm.nih.gov/) was used, prioritizing experimental and observational articles published in the past five years written in any language. The following descriptors were used: “resveratrol”, “molecular mechanisms”, “mechanisms of action”, “neuroinflammation”, “oxidative stress”, “autophagy”, “gene regulation”, “clinical studies”, and “perspectives”.
Neuroinflammation occurs when harmful stimuli affect the central nervous system (CNS), activating microglial cells and astrocytes, which will neutralize these stimuli, remove cellular debris, and promote tissue regeneration. Under physiological conditions, this process protects the CNS by fighting infections and repairing tissues through phagocytosis, the release of cytokines, and neurotrophic factors, such as glial cell-derived neurotrophic factor. Microglia perform different functions in the CNS, ranging from neuroprotective to neurodegenerative, especially when inducing dysfunctional pro-inflammatory activity and chronic M1 (pro-inflammatory) activation, which is associated with the development of NDs such as AD and PD [11].
The inflammatory response in the CNS is associated with several intracellular signaling pathways, such as nuclear factor-kappa B (NF-κB), phosphoinositide 3-kinase (PI3K)/Akt, mitogen-activated protein kinase (MAPK), cAMP response element-binding protein (CREB), and Janus kinase (JAK)/signal transducer and activator of transcription (STAT). The NF-κB pathway can be activated by pro-inflammatory cytokines and pathogen-associated molecular patterns, promoting the expression of pro-inflammatory genes. The PI3K/Akt pathway is related to the differential polarization of microglia, being important for states of neuroprotection and neurodegeneration, in addition to regulating apoptotic and cell survival mechanisms. The MAPK pathway is important in the pathophysiology of NDs, as it modulates the production of pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-1β. Therefore, its persistent activation leads to sustained activation of microglia, excessive cytokine release, and neuronal damage. The CREB pathway is activated by PKA and MAPK, leading to the regulation of genes involved in immune response, cell proliferation, and cell survival. Finally, the JAK/STAT pathway, especially via STAT1, favors M1 inflammatory polarization and is involved in hypoxia-associated neurodegeneration, while STAT3 modulation can promote neuroprotection. Thus, these pathways are strategic targets in the development of therapies for NDs [12].
The pathophysiology of NDs shares the establishment of damage on neuronal cells that can be caused by the accumulation of toxic substances and proteins, cytotoxicity induced by the excess neurotransmitters, or genetic factors, as shown in Figure 1. In AD, there is deposition of β-amyloid (Aβ) proteins and tau fibril arrangements; in PD, there is accumulation of α-synuclein; in HD, a gene mutation produces the defective huntingtin (Htt) protein that accumulates in the basal ganglia and cortex, while in MS and ALS, the cause is multifactorial, being associated with autoimmune conditions, glutamate cytotoxicity, and TAR DNA-binding protein 43 (TDP-43) aggregation. These pathophysiological mechanisms culminate in neuroinflammation through oxidative stress, mitochondrial dysfunction, or activation of pro-inflammatory genes. Moreover, given the pharmacological properties of RSV, this molecule has great potential to regulate these neuroinflammation pathways [13].
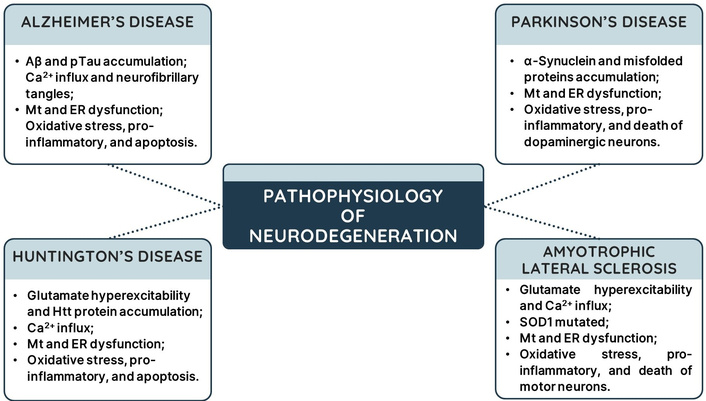
Pathophysiological mechanisms of neurodegenerative diseases. Aβ: β-amyloid; pTau: phosphorylated tau protein; Mt: mitochondria; ER: endoplasmic reticulum; Htt: huntingtin; SOD1: superoxide dismutase 1.
In AD, the accumulation of Aβ plaques and hyperphosphorylation of tau protein caused by autophagy failures generates a sequence of harmful events, such as increased calcium influx and the presence of neurofibrillary tangles (NFTs), which cause mitochondrial and endoplasmic reticulum dysfunction, leading to energy deficits and the production of reactive oxygen species (ROS). This oxidative stress causes damage to genetic material, activates pro-inflammatory genes, and leads to apoptosis activation. RSV presents protective activity, preventing neuroinflammation by promoting NFT autophagy, impairing mitochondrial dysfunction, and promoting the gene expression of antioxidant agents through pathways that will be discussed in the following topics. In post-treatment, RSV acts on Toll-like receptor (TLR) 4, inhibiting both NF-κB/STAT and NLR family pyrin domain containing 3 (NLRP3) pathways, and inducing adaptive immunity, reducing microglia activation, and reversing inflammation [14–16]. It is worth noting that RSV acts as a competitive inhibitor of death-associated protein kinase (DAPK1), which is known for its role in regulating autophagy and apoptosis, as a tumor suppressor protein [17].
In PD, the accumulation of α-synuclein and misfolded proteins, which form the histopathological finding known as Lewy bodies, is caused by defects in the ubiquitin-proteasome pathway due to mutation of the ubiquitin ligase E3 (Parkin). This leads to mitochondrial and endoplasmic reticulum dysfunction, oxidative stress, inflammation, and death of dopaminergic neurons. In addition, α-synuclein inhibits the internalization of dopamine into vesicles, leading to its oxidation in the cytosol and decreasing dopaminergic transmission in the substantia nigra-striatal pathway [18]. RSV and its analogues demonstrate broad anti-Parkinson’s potential by acting on proteasomal function via sirtuin 1 (SIRT1) and inhibiting the NF-κB pathway, which negatively regulates the gene expression of TNF-α, IL-1β, and IL-6 and inducible nitric oxide synthase (iNOS) enzyme [19]. Furthermore, RSV acts preventively by displacing nuclear factor erythroid 2-related factor 2 (Nrf2) and promoting the activation of the antioxidant response element (ARE), increasing the levels of reduced glutathione (GSH), superoxide dismutase (SOD), and heme-oxygenase 1 (HO-1) [20, 21].
In HD, glutamate hyperexcitability acts via NMDA and AMPA receptors, increasing calcium and sodium influx and Htt protein accumulation, leading to mitochondrial and endoplasmic reticulum dysfunction, gene expression suppression, autophagy inhibition, and caspase-9/3 activation. The action of RSV on HD is associated with the activation of Htt autophagy via an increase in autophagy-related protein 4 (ATG4), which promotes the formation of autophagolysosomes [22]. Moreover, RSV reverses immune activation mediated by double-stranded mitochondrial RNA (mt-dsRNA) associated with mitochondrial dysfunction [23]. It has been demonstrated in in vivo experiments (25 mg per animal orally) that RSV acts similarly to neurotrophic factors, by activating the extracellular signal-regulated kinase (ERK) pathway via MAPK/Ras kinase involved in brain function [24]. The activation of SIRT1 by RSV in HD models can improve mitochondrial function, but it was not able to reverse the disease phenotype, such as striatal atrophy [25].
In ALS, the excitotoxicity exerted by glutamate increases calcium influx in motor neurons, causing mitochondrial and endoplasmic reticulum dysfunction and oxidative stress. Furthermore, mutation in SOD1 decreases the antioxidant response, while iNOS expression increases NO and ROS levels, leading the cell to activate apoptotic pathways and microglia activation. In addition, mechanisms such as RNA processing, autophagy, and mitophagy become dysfunctional, impacting neuronal cell survival [26]. RSV improves the function of motor neurons with mutated SOD1 by reducing calcium levels and increasing the expression and activation of AMP-activated protein kinase (AMPK)/SIRT1 and heat shock proteins 25 and 70 [27–30]. However, its action on SIRT1 in the ALS model is controversial. A structural analysis showed that RSV alone does not activate SIRT1, but its presence promotes a closer bond between the enzyme and its substrate [31, 32].
In summary, RSV exhibits anti-inflammatory and neuroprotective activity through various intracellular signaling pathways associated with the gene expression of antioxidant agents and the suppression of pro-inflammatory genes [33]. RSV may modulate the anti-inflammatory response through gene regulation of the NF-κB and JAK/STAT pathways [34]. NF-κB is a transcription factor that regulates a variety of genes associated with inflammation, having two subunits: p65 and p50. Under resting conditions, NF-κB is located in the cytoplasm of cells bound to inhibitory proteins called IκB. After stimuli that induce the inflammatory response, IκB is phosphorylated and subsequently degraded, releasing NF-κB p65 for translocation to the nucleus and subsequent induction of target genes related to inflammation. RSV can act on this pathway by inhibiting the phosphorylation of the IκB protein, preventing the translocation of NF-κB p65 to the nucleus, and consequently reducing the expression of inflammatory genes such as TNF-α, IL-6, and iNOS [35, 36].
In a pro-inflammatory microenvironment, cytokines bind to their receptors on neuronal cells and promote JAK activation, which will perform transphosphorylation and phosphorylation of the receptor. Moreover, phosphorylated JAK will phosphorylate the tyrosine residues of cytoplasmic STAT proteins. After phosphorylation, STAT proteins form dimers from the binding of their SH2 domains, which will act in promoter regions, activating the gene expression of pro-inflammatory cytokines [37]. RSV was able to reduce JAK/STAT phosphorylation in a time- and concentration-dependent manner, leading to reduced IL-6 and iNOS gene expression, which resulted in lower levels of IL-6 and nitrite. Furthermore, inhibition of the JAK/STAT pathway by RSV was able to inhibit NLRP3 inflammasome activation, reducing levels of caspase-1, IL-1β, and IL-18 [36, 38], as illustrated in Figure 2.
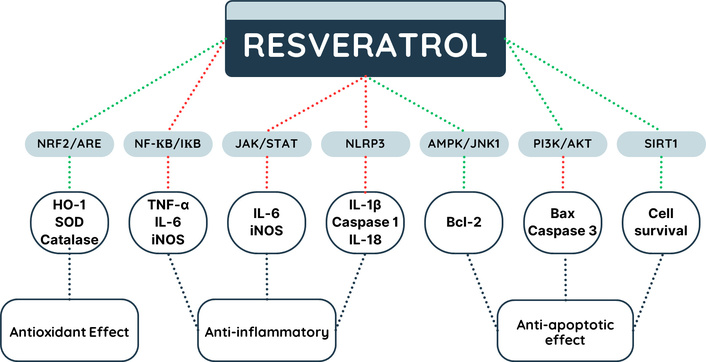
Potential mechanisms of gene regulation by resveratrol. AMPK: AMP-activated protein kinase; ARE: antioxidant response element; HO-1: heme-oxygenase 1; IL-18: interleukin 18; IL-1β: interleukin 1 beta; IL-6: interleukin 6; iNOS: inducible nitric oxide synthase; JAK: Janus kinase; NF-κB: nuclear factor-kappa B; Nrf2: nuclear factor erythroid 2-related factor 2; NLRP3: NLR family pyrin domain containing 3; PI3K: phosphoinositide 3-kinase; SIRT1: sirtuin 1; SOD: superoxide dismutase; STAT: signal transducer and activator of transcription; TNF-α: tumor necrosis factor alpha. Lines in green indicate stimulation and in red inhibition.
Oxidative stress is related to numerous disorders in the human body, including NDs such as Alzheimer’s, Parkinson’s, Huntington’s, and ALS, due to mitochondrial damage caused by ROS, causing progressive structural and functional loss of neurons [39]. Brain functions depend directly on mitochondrial activity because brain tissue has a high energy demand [40]. Under certain conditions, such as ischemia, there is an overproduction of ROS, such as superoxide, hydroxyl radicals, and hydrogen peroxide, which are harmful to brain tissue and play an important role in physiological signaling pathways, such as neuroplasticity, memory, communication, cell proliferation and growth, autophagy, apoptosis, and aging [41–43].
In this context, there are consequences due to ROS imbalance, such as protein dysfunction, activation of glial cells, mitochondrial dysfunction, and cell apoptosis [44]. Therefore, neurons, which are cells highly susceptible to oxidative damage due to their high content of polyunsaturated fatty acids in the plasma membrane, high oxygen consumption, and weak antioxidant defense, require the action of antioxidant molecules such as the enzymes SOD, catalase, and GSH [45]. In addition, the oxidative stress process acts indirectly by activating pathways such as NF-κB, which acts in the activation of pro-inflammatory factors such as TNF-α, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion protein 1 (VCAM-1) [46].
In this sense, regulators of cellular oxidative function are essential for maintaining homeostasis, such as the nuclear transcription of Nrf2, since this factor regulates the expression of antioxidant proteins and protects the cell from apoptotic processes. RSV may participate in the modulation of gene expression in antioxidant responses related to the pathophysiological mechanism of NDs. Among the genes involved in the antioxidant response, the Nrf2/ARE pathway stands out. The transcription factor Nrf2 acts by controlling the gene expression of antioxidant mediators, which are important for the defense mechanism against oxidative stress [47]. Nrf2 has seven functional domains, with the Neh2 domain responsible for its binding to Keap1. The Neh2 domain has two motifs, called ETGE and DLG, responsible for the interaction between Keap1 and Nrf2 [48]. RSV can act on this pathway through its interaction with DLG, resulting in the dissociation of Keap1 and DLG. This impairs the interaction between Nrf2 and Keap1, leaving Nrf2 free to complex with the musculoaponeurotic fibrosarcoma transcription factor (small Maf). This complex binds to ARE, located in the promoter region, activating the gene transcription of antioxidant enzymes such as HO-1, SOD, and catalase, reducing oxidative stress [47, 49, 50], are illustrated in Figure 2.
Oxidative stress is related to multiple cell death pathways, including ferroptosis, an iron-dependent cell death process. In a model using the HT22 cell line derived from mouse hippocampus, the role of RSV (10 μM) in the Nrf2-ARE pathway was investigated, which showed potential in reducing iron levels; however, this effect was probably associated with iron chelation, and not with the activation of this pathway [51]. Although RSV showed this effect on ferroptosis, when SH-SY5Y cells were exposed to oxygen and glucose deprivation, providing an environment rich in oxidative stress, RSV was able to rescue Nrf2, as well as target genes linked to it, such as superoxide oxidase (SOO), glutathione oxidase (GO), catalase, and HO-1 [52].
Furthermore, as already mentioned, oxidative stress leads to mitochondrial damage, which leads to neuronal death characteristic of NDs [53]. In this sense, RSV (75 nM in pretreatment) was evaluated in SN4741 cells, a cell line derived from substantia nigra mouse embryos, induced by 1-methyl-4-phenylpyridinium (MPP+) in a PD model. This study showed that RSV was able to attenuate mitochondrial dysfunction as well as cell apoptosis via Akt/protein kinase-B-glycogen synthase kinase-3 (GSK-3β) by inhibition of GSK-3β activity by Akt, since GSK-3β is critical in cellular functions such as apoptosis, cell signaling, and proliferation [54–57]. RSV can also improve motor dysfunction and cognitive decline when evaluated in MPP+ mice (A53T), being able to reduce the accumulation of ROS in dopaminergic neurons by inhibiting the voltage-dependent anion channel I protein (VDAC1), which favors the opening of the mitochondrial permeability transition pore (mPTP) and the influx of calcium ions, thus improving mitochondrial dysfunction [58]. This also reduces cell apoptosis [54].
In addition, RSV has antioxidant neuroprotective potential when used as a pretreatment. In this context, in a model of AD, RSV was able to reduce the formation of Aβ plaques [59]. This effect is possibly due to its activation of SIRT1, which is linked to energy balance and regulation of gene transcription, leading to activation of α-secretase, which reduces the production of Aβ [60]. Thus, RSV has an important therapeutic potential in the prevention and treatment of oxidative stress, given its antioxidant properties, inhibiting lipid peroxidation, oxidative damage, and DNA damage, as it has the ability to capture superoxide and hydroxyl radiation [61].
Protein aggregates (α-synuclein, Aβ, and tau proteins) contribute to the development of neurodegenerative disorders through disturbances in autophagy processes. Some proteins, including poly ADP-ribose polymerase (PARP) enzymes, and SIRT1 are linked to the overproduction of these unfolded proteins, which worsen of patient’s cognitive symptoms. The buildup of these proteins causes oxidative stress, mitochondrial dysfunction, and death of aged dopaminergic neurons [62, 63]. It is suggested that the binding of RSV to SIRT1 causes structural changes, increasing the strength of interaction with its substrates [64].
Autophagic cell death is characterized by the degradation of protein aggregates, the clearing of organelles, such as mitochondria (mitophagy) and the lysosome, with failure of these processes resulting in ROS bursts, and mitochondrial malfunction in aged neurons [65]. These dysfunctional processes are caused by mammalian target of rapamycin (mTORC1) complex hyperactivation, inhibition of Beclin 1 protein and dysregulated inflammasome activation. However, AMPK activation impairs mTORC1 complex formation and increases autophagic essential proteins, such as microtubule-associated protein 1 light chain 3 (LC3B) and Beclin-1 [66, 67]. RSV stimulates SIRT1-mediated mitophagy and mitochondrial protection, which helps to reduce oxidative stress and mitochondrial damage, hence avoiding neuronal cell death [68–71], are illustrated in Figure 2.
SIRT1, a NAD+-dependent deacetylase, stimulates the production of LC3B proteins. SIRT1 also promotes the development of autophagosomes and mitophagy by deacetylating Beclin 1. Furthermore, SIRT1 upregulation protects DNA from epigenetic modifications, protein aggregation formation, and, as a result, neuronal death during neurodegenerative disorders. SIRT1 also increases the expression of Nrf2, SOD, HO-1, GSH, and catalase, therefore protecting neurons from oxidative stress. SIRT1 supports cell survival by balancing anti-apoptotic and pro-apoptotic signals, as well as reducing NF-κB production. SIRT1 and its associates’ pathways are intensively studied in the therapy of neurodegenerative disorders [72–74].
RSV increases autophagic cell death in AD and PD models via stimulating SIRT1, PARP, tyrosyl-RNA synthetase (TyrRS), and HO-1, as well as MAPK/ERK signaling. Furthermore, RSV promoted SIRT1 expression, which resulted in autophagic cell death and the removal of protein aggregates [68, 70, 71, 74, 75]. Moreover, RSV can inhibit cell death by activating the AMPK/JNK-1 pathway, promoting AMPK/JNK-1 phosphorylation, resulting in Beclin-1 and Bcl-2 phosphorylation and consequent dissociation of the Bcl-2/Beclin-1 complex. Free Bcl-2 exerts anti-apoptotic action by interacting with pro-apoptotic molecules, inhibiting cell apoptosis [76], are illustrated in Figure 2.
Neurodegenerative disorders are related to excessive unfolded proteins and neuroinflammation. NF-κB overexpression in microglia cells stimulates the NLRP3 inflammasome pathway, leading to increased production of IL-1β and IL-18 [77–79]. Also, the continual exacerbation of the immune response inhibits the removal of protein clumps in the hippocampus. Microglia overactivation is also mediated by MAPK/Akt/PI3K, JAK/STAT, and TLR4/NF-κB pathways, and it is counterbalanced by suppressors of cytokine signaling (SOCS-1) protein, whose overexpression is stimulated by RSV in microglia cells, leading to inhibition of pro-inflammatory cytokine release [71, 80–82].
RSV can suppress the gene expression of pro-apoptotic proteins Bax and caspase-3 by activating the PI3K/Akt pathway, which is involved in the inhibition of apoptosis and cell survival, thereby exerting its neuroprotective action [83], as illustrated in Figure 2. Downregulation of PI3K/Akt/mTOR leads to increased autophagy and Aβ protein breakdown. PD is associated with PI3/Akt/mTOR-mediated mitochondrial dysfunction, ROS burst, and oxidative stress. The action of phenolic compounds, such as RSV, on this pathway, as well as Akt/Nrf2, boosts antioxidant defenses by reducing Keap1 expression, which contributes to neuron regeneration and antiapoptotic effects [68, 74, 84]. In addition to cell death events, decreased dopamine levels in neurodegenerative illnesses may be related to a reduction in the TyrRS enzyme, which controls tyrosine production [85].
RSV inhibits the formation of or promotes the removal of unfolded proteins and aggregates. These proteins cannot interact with TLR, leading to NF-κB expression, NLRP3 inflammasome, cytokine release, or NCS inflammation. It is worth noting that RSV increases the synthesis of antioxidant mediators (GSH, SOD, and HO-1), which reduces the quantity of ROS in the CNS. RSV’s anti-apoptotic effect is mediated by modulating the balance of anti-(Bcl-2 and SIRT1) and pro-apoptotic (caspase-3, p53, Bax, and JNK) proteins, as shown in Figure 3 [86].
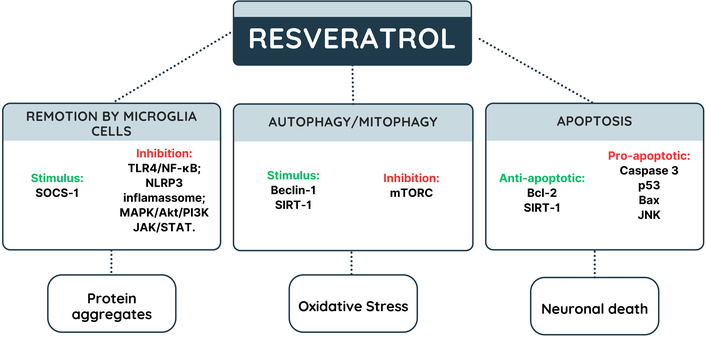
Potential mechanisms of resveratrol in autophagy and cell death. JAK: Janus kinase; MAPK: mitogen-activated protein kinase; mTOR: mammalian target of rapamycin; NF-κB: nuclear factor-kappa B; NLRP3: NLR family pyrin domain containing 3; PI3K: phosphoinositide 3-kinase; SIRT1: sirtuin 1; SOCS-1: suppressors of cytokine signaling; STAT: signal transducer and activator of transcription; TLR4: Toll-like receptor 4. Green indicates stimulation and red indicates inhibition.
Mice were treated with RSV (160 mg/kg daily) for 16 weeks and submitted to the ND experimental model, and evaluation of motor coordination, equilibrium, and strength by the Rotarod locomotion test. When compared to the control group, RSV was able to delay symptoms arising for two weeks. Further, animals treated with polyphenol showed a high improvement in locomotor function [87]. In another preclinical study, with the Rotarod test, rats received cassava (Manihot esculenta Crantz) juice (28.56 mg/kg/day for 28 days) to induce motor change in animals. Concomitant treatment with RSV (10.70 mg/kg/day for 28 days) reversed cassava-induced neuromotor degeneration [88].
Further, recent studies using Tg2576 mice, submitted to an AD-type amyloid beta-protein neuropathology model, which received Cabernet Sauvignon wine (0.2 mg/L—corroborant with FDA-suggested diary ingestion, and 10-fold lower than the minimal effective concentration shown to promote Aβ protein in vitro clearance), showed that a moderate consumption of wine attenuates AD-type deterioration of functional memory and Aβ protein accumulation in the hippocampus [89]. The genetic expression data of C57BL/6xC3HH/He mice that received an RSV-containing diet (4.9 mg/kg day) were analyzed. These animals downregulated genes related to Gfap, dynamin 1, and Vamp2 proteins, involved in glial-neuronal networks, synaptic vesicle transport, neurotransmitter release, and neurodegeneration [90].
SAMP8 mice with 5 months old, were fed with RSV (1 g/kg chow–160 mg/kg for 3 or 4 weeks), showed HDL levels (86%) high-improvement, as well as down-expression of APP mRNA (63%), and BACE1 mRNA (15%) (Aβ formation-related genes), while 7 months-old animals, besides the lower HDL levels, enhanced Aβ clearance-related cellular compounds [91]. When evaluating RSV effect on the adenosinergic pathway of SAMP8 mice, the polyphenol reversed adenosine receptor loss, which is appointed as a promisor target in the context of NDs [92].
When searching the clinicaltrials.gov database using the filters “neurodegenerative diseases” and “resveratrol”, the result returned 17 registered clinical studies, of which 15 were completed, one had not yet started recruitment, and another was withdrawn. Although 15 trials were completed, only one presented the results. This was a double-blind, randomized, placebo-controlled, multicenter phase 2 study that recruited 119 patients with mild to moderate dementia due to probable AD to verify the safety, efficacy, and pharmacokinetic profile of RSV and its metabolites. The experimental design of the 52-week intervention was as follows: RSV group—individuals started by taking 500 mg orally once a day, and every 13 weeks the dose was doubled until reaching the maximum dose of 1 gram orally twice a day, with or without food. The placebo group started with one capsule taken once a day and increased at 13-week intervals to two capsules twice a day [15].
The results demonstrated that high doses of RSV are safe and well tolerated in elderly individuals, with weight loss being the only significant adverse event. RSV and its metabolites were detected in cerebrospinal fluid (CSF), demonstrating that RSV is capable of crossing the blood-brain barrier (BBB). Its effects after 52 weeks were more significant in CSF than in plasma, causing a reduction in human matrix metalloproteinase 9 levels and an increase in macrophage-derived chemokine, fibroblast growth factor, and IL-4. Regarding cognitive decline, treatment with RSV was able to slow it by half compared to placebo. However, it is worth noting that the small sample size of the study reduces the statistical power of the results, requiring phase 3 studies with a larger number of patients with AD [15].
Future research may associate biological effects with mechanistic assays, using molecular docking analyses to help clarify the cellular targets to which RSV can bind with greater affinity. In addition, studies of synergism with other polyphenols may enhance the antioxidant, anti-inflammatory, and anti-apoptotic effects, not only in NDs but also in pro-inflammatory diseases. Furthermore, in order to improve the pharmacokinetic properties of RSV, nanoencapsulation of this active ingredient can be performed to improve its stability in biological tissues and increase its efficacy through the functionalization of nanocarriers for delivery to target organs, such as the brain.
This review has some limitations, such as the difficulty of exhausting all published works. The study focused on describing and discussing the pathophysiological mechanisms of neuroinflammation involving oxidative stress, gene expression, autophagy, and cell death, which have not yet been fully explored in the literature, but did not cover other mechanisms that may be related. Although promising, human studies are needed to define the main mechanisms and the safe and effective concentration range for treating and preventing NDs, considering the pharmacokinetic limitations associated with stability and penetration of the BBB, which has highlighted the encapsulation of RSV in nanocarriers for more efficient delivery to the CNS. In addition, it was not possible to verify updated publications that were in the process of peer review. The review focused on the description and discussion of the pathophysiological mechanisms of neuroinflammation involving oxidative stress, gene expression, autophagy, and cell death, but did not cover other mechanisms that may be related. Although the vast majority of studies are in vitro or in vivo, there is the translational potential to extrapolate the results found to humans, as observed for other polyphenolic compounds.
RSV is a molecule with great potential for the prevention and treatment of NDs, as its antioxidant, anti-inflammatory, anti-apoptotic, and cell survival effects are extensively reported in the literature. These effects can be modulated by various intracellular signaling pathways, especially by the enzymatic and epigenetic regulation of genes involved in protection against oxidative stress caused by proteins and toxic substances and in the immune response. There are some pathways and molecular targets that have been most promising in the action of RSV in NDs, such as SIRT1, NF-κB, JAK/STAT, MAPK/ERK, PI3K/Akt, and Nrf2/Keap1, which are associated with promoting antioxidant response, regulating immune response, promoting autophagy and mitophagy of toxic proteins, and suppressing cell death mechanisms.
AD: Alzheimer’s disease
ALS: amyotrophic lateral sclerosis
AMPK: AMP-activated protein kinase
ARE: antioxidant response element
Aβ: β-amyloid
BBB: blood-brain barrier
CNS: central nervous system
CREB: cAMP response element-binding protein
CSF: cerebrospinal fluid
ERK: extracellular signal-regulated kinase
GSH: reduced glutathione
GSK-3β: protein kinase-B-glycogen synthase kinase-3
HD: Huntington’s disease
HO-1: heme-oxygenase 1
Htt: huntingtin
IL: interleukin
iNOS: inducible nitric oxide synthase
JAK: Janus kinase
LC3B: microtubule-associated protein 1 light chain 3
MAPK: mitogen-activated protein kinase
MPP+: 1-methyl-4-phenylpyridinium
MS: multiple sclerosis
mTOR: mammalian target of rapamycin
NDs: neurodegenerative diseases
NFTs: neurofibrillary tangles
NF-κB: nuclear factor-kappa B
NLRP3: NLR family pyrin domain containing 3
Nrf2: nuclear factor erythroid 2-related factor 2
PARP: poly ADP-ribose polymerase enzymes
PD: Parkinson’s disease
PI3K: phosphoinositide 3-kinase
ROS: reactive oxygen species
RSV: resveratrol
SIRT1: sirtuin 1
SOD: superoxide dismutase
STAT: signal transducer and activator of transcription
TLR: Toll-like receptor
TNF-α: tumor necrosis factor alpha
TyrRS: tyrosyl-RNA synthetase
MDRdC: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. IMMT: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. BRD: Investigation, Writing—original draft. NMLP: Investigation, Writing—original draft, Writing—review & editing. CFA: Investigation, Writing—original draft. MOF: Investigation, Writing—original draft. LHeS: Investigation, Writing—original draft. HLPF: Investigation, Writing—original draft. GdAV: Investigation, Writing—original draft. EPM: Investigation, Writing—original draft, Writing—review & editing. RRPPBdM: Writing—review & editing, Supervision. AMCM: Writing—review & editing, Supervision. TLS: Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Ravi Philip Rajkumar
