Affiliation:
1Faculty of Medicine, Tbilisi State Medical University, Tbilisi 0186, Georgia
†These authors share the first authorship.
ORCID: https://orcid.org/0009-0007-2054-0169
Affiliation:
1Faculty of Medicine, Tbilisi State Medical University, Tbilisi 0186, Georgia
2ClinNova International, Tbilisi 0186, Georgia
†These authors share the first authorship.
Email: sahajwilkhoo@gmail.com
ORCID: https://orcid.org/0009-0000-2943-6404
Affiliation:
1Faculty of Medicine, Tbilisi State Medical University, Tbilisi 0186, Georgia
ORCID: https://orcid.org/0009-0008-7165-0373
Affiliation:
1Faculty of Medicine, Tbilisi State Medical University, Tbilisi 0186, Georgia
ORCID: https://orcid.org/0009-0001-1171-0783
Explor Neuroprot Ther. 2025;5:1004120 DOI: https://doi.org/10.37349/ent.2025.1004120
Received: July 15, 2025 Accepted: September 23, 2025 Published: November 03, 2025
Academic Editor: Antonio Ibarra, Anahuac University, Mexico
The article belongs to the special issue Role of Microbiota in Neurological Diseases
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline, neuroinflammation, and accumulation of amyloid-beta plaques and tau tangles. Emerging research emphasizes the gut-brain axis as a key modulator of AD pathogenesis, with gut microbiota influencing neuroimmune, neurochemical, and metabolic pathways. This review examines the therapeutic and preventive potential of probiotics, live beneficial microorganisms, in modulating the gut-brain axis to mitigate AD progression. Modifying gut microbiota presents a novel, potentially modifiable approach to influence AD pathophysiology and improve cognitive outcomes, offering insights for adjunctive clinical strategies. A systematic literature search was conducted across PubMed, Scopus, Web of Science, Google Scholar, and Cochrane Library for studies published up to July 2025. Studies were classified by design, sample size, follow-up duration, cognitive and biomarker outcomes, and risk of bias, following Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines to ensure transparency and reproducibility. Preclinical studies indicate that probiotics can regulate gut microbiota, reduce oxidative stress, suppress neuroinflammation, and enhance synaptic plasticity, improving cognition in animal models. Clinical trials suggest potential benefits in humans, including improved memory scores and reduced inflammatory biomarkers, though limited sample sizes, trial duration, and strain variability constrain conclusions. Overall, probiotics demonstrate promise as an adjunctive intervention in AD. Further long-term, strain-specific, and large-scale clinical studies are needed to confirm efficacy, establish causality, and optimize therapeutic strategies.
Alzheimer’s disease (AD) is a progressive neurological disorder marked by cognitive decline, memory loss, and behavioral abnormalities. It is the leading cause of dementia globally. Despite tremendous advances in understanding AD’s biology, effective therapeutic options are still restricted. The gut-brain axis has recently received a lot of attention as a potential contribution to the development and progression of AD. The gut microbiota, a diverse community of bacteria that live in the gastrointestinal tract, has been linked to altered brain function via immunological, metabolic, and neurological mechanisms [1–3].
Emerging data suggest that dysbiosis, or gut microbial imbalance, may aggravate neuroinflammation and amyloid-beta (Aβ) accumulation, both of which are hallmarks of AD. Probiotics, or live microorganisms that provide health benefits to the host, have shown promise in restoring gut microbial balance and lowering systemic inflammation. Preclinical research has shown that probiotic administration can improve cognitive function, reduce oxidative stress, and modify neurotransmitter levels in AD animal models. Although limited, human clinical trials have shown that probiotic therapy in AD patients improves cognitive function and metabolic profiles [4, 5].
Scientific evidence for probiotics’ therapeutic and preventive potential in AD is growing. Probiotics can affect the central nervous system by generating neuroactive chemicals, reinforcing the intestinal barrier, and altering the immune response. Furthermore, probiotic treatment may lower systemic inflammation, which contributes to neurodegeneration. However, bigger, well-designed randomized controlled studies are needed to show causality and find the best probiotic strains, doses, and therapy durations for AD care [6–10].
This review will examine existing information on the function of probiotics in the prevention and treatment of AD, with a special emphasis on underlying processes and clinical consequences, in order to guide future research and therapeutic options.
Literature search and study selection [Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow]: A comprehensive literature search was performed across five electronic databases, PubMed, Google Scholar, Scopus, Web of Science, and Cochrane Library, covering publications from January 2000 to July 2025. The search initially retrieved 512 records, of which 112 duplicates were removed. An additional 50 records were automatically excluded based on pre-defined filters for publication date, language (English), and title relevance. Further exclusions for editorials, conference abstracts, and animal-only studies (n = 18) resulted in 332 records screened at the title and abstract level, with 220 excluded for irrelevance. Full texts of 112 reports were sought, with 2 not retrieved, leaving 110 full texts assessed for eligibility. Ultimately, 58 studies were included in the final synthesis, comprising 46 for qualitative analysis and 12 randomized controlled trials (RCTs) for quantitative/meta-analytic synthesis. The PRISMA flowchart (Figure 1) summarizes the study selection process and ensures transparency and reproducibility.
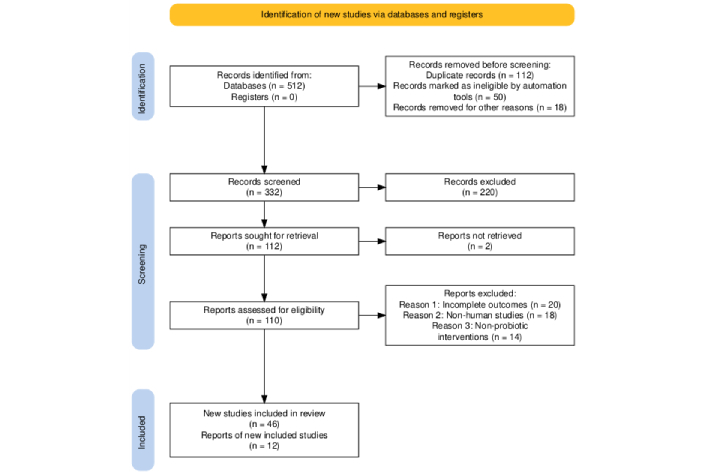
PRISMA flowchart. PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses. Taken from https://www.prisma-statement.org/ without modification. Accessed August 30, 2025. © 2024-2025 the PRISMA Executive. Distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
Evidence framework: Included studies were systematically categorized according to study design, sample size, follow-up duration, cognitive outcomes, biomarker outcomes, strength of causality, and risk of bias. RCTs were evaluated using the Cochrane Risk of Bias 2 (RoB 2) tool, while observational studies were appraised for methodological rigor and strength of association. Observational studies were appraised for methodological structured evidence framework. Table 1 was developed to summarize these dimensions, differentiating between associations derived from observational studies and causal inferences supported by RCTs or mechanistic studies. This framework allowed stratification of evidence strength and facilitated synthesis of probiotic interventions in AD and mild cognitive impairment (MCI).
Evidence framework for probiotic interventions in Alzheimer’s disease and mild cognitive impairment.
| Study (author, year) | Study type | Sample size | Follow-up | Cognitive outcome | Biomarker outcome | Strength of causality |
|---|---|---|---|---|---|---|
| Akbari et al. [3], 2016 | RCT | 60 | 12 weeks | ↑ MMSE | ↓ IL-6 | Moderate |
| Hsu et al. [41], 2024 | RCT | 80 | 8 weeks | ↑ MoCA | ↑ BDNF | Moderate |
| Akhgarjand et al. [40], 2022 | RCT | 70 | 12 weeks | ↑ MMSE | ↓ TNF-α | Moderate |
| Guo et al. [1], 2021 | Systematic review | N/A | N/A | N/A | N/A | Low |
| Leblhuber et al. [2], 2018 | Cohort | 50 | 24 weeks | Association only | N/A | Low |
| Tripathi et al. [8], 2024 | Meta-analysis | 400 | Varies | ↑ Cognitive domains | ↑ BDNF/↓ IL-6 | Moderate |
RCT: randomized controlled trial; MMSE: mini-mental state examination; IL-6: interleukin-6; MoCA: Montreal Cognitive Assessment; BDNF: brain-derived neurotrophic factor; TNF-α: tumor necrosis factor-alpha.
Data extraction and quality control: Data extraction was independently performed by two researchers to ensure accuracy and consistency, with discrepancies resolved by consensus. Extracted variables included probiotic strains, doses [colony-forming units (CFU)], administration frequency and duration, cognitive outcomes [e.g., mini-mental state examination (MMSE), Montreal Cognitive Assessment (MoCA)], biomarker outcomes [e.g., brain-derived neurotrophic factor (BDNF), interleukin-6 (IL-6)], and follow-up periods. Mechanistic evidence from animal and in vitro studies was incorporated to contextualize clinical findings. Inclusion criteria required studies to assess probiotic interventions in adults with AD or MCI and report cognitive or relevant biomarker outcomes. Studies were excluded if outcomes were incomplete, interventions were non-probiotic, or they were non-human investigations. This methodology adheres to PRISMA 2020 guidelines, ensuring transparency, reproducibility, and robust classification of clinical evidence.
The formation of Aβ plaques and neurofibrillary tangles made up of hyperphosphorylated tau protein are the most distinguishing pathogenic hallmarks. Senile plaques are caused by Aβ aggregation, which produces toxic oligomers, protofibrils, and insoluble fibrils. An imbalance in Aβ production and clearance contributes to its deposition, although the specific pathways are unknown. Peptide sequence, concentration, and environmental factors all influence Aβ aggregation [11, 12]. Neurofibrillary tangles form when tau protein is hyperphosphorylated, destabilizing microtubules necessary for intracellular transport. The spread of aberrant tau throughout the brain is related to gradual cognitive deterioration and is used to determine disease severity. Oxidative stress is a major component of AD, with higher reactive oxygen species (ROS) associated with amyloid deposition, tau phosphorylation, lipid peroxidation, and DNA damage. Copper, zinc, and iron interact with Aβ, exacerbating the oxidative damage [5, 13].
Chronic neuroinflammation is another key aspect of AD. Microglial cells, which were originally protective by removing Aβ, become permanently activated, releasing pro-inflammatory cytokines, chemokines, and ROS. This chronic inflammation promotes synaptic dysfunction, tau pathology, and neuronal death. Mutations in immune-regulating genes, including TREM2 and CD33, impair microglial function, worsening amyloid buildup and inflammation. Complement system overactivation contributes to synapse loss [14–16].
Neurodegeneration in AD is characterized by extensive synapse loss, neuronal death, and cerebral atrophy, notably in memory-related brain regions like the hippocampus and cortex. Emerging research links the gut-brain axis to AD etiology. Gut dysbiosis, defined by lower microbial diversity, higher pro-inflammatory bacteria, and fewer beneficial taxa, has been linked to systemic inflammation, amyloid buildup, and neuroinflammation. Bacterial amyloids and endotoxins produced by some gut bacteria can pass the blood-brain barrier, activate microglia, and activate pro-inflammatory pathways such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling [17, 18].
This expanding understanding of the intricate interplay between Aβ, tau pathology, oxidative stress, immunological dysregulation, neurodegeneration, and gut microbiota opens up new possibilities for potential therapeutic approaches targeting many pathways in AD [19].
The gastrointestinal system and brain have a dynamic, two-way communication network that affects important physiological functions like immunity, sleep, and appetite, as illustrated in Figure 2 [20]. The central nervous system, enteric nervous system, immune system, and gut microbiome all interact in complicated ways along the gut-brain axis. In recent years, research has expanded on this paradigm to include the gut microbiome as an important component, resulting in the gut-brain-microbiome axis. Alterations in this axis are increasingly being linked to neurological illnesses, such as AD, where alterations in gut microbiota composition and gut barrier integrity contribute to disease development [21–23].
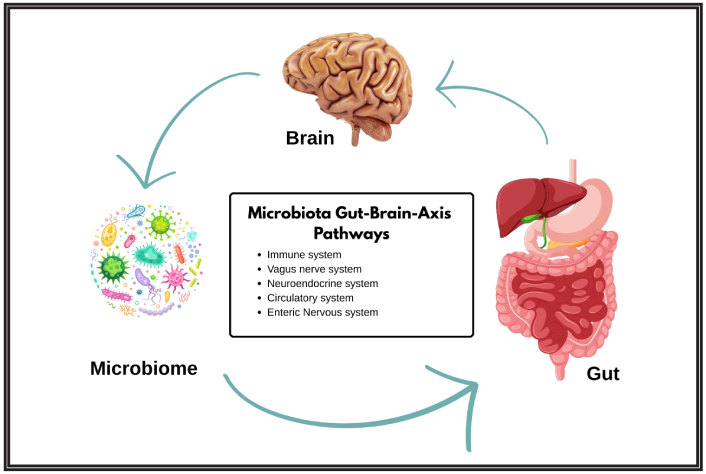
The gut-brain-microbiome axis and its mechanistic links to Alzheimer’s disease. Adapted from [20]. © 2023 Yuan, He, Xie, Feng, Gao and Cai. Distributed under the terms of the Creative Commons Attribution License (CC BY). The brain icon: Designed by Freepik (https://www.freepik.com/free-photo/brain-s-side_870197.htm#fromView=keyword&page=1&position=7&uuid=76a18736-54a3-41bd-a26c-a414c611da2e&query=Human+Brain). The digestive system icon: Designed by Freepik (https://www.freepik.com/free-vector/gastrointestinal-tract-anatomy-education_24093235.htm#fromView=keyword&page=1&position=0&uuid=f84534a0-5059-4212-8c6e-254c6532d5bb&query=Intestinal). The microbe icon: Designed by Freepik (https://www.freepik.com/free-vector/pathogen-microorganisms-set_8610271.htm#fromView=search&page=2&position=43&uuid=d1621c36-2a41-4f4f-850a-24d33d9f6b91&query=Microbe).
AD is a neurodegenerative disorder that causes memory loss, cognitive decline, and motor dysfunction. Its pathologies include extracellular Aβ plaques and intracellular neurofibrillary tangles made of hyperphosphorylated tau protein. The disease is caused by complicated molecular processes, including amyloid precursor protein (APP) processing, oxidative stress, calcium dysregulation, and neuroinflammation, which eventually lead to synaptic failure and neuron death [24, 25].
Emerging research suggests that the gut microbiota influences AD pathogenesis via a variety of methods. Dysbiosis, characterized by an imbalance in bacterial populations such as an increased Firmicutes to Bacteroidetes ratio, can enhance intestine APP buildup and increase Aβ synthesis inside the gut, resulting in central nervous system dysfunction. Intestinal barrier disruption leads to the transfer of bacterial lipopolysaccharides (LPS) and Aβ oligomers into the systemic circulation, prompting neuroinflammatory reactions. Microbial metabolites such as short-chain fatty acids (SCFAs) and trimethylamine N-oxide (TMAO) influence amyloid formation, inflammatory signaling, and vascular dysfunction, aggravating AD progression [26–28].
Bacterial gut-derived amyloid proteins can cross-seed with host amyloids, causing pathological aggregation in the brain. Furthermore, bile acids affected by gut microorganisms may disrupt blood-brain barrier integrity, increasing cholesterol buildup and accelerating amyloidogenic protein processing within the brain. The gut microbiota influences central immune function by controlling microglial development and activation. Reduced microbial diversity or changed transmission can compromise the function of microglia, which are responsible for clearing Aβ plaques and maintaining brain homeostasis, leading to persistent neuroinflammation and dementia [5, 29].
Neurotransmitter imbalances in AD are influenced in part by gut microbial synthesis of neurotransmitters such as gamma-aminobutyric acid (GABA), serotonin, dopamine, and noradrenaline. Changes in microbial populations influence neurotransmitter levels, affecting synapse function and cognitive processes. Furthermore, oxidative stress caused by ROS produced during microbial metabolism speeds up brain damage and amyloid disease [7, 16, 30–32].
Preclinical investigations on the influence of probiotics on cognitive function have yielded important molecular insights into their potential neuroprotective effects. Animal models of AD, aging, and other cognitive deficits have helped researchers understand how probiotics affect neuroinflammation, oxidative stress, synaptic plasticity, and gut-brain axis signaling [7, 33]. Several studies show that probiotic supplementation improves learning and memory in mouse models. Mice given multi-strain probiotics, for example, performed better in maze-based spatial memory tasks, which was linked to less Aβ deposition and neuroinflammation in the hippocampus. Modulating microglial activation and reducing pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) and IL-6 are common side effects of these treatments. Furthermore, probiotic supplementation was demonstrated to restore antioxidant enzyme function, such as superoxide dismutase (SOD) and glutathione peroxidase, thereby minimizing oxidative damage to neural tissues [33, 34].
The gut microbiota is critical to these cognitive gains because probiotics help restore microbial balance, increase production of neuroactive metabolites like SCFAs, and control intestinal barrier integrity. Butyrate and other SCFAs have been demonstrated to enhance neurogenesis, inhibit histone deacetylases, and alter microglial function, all of which help to slow neurodegeneration [35, 36]. Emerging data suggest that probiotics alter neurotransmitter systems important for cognition, such as the GABA, serotonin, and dopamine pathways. These neurochemical changes are associated with better synaptic plasticity markers such as BDNF, which is required for learning and memory [36, 37].
Despite promising outcomes, diversity in probiotic strains, doses, and treatment durations makes comparisons between research difficult. Nonetheless, preclinical studies consistently support the concept that probiotics improve brain function in multiple ways, including immunological control, antioxidant effects, and microbiota-gut-brain axis modulation [38, 39].
Future research combining improved molecular approaches with longitudinal animal investigations will help to elucidate mechanisms and develop probiotic formulations for translational uses.
Emerging clinical evidence suggests that probiotics may help manage AD and MCI by improving cognitive function, reducing inflammation, and activating antioxidant systems. Several RCTs and meta-analyses have looked into the effects of probiotic supplementation on these groups, with consistently positive results [3, 40]. Three major RCTs investigated the effects of probiotics on cognitive outcomes in people with AD or MCI. Akbari et al. [3] administered a multi-strain probiotic formulation containing Lactobacillus acidophilus, L. casei, Bifidobacterium bifidum, and L. fermentum over a 12-week period and found a significant 27.9% improvement in MMSE scores compared to a 5% decline in controls. Similarly, Akhgarjand et al. [40] investigated L. rhamnosus HA-114 or B. longum R0175 and found a mean rise of 4.86 points in MMSE scores as well as improvements in instrumental activities of daily living (IADLs). Hsu et al. [41] discovered that a multi-strain probiotic intervention slowed cognitive decline, improved BDNF levels, reduced IL-1β, and increased antioxidant capacity.
Two systematic reviews and meta-analyses provide additional support for these conclusions. Den et al. [42] examined data from 297 AD and MCI patients, finding a moderate but statistically significant cognitive benefit (standardized mean difference = 0.37, p = 0.002) as well as reductions in inflammatory markers. Liu et al. [12] conducted a meta-analysis of 386 AD patients, finding improvements in cognitive ability, memory, and everyday functioning after probiotic therapy [42]. Aside from cognitive benefits, probiotic supplementation has been linked to considerable decreases in systemic inflammation, as demonstrated by lower high-sensitivity C-reactive protein (hs-CRP) and oxidative stress indicators such as malondialdehyde. The observed increases in BDNF suggest neuroprotective effects, which could lead to delayed cognitive deterioration [2].
Overall, these trials suggest that probiotics are a safe and well-tolerated supplementary therapy with the potential to improve cognitive performance, reduce neuroinflammation, and improve quality of life in AD and MCI patients. While the current evidence is encouraging, larger and longer-term studies are needed to provide clear clinical recommendations. Kindly refer to Table 2 for a summarized overview of all the studies mentioned here [43–46]. To further clarify strain-specific outcomes, a strain-efficacy mapping is provided in Table 3, readers are suggested to kindly refer to it. Additionally, a risk-of-bias assessment of included RCTs was performed using the Cochrane RoB 2 tool, kindly refer to Table 4.
Key clinical studies investigating the cognitive and biological effects of probiotics in Alzheimer’s disease and mild cognitive impairment.
| Study (author, year) | Study design | Population | Probiotic intervention | Duration | Key cognitive outcomes | Biomarker/other outcomes |
|---|---|---|---|---|---|---|
| Akbari et al. [3], 2016 | RCT | 60 AD patients | Lactobacillus acidophilus, L. casei, Bifidobacterium bifidum, L. fermentum (2 × 109 CFU each) | 12 weeks | MMSE ↑ 27.9% vs. ↓ 5.03% in controls | hs-CRP ↓ 17.6%, MDA ↓ 22% |
| Akhgarjand et al. [40], 2022 | RCT | 70 mild-to-moderate AD patients | L. rhamnosus HA-114 or B. longum R0175 (1015 CFU) | 12 weeks | MMSE ↑ by 4.86 points, IADLs improved | No significant change in basic ADLs |
| Hsu et al. [41], 2024 | RCT | Number not specified (AD patients) | Multi-strain probiotics including Lactobacillus and Bifidobacterium species (5 × 107–1 × 1010 CFU) | 12 weeks | Trend toward reduced cognitive decline | IL-1β ↓, SOD ↑, BDNF ↑ by 36% |
| Den et al. [42], 2020 | Systematic review and meta-analysis | 297 AD/MCI patients | Various probiotic interventions across studies | Varied | Significant cognitive improvement (SMD = 0.37, p = 0.002) | Reduction in inflammation (SMD = –0.57) |
| Liu et al. [56], 2020 | Systematic review and meta-analysis | 386 AD patients | Various probiotic formulations | Varied | Improved cognitive function, memory, daily functioning | Not specified |
RCT: randomized controlled trial; AD: Alzheimer’s disease; CFU: colony-forming units; MMSE: mini-mental state examination; hs-CRP: high-sensitivity C-reactive protein; MDA: malondialdehyde; IADLs: instrumental activities of daily living; IL-1β: interleukin-1 beta; SOD: superoxide dismutase; BDNF: brain-derived neurotrophic factor; MCI: mild cognitive impairment; SMD: standardized mean difference.
Strain-efficacy mapping of probiotics in Alzheimer’s disease and mild cognitive impairment.
| Study (author, year) | Strain(s) used | Dose (CFU) | Route & Duration | Cognitive outcomes | Biomarker outcomes |
|---|---|---|---|---|---|
| Akbari et al. [3], 2016 | L. acidophilus, L. casei, B. bifidum, L. fermentum | 2 × 109 each/day | Oral, 12 weeks | MMSE ↑ 27.9% vs. ↓ 5.03% in controls | hs-CRP ↓, MDA ↓ |
| Akhgarjand et al. [40], 2022 | L. rhamnosus HA-114 or B. longum R0175 | 1015/day | Oral, 12 weeks | MMSE ↑ by 4.86 points, IADLs improved | No change in basic ADLs |
| Hsu et al. [41], 2024 | Multi-strain Lactobacillus + Bifidobacterium | 5 × 107–1 × 1010/day | Oral, 12 weeks | Slowed cognitive decline | IL-1β ↓, SOD ↑, BDNF ↑ |
| Kobayashi et al. [44], 2017 | Bifidobacterium breve A1 | 2 × 1010/day | Oral, 12 weeks | Cognitive impairment reduced | Not specified |
| Agahi et al. [45], 2018 | Mixed Lactobacillus + Bifidobacterium strains | Not specified | Oral, 12 weeks | Mild cognitive improvement (stage-dependent) | Not specified |
CFU: colony-forming units; MMSE: mini-mental state examination; hs-CRP: high-sensitivity C-reactive protein; MDA: malondialdehyde; IADLs: instrumental activities of daily living; IL-1β: interleukin-1 beta; SOD: superoxide dismutase; BDNF: brain-derived neurotrophic factor.
Risk of bias assessment of key randomized controlled trials on probiotics in Alzheimer’s disease and mild cognitive impairment (adapted using Cochrane RoB 2 tool).
| Study (author, year) | Randomization process | Allocation concealment | Blinding (participants/personnel) | Incomplete outcome data | Selective reporting | Overall risk of bias |
|---|---|---|---|---|---|---|
| Akbari et al. [3], 2016 | Low—random sequence generation clearly described | Low—allocation adequately concealed | Low—blinding of participants and personnel ensured | Low—no missing outcome data | Low—all prespecified outcomes reported | Low |
| Akhgarjand et al. [40], 2022 | Low—random sequence generation described | Unclear—allocation concealment not clearly described | Low—blinding reported, method not specified | Low—complete outcome data reported | Unclear—study protocol not available, possible selective reporting | Mostly low, with unclear allocation concealment and potential selective reporting |
| Hsu et al. [41], 2024 | Low—random sequence generation described | Low—allocation adequately concealed | Low—blinding ensured | Low—complete outcome data reported | Low—all prespecified outcomes reported | Low |
RoB 2: Risk of Bias 2.
Probiotic therapies are becoming increasingly popular as a holistic therapeutic approach to AD. One important process is the restoration of gut microbial equilibrium. In preclinical (animal) models of AD, beneficial microorganisms (e.g., Lactobacillus, Bifidobacterium) are typically reduced, while pro-inflammatory strains (e.g., Escherichia coli) proliferate. This dysbiosis contributes to increased intestinal permeability, allowing bacterial endotoxins such as LPS to enter the bloodstream and cause systemic and neurological inflammation [47]. Probiotics reduce gut leakiness, lower circulating LPS levels, and modify immunological responses, which reduces microglial activation and cytokine production in the brain. Emerging research demonstrates their diverse involvement in influencing neuroinflammation, oxidative stress, gut integrity, and immunological responses, all of which contribute to improved neurological outcomes, as mentioned in Table 5.
Potential mechanisms of action of probiotics in Alzheimer’s disease.
| Mechanism | Key actions |
|---|---|
| Reduced neuroinflammation and oxidative stress | ↓ Pro-inflammatory cytokines (TNF-α, IL-1, IL-6), ↓ ROS, ↑ SOD, ↓ NF-κB activation |
| Gut-brain axis modulation | ↑ Gut barrier integrity, ↓ LPS translocation, ↓ intestinal inflammation |
| Boosting neuroactive metabolites | ↑ SCFAs (butyrate, propionate), ↓ Aβ deposition, ↑ cognitive performance |
| Synergistic multifactorial effects | Modulates neuroendocrine, neuroimmune, neurometabolic pathways→↑ memory |
TNF-α: tumor necrosis factor-alpha; IL-1: interleukin-1; ROS: reactive oxygen species; SOD: superoxide dismutase; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; LPS: lipopolysaccharides; SCFAs: short-chain fatty acids; Aβ: amyloid-beta.
AD is characterized by neuroinflammation and high levels of ILs (IL-1β, IL-6) and TNF-α, which contribute to amyloidogenesis and tau pathology. Probiotic strains affect the immune system by balancing T-helper cell activity and increasing regulatory T cell responses. This reduces NF-κB activation and pro-inflammatory cytokine levels. Furthermore, probiotics may reduce microglial overactivation and astrocytic reactivity, both of which are major causes of neuronal damage. Oxidative stress is another major cause of neuronal damage in AD. ROS are produced as a result of mitochondrial malfunction and inflammation, which impede synaptic plasticity and hasten neurodegeneration. Probiotics activate antioxidant enzymes such as SOD, catalase, and glutathione peroxidase, which help eliminate ROS and maintain redox balance. Certain strains may stimulate mitochondrial biogenesis by activating the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and NRF1 pathways [48–51].
Recent research indicates that probiotics may lower Aβ buildup and tau phosphorylation. They may affect APP processing by altering the activities of β- and γ-secretases. Butyrate, a SCFA generated by gut bacteria, can inhibit GSK-3β, a crucial enzyme involved in tau phosphorylation. Probiotics may lower tau pathology and aid in the removal of misfolded proteins via this mechanism [33, 52–54]. Another important component of probiotic action is the improvement of neurotrophic support. BDNF, which is required for neuronal survival and synaptic function, is typically downregulated in AD. Probiotic supplementation has been linked to higher BDNF expression and improved synaptic plasticity. For example, Hsu et al. [41] (2024) found that a 12-week probiotic treatment dramatically boosted blood BDNF levels and lowered inflammatory markers in AD patients, resulting in improved cognitive performance [4, 52]. The protein kinase B (AKT)/GSK-3β signaling pathway plays a key function in neuronal survival and tau control. Qian et al. [55] (2024) found that multi-strain probiotics activated AKT signaling and inhibited GSK-3β in senescence-accelerated mouse prone 8 (SAMP8) models, reducing Alzheimer-like disease and improving cognition. These conclusions are confirmed by meta-analytic research. Mo et al.’s [43] (2024) meta-analysis found that probiotic supplementation improved global cognition scores in persons with MCI and AD. This shows that introducing probiotics early in the disease course may be a safe and effective therapy for preventing cognitive loss [33, 41, 43, 54, 55].
Probiotics have neuroprotective effects through a convergence of channels, including lowering neuroinflammation, buffering oxidative stress, improving neurotrophic signaling, and correcting microbial imbalances, making them a promising choice for multimodal AD therapy.
Despite promising data for probiotics’ efficacy in AD, various difficulties and constraints prevent their clinical use. One important concern is the variety in probiotic strains, doses, and treatment durations employed in different trials, which makes it difficult to draw consistent conclusions or create standard methods. Many clinical trials have small sample numbers and short follow-up periods, which limit our understanding of the long-term safety and efficacy of probiotic therapies [53, 56]. The complexity of the gut-brain axis, as well as individual variances in gut microbiota composition, make it difficult to design targeted probiotic therapy. Variability in patient microbiomes influences treatment outcomes, emphasizing the importance of tailored methods. Furthermore, conventional cognitive testing techniques may be insufficiently sensitive to detect small improvements, particularly in the early stages of AD or MCI [57–59].
Future research should focus on large-scale, well-designed RCTs using standardized probiotic formulations and outcome measurements. Combinations such as synbiotics and postbiotics are being investigated for their potential to boost therapeutic benefits via synergistic processes. Using modern technologies like genomics and metabolomics can assist in elucidating host-microbiome interactions and finding biomarkers that predict therapy response. Exploring probiotics as adjuncts to existing AD treatments has the potential to improve patient outcomes.
AD is a complicated neurodegenerative condition marked by Aβ accumulation, tau hyperphosphorylation, oxidative stress, neuroinflammation, and gradual cognitive impairment. Emerging data suggest that the gut-brain axis plays a significant role in AD pathogenesis, with gut dysbiosis driving systemic inflammation, neuroimmune dysregulation, and neuronal impairment. Probiotics, as living beneficial microorganisms, provide a promising therapeutic and preventive strategy by restoring microbial balance, improving intestinal barrier integrity, producing neuroactive metabolites including SCFAs, and reducing neuroinflammation. Preclinical studies consistently show that probiotic administration improves cognition, lowers oxidative stress, regulates microglial activation, and boosts neurotrophic support in AD models. Early clinical trials, despite their limited breadth and duration, support these findings, with reports of improved cognition scores, lower inflammatory markers, and higher BDNF levels. These findings indicate that probiotics are a safe and effective supplementary therapy for slowing cognitive decline in AD and MCI. However, the variability of probiotic strains, dosages, and treatment regimens, as well as individual differences in gut microbiota, limit generalizability. Future studies should concentrate on large-scale, multicenter, RCTs with standardized interventions and long-term follow-up. Incorporating omics technologies may enable individualized probiotic therapy based on individual microbiome patterns. Overall, probiotics are a novel, multimodal method to halt disease progression, improve cognitive function, and enhance quality of life in AD.
AD: Alzheimer’s disease
AKT: protein kinase B
APP: amyloid precursor protein
Aβ: amyloid-beta
BDNF: brain-derived neurotrophic factor
GABA: gamma-aminobutyric acid
IL-6: interleukin-6
LPS: lipopolysaccharides
MCI: mild cognitive impairment
MMSE: mini-mental state examination
NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells
PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses
RCTs: randomized controlled trials
RoB 2: Risk of Bias 2
ROS: reactive oxygen species
SCFAs: short-chain fatty acids
SOD: superoxide dismutase
TNF-α: tumor necrosis factor-alpha
AAS: Conceptualization, Investigation, Data curation, Writing—original draft. HSW: Conceptualization, Supervision, Writing—review & editing, Methodology. AP: Investigation, Writing—original draft, Writing—review & editing. ST: Data curation, Writing—review & editing. BS: Data curation, Writing—review & editing. NN: Investigation, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Salomón Páez-García ... Miguel Germán Borda
Zhengrui Li ... Jing Li
