Affiliation:
1Centro de Investigación en Ciencias de la Salud (CICSA), Facultad de Ciencias de la Salud, Universidad Anáhuac México, Huixquilucan, Méx. 52786, México
†These authors share the first authorship.
ORCID: https://orcid.org/0000-0003-2134-1843
Affiliation:
1Centro de Investigación en Ciencias de la Salud (CICSA), Facultad de Ciencias de la Salud, Universidad Anáhuac México, Huixquilucan, Méx. 52786, México
†These authors share the first authorship.
ORCID: https://orcid.org/0009-0002-1365-2887
Affiliation:
2Secretaría de la Defensa Nacional, Escuela Militar de Graduados de Sanidad, Ciudad de México, CDMX 11200, México
Affiliation:
2Secretaría de la Defensa Nacional, Escuela Militar de Graduados de Sanidad, Ciudad de México, CDMX 11200, México
Affiliation:
2Secretaría de la Defensa Nacional, Escuela Militar de Graduados de Sanidad, Ciudad de México, CDMX 11200, México
Affiliation:
1Centro de Investigación en Ciencias de la Salud (CICSA), Facultad de Ciencias de la Salud, Universidad Anáhuac México, Huixquilucan, Méx. 52786, México
ORCID: https://orcid.org/0000-0001-5559-2339
Affiliation:
1Centro de Investigación en Ciencias de la Salud (CICSA), Facultad de Ciencias de la Salud, Universidad Anáhuac México, Huixquilucan, Méx. 52786, México
2Secretaría de la Defensa Nacional, Escuela Militar de Graduados de Sanidad, Ciudad de México, CDMX 11200, México
Email: jose.ibarra@anahuac.mx
ORCID: https://orcid.org/0000-0003-2489-4689
Explor Neuroprot Ther. 2025;5:1004123 DOI: https://doi.org/10.37349/ent.2025.1004123
Received: August 06, 2025 Accepted: October 22, 2025 Published: November 20, 2025
Academic Editor: Yujie Chen, Third Military Medical University, China
The article belongs to the special issue Therapeutic Targets for Neuroprotection in Ischemic Stroke
The glymphatic system (GS) consists of a paravascular fluid-exchange network that regulates cerebrospinal and interstitial fluid dynamics, clears metabolic waste, and modulates neuroinflammation. Aquaporin-4 (AQP-4), expressed in astrocytic end-feet, is central to GS function and blood-brain barrier integrity, but in cerebral ischemia (CI), GS disruption and AQP-4 mislocalization drive cytotoxic edema, inflammation, and vascular dysfunction, worsening outcomes. This review aimed to examine the role of the GS in CI, focusing on pathophysiology and potential therapeutic targets. A PubMed-based literature review was conducted, selecting 51 studies from 115 screened that addressed GS, AQP-4, and ischemic stroke. Evidence suggests that modulating GS flow, through strategies such as enhancing arterial pulsations or regulating AQP-4, may reduce edema and neuroinflammation, although selectively targeting AQP-4 without impairing waste clearance remains a key challenge. The GS represents a promising therapeutic target in ischemic stroke, and a deeper understanding of its physiology may guide the development of neuroprotective interventions; future research should refine pharmacological strategies to optimize glymphatic function and improve recovery in CI patients.
Cerebral ischemia (CI), or ischemic stroke, is a leading cause of disability and cognitive decline, accounting for over 10.7% of global deaths in 2021 [1]. CI results from intravascular thrombosis, causing tissue hypoxia and neuronal necrosis. About 50% of ischemic strokes are due to rupture of arteriosclerotic plaques, 20% to cardiogenic cerebral infarction, 25% to lacunar infarcts of small vessels, and the remaining 5% to vasculitis or other rare causes [2]. Acute ischemic stroke is a heterogeneous disease; distinguishing subtypes such as cardioembolic, lacunar, and atherothrombotic infarcts is essential because they differ in pathophysiology, severity, and outcomes. Patients with cardioembolic or atherothrombotic strokes have worse short-term prognosis and higher mortality, underscoring the need for tailored research and treatment strategies [3].
In the peripheral system, the lymphatic network clears metabolic waste and excess interstitial fluid (ISF), maintaining fluid balance and homeostasis. The brain lacks identifiable lymphatic vessels [4–6]; instead, the glymphatic system (GS) serves as its waste-removal pathway. Evidence shows that glymphatic dysfunction after CI promotes toxic metabolite accumulation and secondary injury, including cytotoxic edema and neuroinflammation [5]. Impaired glymphatic flow, documented in preclinical and clinical stroke studies, worsens neuronal injury and functional recovery [6, 7]. Moreover, mislocalization and loss of aquaporin-4 (AQP-4) polarity disrupt glymphatic clearance, aggravating edema and pro-inflammatory responses [7].
Animal and human studies consistently link glymphatic impairment to poor waste clearance and long-term cognitive decline [1]. Diffusion tensor imaging along perivascular spaces provides direct clinical evidence of glymphatic dysfunction in acute CI [8]. At the molecular level, dystrophin-71 (DP71) deficiency impairs ionic balance, water flux, and astrocytic response, worsening post-ischemic edema [5]. Similarly, recent neuroimaging shows that increased extracellular water in small vessel disease correlates with perivascular space dilation and other structural changes, supporting the link between disrupted brain fluid exchange and disease progression [9]. Furthermore, cardioembolic and atherothrombotic strokes carry higher short-term mortality, highlighting the need for targeted research and therapies [10]. This highlights the importance of specific research and therapeutic strategies [11].
Overall, these findings highlight the GS as a promising therapeutic target to improve outcomes after CI.
A structured literature search was performed in the PubMed database to identify studies exploring the role of the GS and AQP-4 in CI. The search strategy combined the terms “aquaporin-4 (AQP-4), glymphatic system, ischemia, and ischemic stroke”. Boolean operators (“AND,” “OR”) were used to refine the query and include variations of these keywords. The search covered the last five years (January 2019–May 2025) to ensure up-to-date evidence, but highly relevant older articles describing key concepts of CI pathophysiology or AQP-4 function were also included. Inclusion criteria were: (1) English-language original or review articles; (2) studies on AQP-4 and GS function in ischemia; (3) publication between January 2019 and May 2025. As seen in Figure 1.
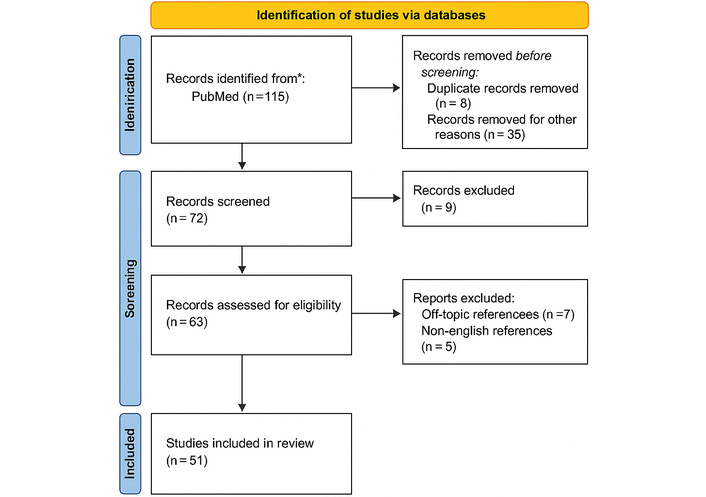
PRISMA diagram. The initial search contained 115 records. After removing 8 duplicates and 35 records for other reasons, 72 records were screened. Of these, 9 were excluded at the title/abstract screening stage. The full text of 63 articles was evaluated for eligibility, leading to the exclusion of 12 articles (7 for being off-topic and 5 for being non-English). A total of 51 articles were included in the final review. Adapted from https://www.prisma-statement.org/. Accessed August 1, 2025. © 2024–2025 the PRISMA Executive. Licensed under CC BY 4.0.
Exclusion criteria: (1) case reports, (2) conference abstracts, (3) studies not involving CI, (4) non-peer-reviewed material. Two independent reviewers screened 115 records, retaining 51 for qualitative synthesis.
Only English-language original research articles and systematic reviews were considered. Conference abstracts, editorials, case reports, and non-peer-reviewed sources were excluded. Two independent reviewers screened titles and abstracts for relevance, followed by full-text assessment to confirm the article’s contribution to understanding glymphatic function and AQP-4 dynamics in CI.
The initial search yielded 115 articles. After applying the eligibility criteria, 51 studies (including both experimental and clinical investigations) were retained for qualitative synthesis and critical analysis in this review.
In the central nervous system (CNS), the blood-brain barrier (BBB) tightly regulates the entry of proteins, ions, and fluids. However, metabolic waste from the brain’s high energy demand can accumulate in the interstitial space. Because the CNS lacks conventional lymphatic vessels [7], it has evolved specialized mechanisms to maintain fluid balance and remove waste.
The GS provides a brain-wide perivascular pathway for fluid exchange and clearance. It drives cerebrospinal fluid (CSF) influx along periarterial spaces and ISF efflux toward the meninges and cervical lymphatics [8]. CSF is produced by the choroid plexus epithelium and enters the subarachnoid space before moving into the brain parenchyma [9]. Experimental studies show that CSF and ISF flow in opposite directions within distinct perivascular spaces—some specialized for CSF influx, others for ISF efflux [4]. CSF travels along periarterial channels, exchanges with ISF in the parenchyma, and ultimately drains along perivenous pathways back to the subarachnoid space [12].
Although details of paravascular CSF–ISF recirculation remain debated, AQP-4 channels at astrocytic endfeet play a key role [13, 14]. AQP-4 supports glymphatic water transport and helps preserve BBB glial integrity [15]. Waste products from CSF–ISF exchange exit via paravenous routes and cervical lymphatics, a process driven by vascular and respiratory pulsations [16]. Notably, AQP-4 knockout, CSF depletion, or even posture changes can suppress glymphatic flow and raise brain lactate levels [17].
These findings suggest that glymphatic dysfunction contributes to neurodegeneration and other CNS injuries, including CI (Figure 2).
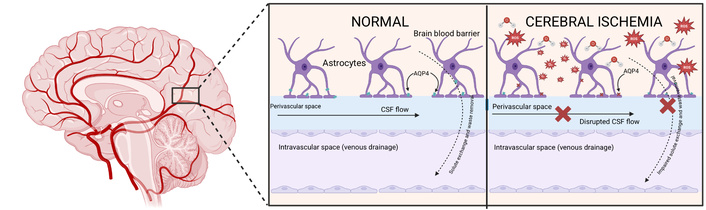
Glymphatic function in normal and ischemic brain. The glymphatic system (GS) regulates cerebrospinal fluid (CSF) influx into the interstitial space (ISF) and mediates the clearance of metabolic waste through aquaporin-4 (AQP-4) channels located in astrocytic endfeet along perivascular pathways. Under physiological conditions, AQP-4 is highly polarized toward the perivascular endfeet, which facilitates efficient solute exchange and directs waste removal toward venous and lymphatic drainage routes. During CI, however, this polarization is disrupted, leading to AQP-4 mislocalization within astrocytes. Concurrently, arterial pulsations that normally drive glymphatic flow are diminished, further reducing fluid exchange. The combined effect of these alterations results in interstitial fluid accumulation, astrocytic swelling, and impaired clearance of neurotoxic molecules, thereby exacerbating cerebral edema, neuroinflammation, and secondary neuronal injury. Created in BioRender. Moreno, E. (2025) https://BioRender.com/9ozdmlr.
The GS plays a key role in the delivery of nutrients across the brain and the astrocytic paracrine signaling with lipid molecules [18]. However, its metabolite clearance capacities are equally crucial. Studies have shown that amyloid-β peptide (Aβ) and accumulated lactate are among the waste products cleared by the GS [19]. This further supports its role in neurodegenerative diseases, such as Alzheimer’s Disease, where its dysfunction is implicated in pathological progression. In the context of cerebrovascular disease, impaired GS function leads to reduced clearance of cortical interstitial solutes, which have been associated with motor and cognitive neurologic decline. Current and future research on the GS is focused on the identification of mechanisms that regulate blood flow through this pathway with the goal of treating diseases characterized by toxic extracellular solute accumulation [7].
CI is a type of stroke that damages brain tissue and neurons, leading to structural lesions, functional deficits, and potentially death [3]. Clinically, CI often causes motor and cognitive impairments, particularly when associated with arteriolosclerosis, cerebral amyloid angiopathy, and microinfarcts [20].
Neuronal injury in CI arises from three main mechanisms: direct ischemic cell death, reactive oxygen species (ROS)—mediated damage causing functional deficits, and an inflammatory response triggered by immune activation [3]. BBB disruption, observed in both animal models and stroke patients, drives this pathological cascade [21]. Post-stroke inflammation involves elevated cytokines, chemokines, and infiltration of immune cells. Activated neutrophils release proteases that damage the extracellular matrix and increase BBB permeability, amplifying inflammation and worsening clinical outcomes [22].
Current treatment focuses on thrombolytic therapy, such as alteplase, tenecteplase, urokinase, recombinant tissue plasminogen activator (r-tPA), or desmoteplase, to recanalize occluded vessels [23]. However, these drugs are effective only within 4.5 hours of stroke onset, and many patients are ineligible. Importantly, no therapies currently promote neurological recovery after CI [24].
CI also disrupts the GS by causing metabolite and excitatory neurotransmitter accumulation in the parenchyma [25]. This impairs cytokine clearance, exacerbates neuroinflammation, and promotes cell death. Some authors propose that restoring glymphatic perfusion could improve outcomes in stroke patients [26].
After cerebral blood flow is interrupted, cerebral edema develops, increasing disability and mortality in ischemic stroke [27]. Capillary dysfunction after CI disrupts the BBB, reducing its ability to retain ions, water, proteins, and blood components [28]. In early CI, cytotoxic edema is the main driver of neuroinflammation: astrocytes take up sodium from the ISF, which pulls in anions and water [29]. This causes cellular swelling and sodium depletion, creating a gradient that drives sodium—and consequently water—from the vasculature into the brain parenchyma. These shifts contribute to vasogenic edema in later stages, further increasing brain volume [30].
Brain edema increases intracranial pressure and reduces blood flow, potentially leading to herniation. Its severity determines stroke outcomes and remains a major therapeutic target [31]. Clinically, cerebral edema is treated with hyperosmotic agents such as mannitol, but evidence guiding fluid management and optimal ICP monitoring remains limited [32].
AQP-4 plays a pivotal role in edema formation. Located in astrocytes, AQP-4 mediates water influx during cytotoxic edema [33]. Its presence worsens edema after ischemic stroke, while its absence reduces BBB permeability and water entry into the parenchyma. Consequently, AQP-4 inhibition is considered a potential therapy for cytotoxic swelling [34]. However, its effect varies: AQP-4 aggravates edema in ischemia, water intoxication, and meningitis, but appears protective in edema caused by tumors, abscesses, or subarachnoid hemorrhage [35].
After cerebral infarction, AQP-4 becomes mislocalized from perivascular astrocytic endfeet to the soma, losing its normal polarity. This impairs metabolite and cytokine clearance, worsening inflammation, cognitive decline, and neuronal loss. Consistent with this, Weng et al. [36] demonstrated that microinfarction-induced vascular dysfunction disrupts waste clearance and promotes neuroinflammation, contributing to progressive cognitive impairment in mice. Proper AQP-4 polarization depends on the dystrophin-associated protein complex (DAPC), including DP71 [37]. Yang et al. [5] showed that DP71 anchors DAPC to AQP-4; its degradation disrupts AQP-4 localization, impairs fluid outflow, and aggravates edema (Table 1). These findings suggest DP71 as a potential therapeutic target to restore glymphatic function and reduce edema in CI. Supporting this, Mestre et al. [38] reported that AQP-4 knockout mice failed to develop cerebral edema during the first 15 minutes after middle cerebral artery occlusion.
Experimental evidence on the role of AQP-4 in cerebral ischemia and edema.
| Study/Author | Model | Intervention | Main findings | Clinical implication |
|---|---|---|---|---|
| Kato et al. [43] | Cerebral infarction in mice | Observation of AQP-4 localization | AQP-4 shifts from perivascular end-feet to astrocytic soma; it reduces metabolite and cytokine clearance. | Loss of AQP-4 polarity worsens inflammation and neuronal damage. However, being an observational study, it does not provide direct evidence on therapeutic interventions. |
| Yang et al. [5] | Cerebral ischemia in mice | DP71 analysis | DP71 stabilizes AQP-4 anchorage; its degradation alters localization and outflow. | DP71 as a potential therapeutic target. Provides mechanistic insight into scaffolding proteins regulating AQP-4. Suggests that protecting DP71 could indirectly preserve glymphatic flow. |
| Mestre et al. [38] | MCA occlusion in mice | AQP-4 knockout | No cerebral edema in the first 15 min. | AQP-4 is essential for early cytotoxic edema. Reinforces the importance of timing: benefits are evident only in acute stages. |
| Li et al. [44] | Ischemia-reperfusion in mice | TGN-020 | Reduces inflammation and apoptosis via GS and ERK1/2. | AQP-4 inhibition may modulate neuroinflammation. |
AQP-4: aquaporin-4; DP71: dystrophin-71; MCA: middle cerebral artery; TGN-020: N-(1,3,4-thiadiazol-2-yl) pyridine-3-carboxamide dihydrochloride.
The studies in Table 1 consistently show that AQP-4 is a central mediator of edema and neuroinflammation in CI. Weng et al. [36] demonstrated that AQP-4 mislocalization impairs cytokine and metabolite clearance, amplifying tissue damage. Similarly, Yang et al. [5] identified DP71 as essential for maintaining AQP-4 polarity; its degradation worsens edema and suggests a potential therapeutic target. Mestre et al. [38] further showed that AQP-4 knockout mice did not develop early edema after middle cerebral artery occlusion. Collectively, these findings highlight AQP-4 as a promising therapeutic target, though the optimal timing for its modulation remains uncertain.
Arterial pulsations drive glymphatic flow, and enhancing them may improve system efficiency. Persistent glymphatic failure contributes to several CNS disorders, including CI. Prolonged GS failure in perivascular drainage contributes to CNS pathologies, including CI [39]. In a human model, β1-adrenergic agents such as dobutamine increased mean arterial pressure and promoted clearance of metabolic waste and cytokines, but their hypertensive effects limit clinical use [40]. Cao et al. [12] studied digoxin, a cardiac glycoside that improves cardiac output without raising blood pressure (BP) and observed improved vascular pulsations and cognitive outcomes. However, whether these benefits stem from direct effects on cerebral blood flow or arterial pulsation remains unclear.
Given its central role in edema and fluid dynamics, AQP-4 is also a promising therapeutic target. Inhibiting AQP-4 at perivascular astrocytic endfeet may reduce water and ion influx into the parenchyma, helping limit post-stroke edema [41, 42]. Yet, complete AQP-4 blockade would also suppress venous glymphatic outflow, impairing waste clearance and posing serious risks [43, 44]. Selective targeting is challenging because the AQP-4 structure is identical on the arterial and venous sides of the GS. Melatonin, however, has shown potential to preserve glymphatic function by enhancing AQP-4 and α-syntrophin interaction and reducing cyclin-dependent kinase 5 activity in neonatal hypoxic-ischemic models [45].
Timing of AQP-4 modulation is critical. Pre-ischemic AQP-4 inhibition reduces edema in animal models, but Kato et al. [43] first tested post-ischemia inhibition. A single dose of TGN-020, 15 minutes after middle cerebral artery occlusion, decreased acute edema by blocking water influx along glial basement membranes. However, TGN-020 is not glymphatic-specific and may alter AQP-4 expression and polarization; its role in inflammation and apoptosis remains uncertain [46]. Further research is needed to define the therapeutic window and clarify effects on ISF and solute clearance before translation to humans. Given the projected global rise in ischemic stroke incidence and mortality [47], identifying interventions that enhance glymphatic clearance and stabilize AQP-4 polarity becomes increasingly urgent.
BP control has also been linked to GS and AQP-4 regulation. Oxidative stress can disrupt the BBB and upregulate AQP-4. He et al. (2020) [46] found that in CI patients treated with rt-PA, strict systolic BP control reduced early neurological deterioration, likely by mitigating oxidative stress and AQP-4 upregulation [48, 49].
Non-pharmacological interventions may complement drug therapy. The GS is influenced by circadian rhythm and posture; adequate sleep and a lateral sleeping position have been shown to improve glymphatic clearance [48]. MRI studies in neonates demonstrate that hypoxic-ischemic injury reduces glymphatic function [49], and sex differences may also influence CSF flow and outcomes after stroke [13].
As summarized in Table 2, both pharmacological (dobutamine, digoxin, TGN-020, melatonin) and non-pharmacological strategies (BP control, sleep optimization, lateral positioning) show potential to modulate glymphatic function after ischemia [39–55]. Yet, no single approach effectively reduces post-ischemic cerebral edema while maintaining safe and efficient waste clearance. Multi-modal strategies and precise therapeutic timing remain key research priorities.
Proposed interventions to modulate the glymphatic system in cerebral ischemia.
| Strategy | Proposed mechanism | Experimental evidence | Limitations | Analytic considerations |
|---|---|---|---|---|
| Arterial pulsation stimulation (dobutamine) | Increase glymphatic flow and metabolite clearance | Improves clearance in the human brain [40] | Hypertension prevents humans from using | While animal studies suggest improved metabolic clearance, clinical application is limited by cardiovascular risk. |
| Digoxin | Increase cardiac output without raising blood pressure | Improves vascular pulsations and glymphatic function in chronic hypoperfusion [12] | Unclear if the effect is direct on cerebral blood flow | Potential benefits might be indirect, related to improved cardiac function rather than direct cerebral effects. |
| AQP-4 inhibition (TGN-020) | Reduce water and ion influx into parenchyma | Decreases early cytotoxic edema [44] | Non-selective; may impair venous clearance | Promising in early ischemia, but prolonged inhibition could compromise physiological drainage. Future research should focus on transient or more selective modulators of AQP-4. |
| Strict blood pressure control | Possible reduction of oxidative stress-induced AQP-4 upregulation | Less neurological deterioration [46] | Lack of direct evidence on GS | Strict blood pressure control is already standard in clinical care, but its direct impact on glymphatic clearance remains uncertain. |
AQP-4: aquaporin-4; TGN-020: N-(1,3,4-thiadiazol-2-yl) pyridine-3-carboxamide dihydrochloride.
Following CI, AQP-4 loses polarization and becomes mislocalized within the astrocytic soma. This disruption impairs glymphatic transport, exacerbates cerebral edema, and contributes to neuroinflammation following the ischemic insult. Several experimental studies have shown that interventions targeting AQP-4 polarization can improve glymphatic function and reduce secondary insult after ischemic insult [52]. Moreover, clinical and translational studies highlight that aim at preserving or restoring AQP-4 polarization, such as stabilizing astrocytic scaffolding proteins, modulating endothelial function, or using pharmacological agents. Several pharmacological strategies have been investigated to modulate AQP-4 polarization. Melatonin has been shown to enhance AQP-4-alpha-syntrophin interactions by inhibiting cyclin-dependent kinase 5 activity, therefore preserving AQP-4 polarization and maintaining glymphatic clearance in ischemic models [45]. In addition, agents acting directly on AQP-4 have emerged as another approach; dobutamine increases arterial pulsations and improves glymphatic transport in experimental models; however, its hypertensive effects limit clinical applicability in the context of thrombolization and ischemic stroke [40]. Selective modulation of AQP-4 remains challenging due to its dual role in clearance and edema control, especially considering the different subtypes of stroke [55, 56]. In contrast, digoxin enhances myocardial contractility and boosts cerebral vascular pulsations without hypertensive effect, resulting in improved glymphatic function in hypoperfusion models after initial ischemic insults [39].
In CI, glymphatic disruption and AQP-4 mislocalization lead to edema, BBB failure, and poor recovery. Rather than simply describing these associations, recent experimental and clinical evidence positions the GS as a modulatable therapeutic axis in ischemic stroke.
Future progress will depend on translating this mechanistic understanding into safe, targeted interventions. One priority is the selective modulation of AQP-4—limiting harmful water influx during acute edema while preserving its critical role in waste clearance. This requires deeper structural insights into AQP-4 isoforms and regulators, such as the DAPC, to design precision modulators. Another promising avenue is the controlled enhancement of vascular pulsatility, potentially through cardiac output support or neuromodulatory agents that increase glymphatic perfusion without causing systemic hypertension.
Non-invasive imaging tools (e.g., advanced diffusion MRI) should be used to define the temporal dynamics of GS impairment and identify the therapeutic window for glymphatic-targeted therapy. Comparative studies across stroke subtypes—particularly lacunar versus non-lacunar infarcts—are essential to determine whether glymphatic dysfunction is universal or subtype-specific, which would inform patient selection for targeted treatments.
Finally, combining vascular, glial, and hemodynamic modulation with reperfusion therapy may transform glymphatic research into actionable neuroprotective strategies.
This review has the following limitations: First, the majority of the studies included were preclinical, and their direct translation to human pathophysiology is uncertain. Second, clinical evidence is still limited, and the available imaging approaches require further validation. Third, experimental models analyzed were heterogenous, thus the comparability of the results could be influenced by the nature of each study.
AQP-4: aquaporin-4
BBB: blood-brain barrier
BP: blood pressure
CI: cerebral ischemia
CNS: central nervous system
CSF: cerebrospinal fluid
DAPC: dystrophin-associated protein complex
DP71: dystrophyn-71
GS: glymphatic system
ISF: interstitial fluid
RMP: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. IIM: Formal analysis, Validation. HFNV: Formal analysis, Validation. YCM: Methodology, Supervision, Validation, Writing—review & editing. AI: Supervision, Validation, Writing—review & editing. EMAM: Conceptualization, Writing—review & editing. EMG: Visualization, Writing—review & editing. All authors read and approved the submitted version.
Antonio Ibarra, who is the Editorial Board Member and Guest Editor of Exploration of Neuroprotective Therapy, had no involvement in the decision-making or the review process of this manuscript. The other authors declare no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2693
Download: 36
Times Cited: 0
Said Hachimi-Idrissi
Shafiq Dexter B. Abou Zaki, Johnny K. Lokin
Yang Yang ... Yi Li
Lidija Radenovic
Poppy Kristina Sasmita ... Bernadus Bernardino Bramantyo
