Affiliation:
1Centro de Investigación Biomédica en Red Enfermedades Neurodegenerativas (CiberNed), Spanish National Institute of Health Carlos iii, 28031 Madrid, Spain
2Department of Biochemistry and Molecular Biomedicine, University of Barcelona, 08028 Barcelona, Spain
3Institut de Química Teòrica i Computacional (IQTCUB), School of Chemistry, University of Barcelona, 08028 Barcelona, Spain
Email: rfranco123@gmail.com; rfranco@ub.edu
ORCID: https://orcid.org/0000-0003-2549-4919
Explor Neuroprot Ther. 2025;5:1004118 DOI: https://doi.org/10.37349/ent.2025.1004118
Received: August 09, 2025 Accepted: October 09, 2025 Published: October 16, 2025
Academic Editor: Jesus Avila, Consejo Superior de Investigaciones Cientificas (CSIC), Spain
Tau phosphorylated at threonine 217 (p-tau217) has moved from research novelty to clinical reality, but its greatest value lies in dynamic monitoring, not static stratification. The pace of adoption of the plasma measurement of p-tau217 now demands clear guidance on optimal use. Two complementary evidence strands inform this perspective. First, a multi-cohort evaluation of a commercial assay shows high concordance with amyloid and tau reference standards and supports a pragmatic three-zone interpretation, rule-out, indeterminate, and rule-in, that can streamline diagnostic pathways while preserving accuracy. Second, longitudinal analyses in amyloid-positive individuals reveal that the most informative property of p-tau217 is dynamic: steeper rises occur in those who decline faster, whereas baseline values substantially overlap across outcome groups. These findings show that plasma p-tau217 levels can be a complementary tool for triage, enrichment, and longitudinal monitoring, but not as a time-stable baseline stratifier for defining trial cohorts or assessing therapeutic efficacy. Stratification should instead anchor to independent, stable measures such as tau burden measured by positron emission tomography (PET), structural magnetic resonance imaging (MRI), and cognitive history, reducing misclassification and avoiding circular validation. Comparable scrutiny should be applied to other p-tau biomarkers and to composite measures, such as the p-tau217/Aβ1–42 ratio, to rigorously define their risk-benefit profile, guide therapeutic evaluation, and maximize translational impact.
For most of its history, the diagnosis of Alzheimer’s disease could only be confirmed postmortem, limiting both patient care and research progress [1, 2]. In recent years, in vivo methods such as positron emission tomography (PET) or functional magnetic resonance imaging (MRI) and determination of parameters in cerebrospinal fluid (CSF) have led to unprecedented advances in diagnostic accuracy, but their high cost, limited availability, and invasive nature restrict broad clinical implementation [3, 4]. Recent multimodal frameworks, such as those integrating Diffusion Tensor Imaging-Analysis along the Perivascular Space (DTI-ALPS), hippocampal microstructural measures, and CSF profiles, further illustrate the potential of combining imaging and fluid biomarkers to stage disease progression across the continuum from healthy controls to Alzheimer’s disease [5]. Whichever strategy is adopted, a central requirement is the ability to capture the different stages of the disease in a clinically meaningful way [6, 7]. The prospect of plasma-based biomarkers as a more practical and scalable alternative has therefore become a central focus [8]. The challenge, however, lies in selecting plasma biomarkers that can reliably capture the same disease biology defined by imaging or altered CSF composition, recognizing that perfect accuracy is unlikely to be achieved.
The Alzheimer’s disease field has reached an inflection point in which blood tests can be ordered as part of routine evaluation for cognitive complaints [8]. That achievement carries a responsibility to match the right assay to the right clinical question. A biomarker earns its place by helping clinicians and investigators make better decisions. In practice, that means three distinct tasks. Screening and triage identify who merits further work-up. Stratification at baseline creates cohorts that remain meaningfully distinct while a study unfolds. Monitoring quantifies disease dynamics and treatment effects over time. Confusion arises when a single biomarker is asked to perform all three tasks equally well. The most robust evidence to date suggests a sharper allocation.
Against the backdrop of persistent challenges in biomarker discovery and the need for stronger clinical-chemistry grounding [9], recent studies have now clarified the role of tau phosphorylated at threonine 217 (p-tau217) within the diagnostic workflow. Specifically, analyses from two independent cohorts [10, 11] demonstrate that p-tau217 performs well for screening and sensitive longitudinal monitoring, yet falls short as a reliable tool for baseline stratification. The distinction is more than semantic. Stratification asks for time-stable separability. Groups carved at day zero should still be coherent months later; otherwise, the logic of randomization and prespecified subgroup analysis begins to fray. A marker whose signal-to-noise ratio improves with slope rather than level is naturally mismatched to that role. Conversely, a dynamic biomarker can be extraordinarily valuable once we accept that its highest information content is revealed by change.
The clinical evaluation of a commercial p-tau217 assay conducted across independent cohorts provides a picture of performance in real workflows. When compared with amyloid positivity by PET or in tests using CSF, and with tau burden by PET or CSF, the assay achieves high agreement [11], something that is required of a screening tool. Importantly, the investigators did not rely on a single threshold. They proposed a three-range interpretation with a lower zone that identifies individuals very unlikely to be amyloid positive, an intermediate zone that acknowledges uncertainty and routes those people to confirmatory testing, and an upper zone that identifies individuals very likely to be amyloid positive who can be prioritized for tau staging, counseling, and trial screening. In practice, this approach reduces the proportion who need immediate PET or CSF tests in moderate-to-high prevalence settings, while in population or community contexts, the intermediate band expands and the algorithm naturally sends more people for confirmation, which is appropriate for the lower pretest probability [11].
Two aspects of this evaluation deserve emphasis because they often get lost once headline performance metrics are quoted. First, the investigators demonstrated transportability across cohorts with different reference standards. Rather than insist that amyloid PET and CSF tests represent interchangeable gold standards, they accepted these methods as useful but incomplete anchors and reported agreement with both. That framing is honest about uncertainty while still being operationally helpful. Second, they examined longitudinal behavior within the same dataset. p-tau217 increased over time in amyloid-positive individuals and increased most in those with evidence of elevated tau, which situates the marker as a reporter of ongoing pathophysiology rather than a static label. The cross-sectional results justify using p-tau217 early in the pathway to manage referrals and resources. The longitudinal signal justifies bringing the same assay back as a follow-up.
A complementary line of evidence considers a different question. If we set biomarker labels aside and group people by how they actually change over the years, how does p-tau217 behave relative to those trajectories? In amyloid-positive cohorts followed for up to a decade, investigators extracted person-specific cognitive slopes and then used an unsupervised approach to identify three robust clusters: stable cognition, slow decline, and rapid decline. A crucial observation emerged immediately. Each cluster contained both individuals labeled cognitively normal or subjectively impaired and individuals labeled mild cognitive impairment (MCI) at baseline. The clinical label at time zero did not determine the future path [10]. Within that framework, baseline p-tau217 values were, on average, lowest in the stable cluster and stepwise higher in slow and rapid decliners, but the distributions overlapped substantially. Longitudinal analyses demonstrate diverging slopes despite overlapping baselines (Figure 1). When p-tau217 was measured repeatedly, all clusters showed increases over time, and the steepest increases appeared in the rapid decliners (Figure 1). The pattern held across two assay implementations, an in-house method and a commercial kit, which supports biological coherence while reminding us that absolute scales differ by platform. In other words, the strongest information resides in change rather than in a single draw. These findings explain, in data rather than in theory, why baseline stratification by p-tau217 is a fragile proposition.
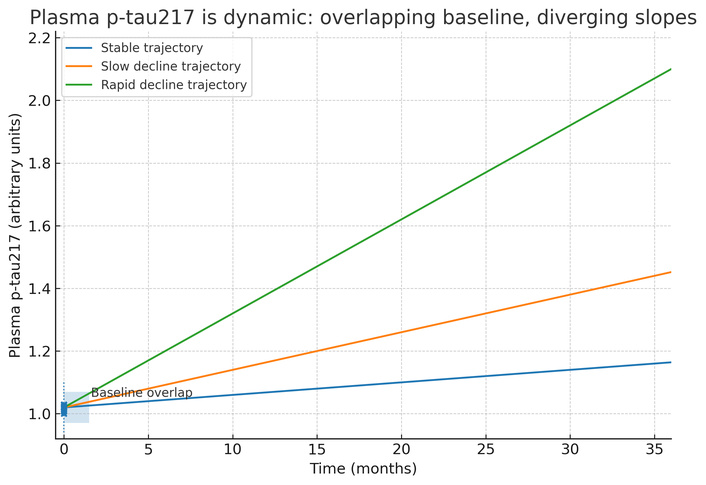
Diverging plasma p-tau217 slopes across progression clusters (stable/slow/rapid decline), based on data in the Kirsebom et al. (2025) report [10]. While baselines overlap, slopes diverge, showing that repeated measures enable effective monitoring, whereas a single cut-off is inadequate for patient stratification. p-tau217: tau phosphorylated at threonine 217.
Stratification is often conflated with enrichment, yet they are different problems. Enrichment tilts probabilities on average, for example, by favoring amyloid-positive individuals or those more likely to harbor a higher tau burden, and plasma p-tau217 is well suited to that role. Stratification intends to create entry strata that remain valid for the duration of a study so that randomization balances disease stage and prespecified subgroup analyses preserve their meaning as data accrue. Several practical issues arise if a single p-tau217 cut-off is used for that purpose. The first problem is overlap at baseline. Group means differ, which tempts the use of a cut-point, yet individual values intermingle (Figure 1). A universal threshold will misclassify a nontrivial fraction in both directions. The second problem is drift. Because association with clinical course is driven by slope, any strata created at baseline begin to erode as soon as data accumulate. The third problem is portability. Absolute values vary by platform and by cohort composition, including age, comorbidity, and sample handling. A cut-point that behaves well in one setting can falter in another. The fourth problem is incompleteness. Progression is shaped by co-pathologies such as vascular disease, Lewy body pathology, or abnormal TAR (trans-activation response) DNA-binding protein 43 kDa (TDP-43) levels, by cognitive reserve, by sleep and systemic health, and by medications that influence protein turnover and clearance. For instance, TDP-43 co-pathology may dilute p-tau217’s predictive value [12]. No single epitope-specific plasma signal will capture all that variance.
In summary, some amyloid-positive individuals who appear cognitively normal at entry will belong to the rapid decliner cluster, while many labeled MCIs will remain stable for years. If the aim is to construct trial strata that remain valid for the duration of follow-up, several practical problems arise.
A disciplined pathway contains three phases. The first phase is triage. A blood draw early in the work-up, with a three-range interpretation, channels people efficiently. Rule-out results justify a watchful waiting approach or further evaluation for non-Alzheimer’s disease causes when the clinical picture is not compelling. Indeterminate results should prompt additional assessment with CSF biomarkers or PET imaging. Rule-in results justify downstream staging and, when appropriate, discussion of disease-modifying therapy eligibility and trial participation. This is the point at which p-tau217 pays for itself in service access and patient convenience (Figure 2).
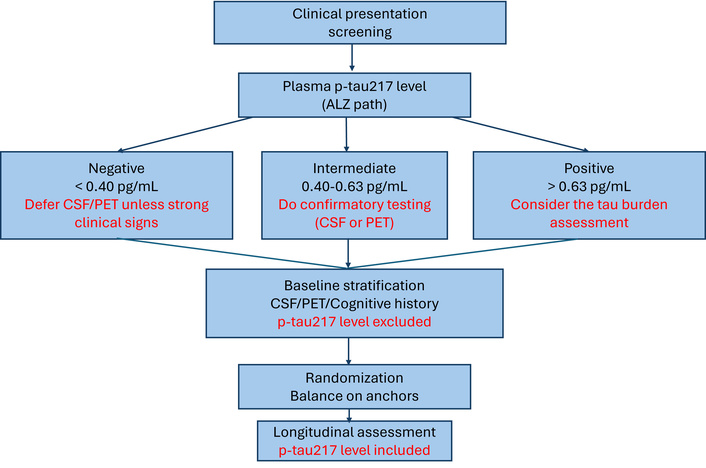
Triage-and-stratify workflow using plasma p-tau217 levels. Triage: p-tau217 thresholds (rule-out < 0.40 pg/mL; indeterminate 0.40–0.63 pg/mL; rule-in > 0.63 pg/mL) guide confirmatory testing. Stratification: time-stable anchors (tau-PET, MRI). Monitoring at 3, 6, 12, etc. months: p-tau217 level as a time-varying covariate. ALZ: Alzheimer’s; PET: positron emission tomography; CSF: cerebrospinal fluid; p-tau217: tau phosphorylated at threonine 217; MRI: magnetic resonance imaging.
The second phase is stratification. Here, time-stable, orthogonal anchors do the heavy lifting. Tau-PET burden categories index tangle load and tend to change more slowly than visit schedules. Structural MRI patterns provide an anatomical readout of neurodegeneration and help disentangle mixed etiologies. Cognitive history, particularly when prior slopes or composite scores are available, captures the trajectory that preceded screening rather than a single snapshot. These anchors can be combined with simple clinical variables such as age and the expressed variant(s) of the APOE gene to define entry strata and to balance randomization. p-tau217 can be kept out of the gatekeeping role at this stage for the reasons already outlined (Figure 2).
The third phase is monitoring. p-tau217 returns to the foreground as a time-varying covariate. Prespecified measurements at three, six, and twelve months, or at a cadence appropriate to the clinic or trial, are modeled jointly with cognitive and functional outcomes. If a therapy is expected to reduce downstream tau phosphorylation, p-tau217 becomes a natural pharmacodynamic readout. Mediation analysis can then ask whether the biomarker’s on-treatment change explains part of the observed clinical benefit. A null result on that mediation question is equally informative because it signals that the biomarker is not a viable surrogate for that mechanism, at least in that population.
This workflow (Figure 2) acknowledges what is the usefulness of the plasma p-tau217, showing its strengths at two points in the pathway, without forcing it into a stratification job that it cannot perform reliably.
A persistent problem in the Alzheimer’s disease biomarker literature is the temptation to treat agreement with PET or CSF parameters as sufficient proof of clinical accuracy. These references are useful, but they are not perfect, and they are not interchangeable across all stages of disease and across all clinical contexts. Agreement with an imperfect reference is evidence of internal coherence, not of external truth. The cross-sectional evaluation of p-tau217 acknowledges this reality by benchmarking against both amyloid and tau standards and by reporting performance transparently across them. The trajectory-based analysis goes a step further by anchoring utility to future clinical change, which is an outcome that matters to patients and caregivers.
Trial design should reflect these lessons. If a p-tau217 threshold is tuned to mimic PET data and then used to define entry, the result is a validation loop embedded in randomization. If, instead, p-tau217 is used to screen and to monitor while entry strata are defined by independent anchors, the loop is broken. Clinical validation then proceeds by demonstrating that biomarker trajectories forecast or mediate clinical change, not simply that they correlate with other biomarkers.
No biomarker survives careless handling or casual modeling. For plasma p-tau217 to deliver portable value, laboratories and investigators need to standardize the routine details that are easy to overlook. Matrix selection should be consistent, with EDTA plasma as a common choice. Time to centrifugation, spin conditions, storage temperature, and the number of freeze-thaw cycles should be pre-specified and recorded. Hemolysis needs to be documented, and out-of-range specimens should be flagged. Assay specificity should be defined clearly, including epitope mapping and cross-reactivity to adjacent phospho-sites, and programs for external quality assessment should be used to quantify inter-lot and inter-laboratory variability. When possible, shared reference materials should be employed to facilitate calibration transfer across platforms [13–16].
Modeling choices deserve the same attention. Dichotomizing continuous data is seductive because it simplifies communication, but it throws away information and makes analyses fragile to small shifts in distribution. Trajectory models, which estimate person-specific slopes and relate them to future change, align more closely with the way p-tau217 behaves. Decision-curve analysis can make explicit the clinical consequences of choosing one threshold over another in a given setting, which is often more helpful to clinicians and payers than a single AUC value. Confounders such as renal function, systemic inflammation, body mass index, and common medications can influence circulating proteins and should be measured and incorporated into interpretation [12]. These measures reflect routine best practice, underpinning the analytical validity necessary for an assay to function as a clinical instrument.
Clinicians may wonder whether plasma p-tau217 should be used alone or combined with other plasma measures such as Aβ1–42 to Aβ1–40 ratio, glial fibrillary acidic protein (GFAP), or neurofilament light chain (NfL) levels [17]. The short answer is that combinations may add value for specific questions, but they do not change the fundamental logic argued here. If the goal is triage for amyloid biology, plasma p-tau217 performs well on its own and can be paired with other plasma parameters in settings where the incremental gain matters. If the goal is to monitor disease activity, serial p-tau217 already carries a strong trajectory signal, and adding GFAP or NfL may refine interpretation when mechanisms not related to Alzheimer’s disease are involved. None of these panels, however, turns a dynamic plasma signal into a time-stable stratifier. The complementary role of tau-PET, MRI, and cognitive history remains.
The promise of a blood-first pathway is not only scientific. It is logistical and ethical. A three-range p-tau217 strategy can reduce travel and waiting time for patients and can concentrate PET and lumbar puncture resources where they deliver the most value. That effect is likely to be largest in health systems where imaging capacity is constrained or unevenly distributed. At the same time, equity requires vigilance. If access to confirmatory testing is poor in a given region, a large intermediate band could translate into delays or uncertainty. Implementations should therefore be paired with pragmatic solutions, for example, shared PET lists across centers, mobile phlebotomy with standardized handling, and clear communication materials that explain what a rule-out or rule-in result means in plain language. Laboratories should report not just a number, but also the context, including assay platform, reference ranges, and any pre-analytical deviations.
Blood tests for brain disease carry emotional weight. Clinicians should communicate probabilistically and longitudinally. A single elevated plasma p-tau217 level should prompt discussion of the next step rather than a definitive label. Serial results can be framed in terms of changing probability. An upward trend increases the likelihood of near-term decline and can justify closer follow-up or early therapy, while a flat trajectory justifies watchful waiting and attention to reversible contributors. Families benefit when they hear that the biomarker is informative but not omniscient, that it is used alongside clinical examination and imaging, and that its greatest strength lies in how it changes rather than in what it says on a single day.
A common first objection is cost-effectiveness. Another is that baseline p-tau217 is higher on average in people who decline; therefore, it should be used to stratify. The problem is that averages do not govern individuals. Overlap at entry is large enough that a hard cut-point will misclassify too many people, and the misclassification will worsen as time goes on because the biology is dynamic. In addition, health systems need a blood-only replacement for PET imaging and CSF tests. p-tau217 does reduce dependence on imaging and lumbar puncture by routing people intelligently, but replacement is the wrong aim. The right aim is to use blood to screen and monitor while reserving imaging or CSF tests for the staging and adjudication questions that still require time-stable anchors. Last but not least, clinicians must go beyond simple yes-or-no answers. In truth, clinicians need answers that are both honest and actionable. Statements that reflect trajectories are more useful than rigid labels. This patient’s biomarker is rising on repeat testing, which increases the probability of near-term decline, says more about what comes next than a yes or no assigned at baseline.
Clear positioning matters for policy. If p-tau217 is labeled and reimbursed as a triage and monitoring tool, documentation should specify appropriate matrices, pre-analytical limits, recommended repeat intervals, and interpretive ranges by clinical setting. Coverage policies should anticipate the intermediate band and reimburse confirmatory testing for those individuals, because that is where diagnostic uncertainty is intentionally concentrated. For trials, guidance that encourages biomarker anchored monitoring and mediation, while reserving baseline stratification for orthogonal, time-stable anchors, will avoid locking circular validation into pivotal studies.
The arguments made here rest on convergent findings rather than on a single dataset. The recent approval by the FDA of Lumipulse G p-tau217/Aβ1–42 adds another perspective consisting of ratios of two parameters in plasma. Further analyses are needed to evaluate whether such a ratio can truly serve as a reliable marker, but current evidence does not make it clear that this ratio could serve for patient stratification, monitoring disease progression, or assessing drug efficacy in clinical trials.
Head-to-head comparisons of p-tau217 alone versus multimarker panels for prediction of clinical outcomes will clarify marginal gains and inform cost-effectiveness. In this sense, rapid technological advances in metabolomics should identify reliable plasma biomarkers of this and other neurodegenerative diseases [9]. Concrete future work includes prospective validation in diverse cohorts (e.g., very elderly, high-comorbidity populations) to test workflow robustness, as well as expansion in cohorts where confounders and competing risks are common. Interventional work should prospectively test whether changes in plasma p-tau217 mediate clinical benefit, which would strengthen the case for using it as part of a surrogate endpoint framework. Finally, continued progress on assay harmonization is needed so that values can be interpreted across platforms without overreliance on site-specific thresholds.
Plasma p-tau217 has progressed from discovery to clinical application. Recent findings indicate that it can streamline diagnostic pathways, reduce reliance on invasive and costly procedures, and serve as a sensitive marker of disease dynamics. However, using p-tau217 solely as a baseline stratification tool in trials of novel therapies risks overlooking the insights it provides into disease progression itself. Recognizing its dynamic nature and integrating it thoughtfully into study designs offers the most direct path from molecular insight to meaningful clinical benefit. Similar scrutiny should be applied to other p-tau biomarkers and to composite measures, such as the p-tau217/Aβ1–42 ratio, to ensure their risk-benefit profile is fully understood and to maximize their translational potential.
CSF: cerebrospinal fluid
GFAP: glial fibrillary acidic protein
MCI: mild cognitive impairment
MRI: magnetic resonance imaging
NfL: neurofilament light chain
PET: positron emission tomography
p-tau217: tau phosphorylated at threonine 217
TDP-43: trans-activation response DNA-binding protein 43 kDa
RF: Writing—original draft, Writing—review & editing, Conceptualization. The author read and approved the submitted version of the manuscript.
Rafael Franco, who is the Editor-in-Chief of Exploration of Neuroprotective Therapy, had no involvement in the decision-making or the review process of this manuscript.
Not applicable.
Not applicable.
Not applicable.
The data in this study were obtained from [10], which was published under the CC BY 4.0 license.
No external funding was received for this work.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
