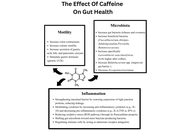
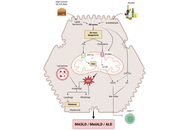







This review goes over the impact of caffeine consumption in inflammatory bowel disease (IBD), examining epidemiology, clinical outcomes, mechanistic studies, and translational research. Caffeine, a widely consumed methylxanthine, exerts diverse physiological effects on the gastrointestinal tract. Mechanistic and preclinical data offer plausible biological pathways by which caffeine could influence the IBD course. Caffeine’s antagonism of adenosine receptors may modulate immune cell activation and cytokine release; its effects on gut motility and secretion can alter symptom perception, and caffeine-mediated changes in intestinal epithelial barrier function, oxidative stress, and the gut microbiome have been demonstrated. These effects make it a lucrative investigational option, and various studies have demonstrated that caffeine intake may reduce the incidence of IBD and may even have disease-modifying effects in regular consumers. However, differences in caffeine source (coffee, tea, soda), dose, concurrent dietary patterns, and disease subtype (Crohn’s disease versus ulcerative colitis) limit definitive causal inference. Clinical implications remain cautious: while moderate caffeine intake may be tolerable and even helpful for many patients, individualized assessment is advisable, particularly for those with symptom-triggering sensitivity or overlapping functional bowel disorders. Future research should target mechanistic links and clinically meaningful outcomes to inform evidence-based dietary guidance for people with IBD.
This review goes over the impact of caffeine consumption in inflammatory bowel disease (IBD), examining epidemiology, clinical outcomes, mechanistic studies, and translational research. Caffeine, a widely consumed methylxanthine, exerts diverse physiological effects on the gastrointestinal tract. Mechanistic and preclinical data offer plausible biological pathways by which caffeine could influence the IBD course. Caffeine’s antagonism of adenosine receptors may modulate immune cell activation and cytokine release; its effects on gut motility and secretion can alter symptom perception, and caffeine-mediated changes in intestinal epithelial barrier function, oxidative stress, and the gut microbiome have been demonstrated. These effects make it a lucrative investigational option, and various studies have demonstrated that caffeine intake may reduce the incidence of IBD and may even have disease-modifying effects in regular consumers. However, differences in caffeine source (coffee, tea, soda), dose, concurrent dietary patterns, and disease subtype (Crohn’s disease versus ulcerative colitis) limit definitive causal inference. Clinical implications remain cautious: while moderate caffeine intake may be tolerable and even helpful for many patients, individualized assessment is advisable, particularly for those with symptom-triggering sensitivity or overlapping functional bowel disorders. Future research should target mechanistic links and clinically meaningful outcomes to inform evidence-based dietary guidance for people with IBD.
DOI: https://doi.org/10.37349/edd.2026.1005112
This article belongs to the special issue Inflammatory Diseases of the Gastrointestinal Tract

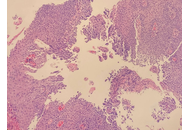
Herpes simplex esophagitis (HSE) is a viral infection of the esophagus caused by the herpes simplex virus (HSV), most commonly HSV-1. It predominantly presents among immunosuppressed individuals. Eosinophilic esophagitis (EoE) is a chronic, inflammatory, immune-mediated disease characterized by significant eosinophilic infiltration in the esophageal mucosa. It is often associated with atopic diseases, including asthma, food allergies, and eczema. Coexistence of HSE and EoE is rare and may be underdiagnosed due to challenges in diagnosing both conditions simultaneously. A major diagnostic dilemma can be traced to their histopathological similarities and differences. HSE is typically characterized by multinuclear giant cells containing intranuclear inclusions, while EoE involves eosinophilic infiltration in the esophageal epithelium. This report highlights the rare but remarkable coexistence between HSE and EoE secondary to a unique patient case. Although each condition may cause esophagitis individually, together—particularly in immunocompetent individuals—they do present a different diagnostic and therapeutic challenge.
Herpes simplex esophagitis (HSE) is a viral infection of the esophagus caused by the herpes simplex virus (HSV), most commonly HSV-1. It predominantly presents among immunosuppressed individuals. Eosinophilic esophagitis (EoE) is a chronic, inflammatory, immune-mediated disease characterized by significant eosinophilic infiltration in the esophageal mucosa. It is often associated with atopic diseases, including asthma, food allergies, and eczema. Coexistence of HSE and EoE is rare and may be underdiagnosed due to challenges in diagnosing both conditions simultaneously. A major diagnostic dilemma can be traced to their histopathological similarities and differences. HSE is typically characterized by multinuclear giant cells containing intranuclear inclusions, while EoE involves eosinophilic infiltration in the esophageal epithelium. This report highlights the rare but remarkable coexistence between HSE and EoE secondary to a unique patient case. Although each condition may cause esophagitis individually, together—particularly in immunocompetent individuals—they do present a different diagnostic and therapeutic challenge.
DOI: https://doi.org/10.37349/edd.2026.1005111

Digestive diseases comprise a diverse range of illnesses, which are prevalent worldwide and represent an important health issue. This is particularly relevant for the impact of metabolic dysfunction-associated steatotic liver disease (MASLD) due to its close association with the obesity pandemic, contributing to the escalation of MASLD as the most common form of chronic liver disease, and the main cause of liver cancer. Not only does MASLD reflect the deterioration of liver health, but it also has far-reaching consequences for the development of extrahepatic digestive diseases. Along with the progression of liver and digestive diseases to liver, colorectal and pancreatic cancer, the onset of inflammation in diseases of the digestive tract, drug-induced liver injury, and cholestasis, drives and contributes to the rise of these diseases in the future, which merit the attention of clinical and translational research to increase our understanding of the pathogenic mechanisms underlying these disorders in order to improve the diagnosis, management, and treatment. With this goal in mind, the current collaborative review gathers experts in a wide range of liver and digestive diseases to provide an up-to-date overview of the mechanisms of disease and identify novel strategies for the improvement of these important health issues.
Digestive diseases comprise a diverse range of illnesses, which are prevalent worldwide and represent an important health issue. This is particularly relevant for the impact of metabolic dysfunction-associated steatotic liver disease (MASLD) due to its close association with the obesity pandemic, contributing to the escalation of MASLD as the most common form of chronic liver disease, and the main cause of liver cancer. Not only does MASLD reflect the deterioration of liver health, but it also has far-reaching consequences for the development of extrahepatic digestive diseases. Along with the progression of liver and digestive diseases to liver, colorectal and pancreatic cancer, the onset of inflammation in diseases of the digestive tract, drug-induced liver injury, and cholestasis, drives and contributes to the rise of these diseases in the future, which merit the attention of clinical and translational research to increase our understanding of the pathogenic mechanisms underlying these disorders in order to improve the diagnosis, management, and treatment. With this goal in mind, the current collaborative review gathers experts in a wide range of liver and digestive diseases to provide an up-to-date overview of the mechanisms of disease and identify novel strategies for the improvement of these important health issues.
DOI: https://doi.org/10.37349/edd.2026.1005110

Interventional radiology (IR) is an ideal domain for artificial intelligence (AI) due to its data-intensive nature. This review provides a targeted guide for clinicians on AI applications in liver interventions, specifically focusing on hepatocellular carcinoma and portal hypertension. Key findings from recent literature demonstrate that AI models achieve high accuracy in predicting the response to transarterial chemoembolization and in non-invasively estimating the hepatic venous pressure gradient. Furthermore, emerging deep learning architectures, such as Swin Transformers, are outperforming traditional mRECIST criteria in longitudinal treatment monitoring. Despite these technical successes, the transition from “code to bedside” is hindered by limited external validation and the “black box” nature of complex algorithms. We conclude that the future of IR lies in the “AI-augmented” interventional radiologist paradigm, in which AI serves as a precision tool for patient selection and procedural safety rather than as a replacement for clinical judgment.
Interventional radiology (IR) is an ideal domain for artificial intelligence (AI) due to its data-intensive nature. This review provides a targeted guide for clinicians on AI applications in liver interventions, specifically focusing on hepatocellular carcinoma and portal hypertension. Key findings from recent literature demonstrate that AI models achieve high accuracy in predicting the response to transarterial chemoembolization and in non-invasively estimating the hepatic venous pressure gradient. Furthermore, emerging deep learning architectures, such as Swin Transformers, are outperforming traditional mRECIST criteria in longitudinal treatment monitoring. Despite these technical successes, the transition from “code to bedside” is hindered by limited external validation and the “black box” nature of complex algorithms. We conclude that the future of IR lies in the “AI-augmented” interventional radiologist paradigm, in which AI serves as a precision tool for patient selection and procedural safety rather than as a replacement for clinical judgment.
DOI: https://doi.org/10.37349/edd.2026.1005109
This article belongs to the special issue Advances in Hepato-gastroenterology: Diagnosis, Prognostication, and Disease Stratification

Aim:
Hepatocellular carcinoma (HCC) accounts for 90% of liver tumors and is the fourth leading cause of cancer-related deaths worldwide. Current treatments have poor outcomes for HCC, highlighting the urgent need for new and effective therapies. Growth differentiation factor 11 (GDF11), a member of the TGF-β superfamily, regulates differentiation, proliferation, and migration processes, effects observed in cancer, including HCC. In this study, we aimed to investigate the chemosensitizing effects on human liver cancer cells.
Methods:
We pre-treated Huh7 and Hep3B cells with GDF11 50 ng/mL for 72 h in the presence of sorafenib (Sfb) or cisplatin (CDDP) and evaluated cellular response.
Results:
Pre-treatment with GDF11 lowered the IC50 of CDDP and Sfb in Huh7 cells. Similar effects were observed in Hep3B cells. Additionally, combining GDF11 with CDDP or Sfb significantly reduced cell viability and decreased the size and number of spheroids. Furthermore, we found that the chemosensitizing effect is initiated by GDF11 binding to the type I receptor ALK5. Inhibition of ALK5 abolished SMAD2 activation, impacting the chemosensitizing effects. Finally, GDF11, combined with Sfb or CDDP, reduced the activity of drug transporters MRP2, MRP3, and MRP4, which explains its chemosensitizing properties.
Conclusions:
GDF11 increases the sensitivity of HCC-derived cell lines to Sfb and CDDP by modulating the drug-efflux transporters MRP2, MRP3, and MRP4.
Aim:
Hepatocellular carcinoma (HCC) accounts for 90% of liver tumors and is the fourth leading cause of cancer-related deaths worldwide. Current treatments have poor outcomes for HCC, highlighting the urgent need for new and effective therapies. Growth differentiation factor 11 (GDF11), a member of the TGF-β superfamily, regulates differentiation, proliferation, and migration processes, effects observed in cancer, including HCC. In this study, we aimed to investigate the chemosensitizing effects on human liver cancer cells.
Methods:
We pre-treated Huh7 and Hep3B cells with GDF11 50 ng/mL for 72 h in the presence of sorafenib (Sfb) or cisplatin (CDDP) and evaluated cellular response.
Results:
Pre-treatment with GDF11 lowered the IC50 of CDDP and Sfb in Huh7 cells. Similar effects were observed in Hep3B cells. Additionally, combining GDF11 with CDDP or Sfb significantly reduced cell viability and decreased the size and number of spheroids. Furthermore, we found that the chemosensitizing effect is initiated by GDF11 binding to the type I receptor ALK5. Inhibition of ALK5 abolished SMAD2 activation, impacting the chemosensitizing effects. Finally, GDF11, combined with Sfb or CDDP, reduced the activity of drug transporters MRP2, MRP3, and MRP4, which explains its chemosensitizing properties.
Conclusions:
GDF11 increases the sensitivity of HCC-derived cell lines to Sfb and CDDP by modulating the drug-efflux transporters MRP2, MRP3, and MRP4.
DOI: https://doi.org/10.37349/edd.2025.1005108

Diverticulitis is one of the most common gastrointestinal causes of hospitalization in Western society. While previously characterized as a disease of older patients, new literature highlights an increasing incidence among the younger population. Over the past few decades, the understanding of etiology and management of diverticulitis has changed drastically. New data refute past beliefs while promoting other novel recommendations to mitigate incidence and subsequent complications. Data now confirms the safety and possible protective benefit of particulate food, while highlighting evidence-based approaches for the use of diagnostic imaging and antibiotics. We recognize modifiable and non-modifiable risk factors that are commonly seen throughout the literature and play a significant role in the management and prevention of diverticulitis. Emerging evidence also links chronic inflammation with subsequent microbial dysbiosis and alterations in the neuroendocrine system, leading to visceral hypersensitivity and perturbation of the gut-brain axis. This review provides a comprehensive update on acute uncomplicated diverticulitis according to the most recent evidence-based literature, encompassing the risks, diagnostic modalities, and management treatment regimens.
Diverticulitis is one of the most common gastrointestinal causes of hospitalization in Western society. While previously characterized as a disease of older patients, new literature highlights an increasing incidence among the younger population. Over the past few decades, the understanding of etiology and management of diverticulitis has changed drastically. New data refute past beliefs while promoting other novel recommendations to mitigate incidence and subsequent complications. Data now confirms the safety and possible protective benefit of particulate food, while highlighting evidence-based approaches for the use of diagnostic imaging and antibiotics. We recognize modifiable and non-modifiable risk factors that are commonly seen throughout the literature and play a significant role in the management and prevention of diverticulitis. Emerging evidence also links chronic inflammation with subsequent microbial dysbiosis and alterations in the neuroendocrine system, leading to visceral hypersensitivity and perturbation of the gut-brain axis. This review provides a comprehensive update on acute uncomplicated diverticulitis according to the most recent evidence-based literature, encompassing the risks, diagnostic modalities, and management treatment regimens.
DOI: https://doi.org/10.37349/edd.2025.1005107
This article belongs to the special issue Diverticulitis: Pathomechanism, Diagnosis and Treatment

Gastric cancer (GC) is one of the most common malignant tumors, ranking fifth in incidence and third in mortality worldwide. China also bears a high burden of GC, second only to lung cancer. With the advancement of clustered regularly interspaced short palindromic repeats (CRISPR) technology, the mechanisms underlying the development and progression of various tumor types have been elucidated. This article summarizes the application of CRISPR technology in the functional genomics identification and target screening of GC genes, explores the use of chimeric antigen receptor T (CAR-T) cell therapy for solid gastric tumors, and discusses the progress and significance of CRISPR technology in constructing GC models using organoids.
Gastric cancer (GC) is one of the most common malignant tumors, ranking fifth in incidence and third in mortality worldwide. China also bears a high burden of GC, second only to lung cancer. With the advancement of clustered regularly interspaced short palindromic repeats (CRISPR) technology, the mechanisms underlying the development and progression of various tumor types have been elucidated. This article summarizes the application of CRISPR technology in the functional genomics identification and target screening of GC genes, explores the use of chimeric antigen receptor T (CAR-T) cell therapy for solid gastric tumors, and discusses the progress and significance of CRISPR technology in constructing GC models using organoids.
DOI: https://doi.org/10.37349/edd.2025.1005106

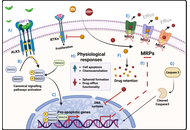
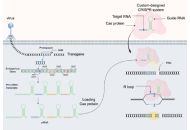
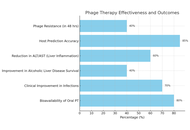
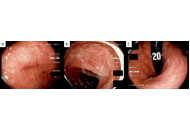
Liver cirrhosis is a condition characterized by scarring of liver tissue resulting from impaired liver function and systemic complications. It shows symptoms like jaundice, ascites, and hepatic encephalopathy. It has significant mortality and morbidity worldwide. As the study of microbial dysbiosis grows, it investigates how an imbalance in gut bacteria can speed up the progression of liver cirrhosis by spreading bacteria, endotoxins, and inflammation all over the body. Dysbiosis damages the gut–liver axis and eventually the liver. The study aims to analyze the therapeutic potential of bacteriophage therapy in liver cirrhosis. Bacteriophage treatment is a new focused method for treating microbial dysbiosis. Bacteriophages are viruses that target and attack harmful pathogens without affecting the helpful ones or causing an imbalance in the gut microbiota’s equilibrium. Since broad-spectrum antibiotics can affect the gut microbiota and lead to antibiotic resistance, phages are a better alternative due to their selectivity. According to preclinical research conducted in animal models, bacteriophage therapy can lower the bacterial load, enhance liver function tests, and decrease the systemic inflammatory indicators. Bacteriophage safety, as well as potential effectiveness in balancing gut microbiota, reducing systemic inflammation, and relieving symptoms such as hepatic encephalopathy, has been shown by preliminary clinical trials and case reports. However, issues like phage-resistant bacteria, patient-specific gut microbiota variation, and lack of clinical trials continue to prevent general use. Additional research is required to determine if it can be used in clinical practice, including large clinical trials and individualized strategies. Bacteriophage therapy is a promising and new technique for improving liver cirrhosis outcomes.
Liver cirrhosis is a condition characterized by scarring of liver tissue resulting from impaired liver function and systemic complications. It shows symptoms like jaundice, ascites, and hepatic encephalopathy. It has significant mortality and morbidity worldwide. As the study of microbial dysbiosis grows, it investigates how an imbalance in gut bacteria can speed up the progression of liver cirrhosis by spreading bacteria, endotoxins, and inflammation all over the body. Dysbiosis damages the gut–liver axis and eventually the liver. The study aims to analyze the therapeutic potential of bacteriophage therapy in liver cirrhosis. Bacteriophage treatment is a new focused method for treating microbial dysbiosis. Bacteriophages are viruses that target and attack harmful pathogens without affecting the helpful ones or causing an imbalance in the gut microbiota’s equilibrium. Since broad-spectrum antibiotics can affect the gut microbiota and lead to antibiotic resistance, phages are a better alternative due to their selectivity. According to preclinical research conducted in animal models, bacteriophage therapy can lower the bacterial load, enhance liver function tests, and decrease the systemic inflammatory indicators. Bacteriophage safety, as well as potential effectiveness in balancing gut microbiota, reducing systemic inflammation, and relieving symptoms such as hepatic encephalopathy, has been shown by preliminary clinical trials and case reports. However, issues like phage-resistant bacteria, patient-specific gut microbiota variation, and lack of clinical trials continue to prevent general use. Additional research is required to determine if it can be used in clinical practice, including large clinical trials and individualized strategies. Bacteriophage therapy is a promising and new technique for improving liver cirrhosis outcomes.
DOI: https://doi.org/10.37349/edd.2025.1005105
This article belongs to the special issue Gut Microbiota towards Personalized Medicine in Metabolic Disease

Pediatric cirrhosis differs significantly from adult liver disease in terms of etiology, progression, and management. The unique physiological, nutritional, and developmental needs of children require specialized diagnostic and therapeutic strategies. This review underscores the distinct challenges in diagnosing and managing pediatric cirrhosis, focusing on its complications, management, and outcomes. Unlike adults, where cirrhosis often results from viral hepatitis or alcohol use, pediatric cases are predominantly cholestatic, with biliary atresia being the most common cause. Complications mainly involve portal hypertension and impaired liver function, leading to malnutrition and neurodevelopmental delay. Nutritional management is complex and requires increased caloric and protein intake, supplementation with fat-soluble vitamins, and the use of medium-chain triglycerides. Although hepatocellular carcinoma is rare in children, it remains a severe complication with a higher incidence in certain genetic and metabolic disorders. Surveillance is challenging due to diagnostic limitations and the lack of standardized pediatric screening protocols. Treatment is further complicated by constraints related to size and developmental stage, particularly in the management of portal hypertension. Pediatric cirrhosis requires an individualized multidisciplinary approach to address the interplay between growth, nutrition, and liver function. Early diagnosis, nutritional optimization, malignancy surveillance, and timely referral for liver transplantation are crucial. Ongoing research on pediatric-specific therapies and outcomes is essential for improving prognosis and quality of life.
Pediatric cirrhosis differs significantly from adult liver disease in terms of etiology, progression, and management. The unique physiological, nutritional, and developmental needs of children require specialized diagnostic and therapeutic strategies. This review underscores the distinct challenges in diagnosing and managing pediatric cirrhosis, focusing on its complications, management, and outcomes. Unlike adults, where cirrhosis often results from viral hepatitis or alcohol use, pediatric cases are predominantly cholestatic, with biliary atresia being the most common cause. Complications mainly involve portal hypertension and impaired liver function, leading to malnutrition and neurodevelopmental delay. Nutritional management is complex and requires increased caloric and protein intake, supplementation with fat-soluble vitamins, and the use of medium-chain triglycerides. Although hepatocellular carcinoma is rare in children, it remains a severe complication with a higher incidence in certain genetic and metabolic disorders. Surveillance is challenging due to diagnostic limitations and the lack of standardized pediatric screening protocols. Treatment is further complicated by constraints related to size and developmental stage, particularly in the management of portal hypertension. Pediatric cirrhosis requires an individualized multidisciplinary approach to address the interplay between growth, nutrition, and liver function. Early diagnosis, nutritional optimization, malignancy surveillance, and timely referral for liver transplantation are crucial. Ongoing research on pediatric-specific therapies and outcomes is essential for improving prognosis and quality of life.
DOI: https://doi.org/10.37349/edd.2025.1005104
This article belongs to the special issue Cirrhosis and Its Complications

Interleukin-17 inhibitors (IL-17i) are used for dermatologic and rheumatologic immune-mediated inflammatory diseases (IMIDs), yet paradoxical inflammatory bowel disease (IBD) can occur. Although trials report low incidence, recognition and management remain difficult outside tertiary care centers. A 54-year-old woman treated with ixekizumab (IXE) for presumptive psoriatic arthritis (PsA) without definitive confirmation developed anorexia, weight loss, abdominal pain, rectal urgency, and hematochezia 16 weeks after IXE initiation. Limited access to gastroenterology contributed to the delayed workup. Catastrophic complications, including bowel perforation, postoperative abscesses, and severe malnutrition, resulted from the cumulative effects of longstanding, inadequately treated disease; excessive immunosuppression with high-dose corticosteroids and infliximab; and concurrent use of opioids and antidiarrheals, among other factors. On transfer to a center skilled in IBD, care included withdrawal of excessive immunosuppression, targeted antimicrobials, and nutrition rehabilitation. Histopathology of the surgical specimen was most consistent with features of Crohn’s disease (CD). After recovery, she achieved clinical, endoscopic, and histologic remission. On rheumatologic reassessment at an independent practice, she did not meet classification criteria for PsA. With continued specialty follow-up, the patient has remained in sustained clinical, laboratory, and endoscopic remission for 16 months, underscoring that timely recognition and disciplined, evidence-based care grounded in the principles used for severe IBD and drug-induced colitis can deliver favorable long-term outcomes. This case highlights the need for structured, accessible clinical guidance, not only to support non-IBD specialists in managing IL-17i-associated complications but also to guide clinicians during the pre-therapy phase in selecting appropriate candidates for treatment and assessing potential gastrointestinal risks before initiating therapy. We present an evidence-informed framework for resource-limited settings that addresses screening, early recognition, diagnostic workup, and therapeutic decision-making to guide safer IL-17i use and improve outcomes.
Interleukin-17 inhibitors (IL-17i) are used for dermatologic and rheumatologic immune-mediated inflammatory diseases (IMIDs), yet paradoxical inflammatory bowel disease (IBD) can occur. Although trials report low incidence, recognition and management remain difficult outside tertiary care centers. A 54-year-old woman treated with ixekizumab (IXE) for presumptive psoriatic arthritis (PsA) without definitive confirmation developed anorexia, weight loss, abdominal pain, rectal urgency, and hematochezia 16 weeks after IXE initiation. Limited access to gastroenterology contributed to the delayed workup. Catastrophic complications, including bowel perforation, postoperative abscesses, and severe malnutrition, resulted from the cumulative effects of longstanding, inadequately treated disease; excessive immunosuppression with high-dose corticosteroids and infliximab; and concurrent use of opioids and antidiarrheals, among other factors. On transfer to a center skilled in IBD, care included withdrawal of excessive immunosuppression, targeted antimicrobials, and nutrition rehabilitation. Histopathology of the surgical specimen was most consistent with features of Crohn’s disease (CD). After recovery, she achieved clinical, endoscopic, and histologic remission. On rheumatologic reassessment at an independent practice, she did not meet classification criteria for PsA. With continued specialty follow-up, the patient has remained in sustained clinical, laboratory, and endoscopic remission for 16 months, underscoring that timely recognition and disciplined, evidence-based care grounded in the principles used for severe IBD and drug-induced colitis can deliver favorable long-term outcomes. This case highlights the need for structured, accessible clinical guidance, not only to support non-IBD specialists in managing IL-17i-associated complications but also to guide clinicians during the pre-therapy phase in selecting appropriate candidates for treatment and assessing potential gastrointestinal risks before initiating therapy. We present an evidence-informed framework for resource-limited settings that addresses screening, early recognition, diagnostic workup, and therapeutic decision-making to guide safer IL-17i use and improve outcomes.
DOI: https://doi.org/10.37349/edd.2025.1005103

DOI: https://doi.org/10.37349/edd.2025.1005102
This article belongs to the special issue Advances in Hepato-gastroenterology: Diagnosis, Prognostication, and Disease Stratification

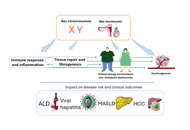
Females are more susceptible to alcohol-related liver disease (ALD) owing to increased risk of alcohol dependence; decreased gastric first-pass effect and increased risk of producing hepatotoxic metabolites, higher alcohol bioavailability, and hormonal fluctuations affecting ethanol metabolism. Male sex is independently associated with hepatitis B virus (HBV) infection and hypertransaminasemia in HBV chronic infection. Compared to women, men have higher risks of being hepatitis B surface antigen (HBsAg) carriers, exhibit higher non-response and lower long-term immunity after prophylactic vaccination, have a higher risk of chronic hepatitis, and fibrotic and hepatocellular carcinoma (HCC). Females have higher spontaneous hepatitis C virus (HCV) clearance and reduced risk of fibrosis, cirrhosis, and HCC than men. However, post-menopausal women experience more rapid progression of hepatic fibrosis and HCC development and lower response rates to antiviral regimens compared to younger women. Hormonal and immunological mechanisms explain these sex differences observed in chronic viral hepatitis B and C. Sex and reproductive status affect the risk of metabolic dysfunction-associated steatotic liver disease (MASLD) development and progression. Genetic sex and sex hormones are involved in the pathogenesis of sex differences in MASLD by differential effects on body fat distribution, insulin sensitivity, and oxidative stress. HCC may arise as a complication of ALD, HBV, HCV, and MASLD and has a definite prevalence in the male sex because of the most robust inflammatory response of the male sex and the anti-inflammatory activity of estrogens. We conclude that those major sex differences which are identifiable in the epidemiology and clinical course of ALD, viral hepatitis owing to HBV and HCV, MASLD, and HCC. These sex disparities are explained by biological sex and sex hormones affecting metabolism, immunity, fibrogenesis, and cancer, and are the foundations for precision medicine approaches in these common hepatological conditions.
Females are more susceptible to alcohol-related liver disease (ALD) owing to increased risk of alcohol dependence; decreased gastric first-pass effect and increased risk of producing hepatotoxic metabolites, higher alcohol bioavailability, and hormonal fluctuations affecting ethanol metabolism. Male sex is independently associated with hepatitis B virus (HBV) infection and hypertransaminasemia in HBV chronic infection. Compared to women, men have higher risks of being hepatitis B surface antigen (HBsAg) carriers, exhibit higher non-response and lower long-term immunity after prophylactic vaccination, have a higher risk of chronic hepatitis, and fibrotic and hepatocellular carcinoma (HCC). Females have higher spontaneous hepatitis C virus (HCV) clearance and reduced risk of fibrosis, cirrhosis, and HCC than men. However, post-menopausal women experience more rapid progression of hepatic fibrosis and HCC development and lower response rates to antiviral regimens compared to younger women. Hormonal and immunological mechanisms explain these sex differences observed in chronic viral hepatitis B and C. Sex and reproductive status affect the risk of metabolic dysfunction-associated steatotic liver disease (MASLD) development and progression. Genetic sex and sex hormones are involved in the pathogenesis of sex differences in MASLD by differential effects on body fat distribution, insulin sensitivity, and oxidative stress. HCC may arise as a complication of ALD, HBV, HCV, and MASLD and has a definite prevalence in the male sex because of the most robust inflammatory response of the male sex and the anti-inflammatory activity of estrogens. We conclude that those major sex differences which are identifiable in the epidemiology and clinical course of ALD, viral hepatitis owing to HBV and HCV, MASLD, and HCC. These sex disparities are explained by biological sex and sex hormones affecting metabolism, immunity, fibrogenesis, and cancer, and are the foundations for precision medicine approaches in these common hepatological conditions.
DOI: https://doi.org/10.37349/edd.2025.1005101

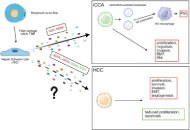
Recent studies demonstrate that peripheral innervation and Schwann cells (SCs) activity within the tumor microenvironment (TME) have a significant influence on liver cancer, specifically hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA). Even though the role of SCs in HCC behavior is only hypothetical, neurotrophic factors like brain-derived neurotrophic factor (BDNF) and artemin (ARTN) enhance tumor growth, angiogenesis, and metastasis. Thus, targeting these pathways has a promising therapeutic potential. Conversely, receptors such as glial cell line-derived neurotrophic factor family receptor α-1 (GFRα1) and factors like neurotrophin-3 (NTF3) exhibit tumor-suppressive roles, indicating a context-dependent, dual impact of neurotrophic signaling in liver cancer. In iCCA, perineural invasion (PNI) correlates with poor prognosis, with SCs promoting tumor progression through the secretion of neurotrophic factors such as nerve growth factor (NGF) and BDNF, which activate signaling pathways downstream. These pathways facilitate epithelial-mesenchymal transition (EMT), invasion, and neural infiltration. This review emphasizes the complex roles of neurotrophic factors in hepatic tumors and their future potential as biomarkers and therapeutic targets via disruption of tumor-nerve interactions.
Recent studies demonstrate that peripheral innervation and Schwann cells (SCs) activity within the tumor microenvironment (TME) have a significant influence on liver cancer, specifically hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA). Even though the role of SCs in HCC behavior is only hypothetical, neurotrophic factors like brain-derived neurotrophic factor (BDNF) and artemin (ARTN) enhance tumor growth, angiogenesis, and metastasis. Thus, targeting these pathways has a promising therapeutic potential. Conversely, receptors such as glial cell line-derived neurotrophic factor family receptor α-1 (GFRα1) and factors like neurotrophin-3 (NTF3) exhibit tumor-suppressive roles, indicating a context-dependent, dual impact of neurotrophic signaling in liver cancer. In iCCA, perineural invasion (PNI) correlates with poor prognosis, with SCs promoting tumor progression through the secretion of neurotrophic factors such as nerve growth factor (NGF) and BDNF, which activate signaling pathways downstream. These pathways facilitate epithelial-mesenchymal transition (EMT), invasion, and neural infiltration. This review emphasizes the complex roles of neurotrophic factors in hepatic tumors and their future potential as biomarkers and therapeutic targets via disruption of tumor-nerve interactions.
DOI: https://doi.org/10.37349/edd.2025.1005100
This article belongs to the special issue Fibrosis and Hepatobiliary Cancer

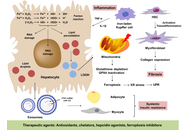
Metabolic dysfunction-associated steatotic liver disease (MASLD) is increasingly recognized as a multisystem disorder in which iron acts as both a metabolic “spark” and an accelerant of liver injury. This integrates emerging evidence that iron-driven oxidative stress and low-grade inflammation are mutually reinforcing processes in metabolic liver disease. In this perspective article, epidemiological evidence, molecular insights, and emerging clinical data are integrated to clarify how hyperferritinemia, often dismissed as a mere inflammatory marker, maps onto genuine iron redistribution and overload in the metabolic liver. Physiological iron homeostasis and its disruption by adiposity-related inflammation, hyperinsulinemia, sex hormones, and common HFE variants, creating a labile catalytic iron pool that fuels Fenton chemistry in lipid-laden hepatocytes. Population studies and expert-panel criteria are summarized that define “metabolic hyperferritinemia” and stratify dysmetabolic iron accumulation into three magnetic resonance imaging (MRI)-based grades, each linked to stepwise increases in steatosis, fibrosis, and clinical events. Mechanistically, excess Fe2+ triggers lipid peroxidation, mitochondrial dysfunction, ferroptosis, Kupffer cell activation, endoplasmic reticulum stress, and hepatic stellate cell sensitization to TGF-β, thereby accelerating the transition from steatosis to steatohepatitis and fibrosis. Finally, the diagnostic algorithms, iron-modulating therapies (phlebotomy, hepcidin agonists, diet), and prospective data supporting ferritin-based triage in clinics are discussed. Collectively, the outlined evidence positions iron not only as a biomarker but also as a modifiable driver of MASLD progression, underscoring the need for randomized trials that test whether targeted iron reduction improves hard hepatic outcomes.
Metabolic dysfunction-associated steatotic liver disease (MASLD) is increasingly recognized as a multisystem disorder in which iron acts as both a metabolic “spark” and an accelerant of liver injury. This integrates emerging evidence that iron-driven oxidative stress and low-grade inflammation are mutually reinforcing processes in metabolic liver disease. In this perspective article, epidemiological evidence, molecular insights, and emerging clinical data are integrated to clarify how hyperferritinemia, often dismissed as a mere inflammatory marker, maps onto genuine iron redistribution and overload in the metabolic liver. Physiological iron homeostasis and its disruption by adiposity-related inflammation, hyperinsulinemia, sex hormones, and common HFE variants, creating a labile catalytic iron pool that fuels Fenton chemistry in lipid-laden hepatocytes. Population studies and expert-panel criteria are summarized that define “metabolic hyperferritinemia” and stratify dysmetabolic iron accumulation into three magnetic resonance imaging (MRI)-based grades, each linked to stepwise increases in steatosis, fibrosis, and clinical events. Mechanistically, excess Fe2+ triggers lipid peroxidation, mitochondrial dysfunction, ferroptosis, Kupffer cell activation, endoplasmic reticulum stress, and hepatic stellate cell sensitization to TGF-β, thereby accelerating the transition from steatosis to steatohepatitis and fibrosis. Finally, the diagnostic algorithms, iron-modulating therapies (phlebotomy, hepcidin agonists, diet), and prospective data supporting ferritin-based triage in clinics are discussed. Collectively, the outlined evidence positions iron not only as a biomarker but also as a modifiable driver of MASLD progression, underscoring the need for randomized trials that test whether targeted iron reduction improves hard hepatic outcomes.
DOI: https://doi.org/10.37349/edd.2025.100599
This article belongs to the special issue Advances in Hepato-gastroenterology: Diagnosis, Prognostication, and Disease Stratification

This review explores recent advancements in the management of Helicobacter pylori infection, a widespread bacterial pathogen associated with various gastrointestinal disorders. The paper discusses improved diagnostic techniques, including molecular methods and non-invasive tests, which have enhanced detection accuracy and antibiotic resistance profiling. New treatment strategies, such as individualized therapy based on antimicrobial susceptibility testing (AST) and the use of probiotics as adjunctive therapy, are examined. The review also addresses the challenges of antibiotic resistance, highlighting the importance of surveillance and monitoring strategies. Novel antibiotic combinations and non-antibiotic therapies, including antibiofilm agents, are presented as potential solutions. The paper concludes by discussing post-treatment follow-up, management of persistent infections, and considerations for special patient populations. Future directions in Helicobacter pylori management, including emerging technologies and global eradication efforts, are briefly outlined.
This review explores recent advancements in the management of Helicobacter pylori infection, a widespread bacterial pathogen associated with various gastrointestinal disorders. The paper discusses improved diagnostic techniques, including molecular methods and non-invasive tests, which have enhanced detection accuracy and antibiotic resistance profiling. New treatment strategies, such as individualized therapy based on antimicrobial susceptibility testing (AST) and the use of probiotics as adjunctive therapy, are examined. The review also addresses the challenges of antibiotic resistance, highlighting the importance of surveillance and monitoring strategies. Novel antibiotic combinations and non-antibiotic therapies, including antibiofilm agents, are presented as potential solutions. The paper concludes by discussing post-treatment follow-up, management of persistent infections, and considerations for special patient populations. Future directions in Helicobacter pylori management, including emerging technologies and global eradication efforts, are briefly outlined.
DOI: https://doi.org/10.37349/edd.2025.100598
This article belongs to the special issue Helicobacter Pylori and Infection: Genomics, Diagnosis, Pathogenesis, Antibiotic Resistance, Microbiota, Cancer, Prevention and Therapeutics

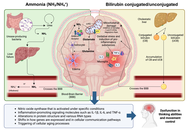
Hepatic encephalopathy (HE) is a debilitating neuropsychiatric complication of liver dysfunction that spans a continuum from subtle cognitive impairment to deep coma. While historically attributed to hyperammonemia, current insights reveal a multifactorial pathogenesis involving systemic inflammation, astrocyte dysfunction, blood-brain barrier (BBB) disruption, and altered neurotransmission. Central to this complex network is the gut-liver axis—a bidirectional system that links the gut microbiota, intestinal barrier integrity, bile acid metabolism, and hepatic immune responses. In cirrhosis, dysbiosis and increased intestinal permeability facilitate the translocation of microbial products—such as endotoxins and ammonia—that trigger hepatic and systemic immune activation, amplifying neurotoxicity through the gut-liver-brain axis. Experimental and clinical evidence has shown that ammonia and bilirubin synergistically promote neuroinflammation, mitochondrial dysfunction, and glial activation. Multiomics data further support the role of the microbiota as an active modulator of liver-brain homeostasis. Microbiota-targeted therapies—including rifaximin, probiotics, synbiotics, and fecal microbiota transplantation (FMT)—demonstrate efficacy in reducing HE recurrence, improving cognition, and restoring microbial balance. Novel receptor-based strategies targeting the farnesoid X receptor (FXR), Takeda G-protein-coupled receptor 5 (TGR5), and aryl hydrocarbon receptor (AhR) show promise for modulating bile acid pathways and mitigating neuroinflammation. Emerging approaches also focus on dietary interventions, the reinforcement of epithelial barrier function, and artificial intelligence (AI)-driven tools for personalized monitoring. Despite these advances, challenges persist regarding FMT standardization, long-term safety, and the integration of digital diagnostics into routine care.
Hepatic encephalopathy (HE) is a debilitating neuropsychiatric complication of liver dysfunction that spans a continuum from subtle cognitive impairment to deep coma. While historically attributed to hyperammonemia, current insights reveal a multifactorial pathogenesis involving systemic inflammation, astrocyte dysfunction, blood-brain barrier (BBB) disruption, and altered neurotransmission. Central to this complex network is the gut-liver axis—a bidirectional system that links the gut microbiota, intestinal barrier integrity, bile acid metabolism, and hepatic immune responses. In cirrhosis, dysbiosis and increased intestinal permeability facilitate the translocation of microbial products—such as endotoxins and ammonia—that trigger hepatic and systemic immune activation, amplifying neurotoxicity through the gut-liver-brain axis. Experimental and clinical evidence has shown that ammonia and bilirubin synergistically promote neuroinflammation, mitochondrial dysfunction, and glial activation. Multiomics data further support the role of the microbiota as an active modulator of liver-brain homeostasis. Microbiota-targeted therapies—including rifaximin, probiotics, synbiotics, and fecal microbiota transplantation (FMT)—demonstrate efficacy in reducing HE recurrence, improving cognition, and restoring microbial balance. Novel receptor-based strategies targeting the farnesoid X receptor (FXR), Takeda G-protein-coupled receptor 5 (TGR5), and aryl hydrocarbon receptor (AhR) show promise for modulating bile acid pathways and mitigating neuroinflammation. Emerging approaches also focus on dietary interventions, the reinforcement of epithelial barrier function, and artificial intelligence (AI)-driven tools for personalized monitoring. Despite these advances, challenges persist regarding FMT standardization, long-term safety, and the integration of digital diagnostics into routine care.
DOI: https://doi.org/10.37349/edd.2025.100597

Aim:
Functional defecatory disorder (FDD) is associated with impaired defecation not only due to abnormalities in recto-anal coordination, but also due to abnormalities in anal tone and rectal sensation. This study aimed to characterize the spectrum of anorectal motor and sensory abnormalities in patients with FDD using the London classification.
Methods:
In this single-center prospective study, 100 consecutive patients fulfilling Rome IV criteria for FDD were included. Secondary causes of constipation were excluded. High-resolution anorectal manometry, rectal sensory testing, and a balloon expulsion test were performed as per the International Anorectal Physiology Working Group (IAPWG) protocol. Patients were classified using the London classification, which identifies multiple subtypes of anorectal dysfunction.
Results:
The median age was 34 years (range: 18–78), with 64% males and 36% females. Among the cohort, 85% had abnormal expulsion with dyssynergia, while 15% had abnormal expulsion with poor propulsion and dyssynergia. Anal hypotension with normal contractility was seen in 9%, while 4% had anal normotension with hypocontractility. Rectal hyposensitivity and borderline rectal hyposensitivity were noted in 4% and 5% of patients, respectively.
Conclusions:
This study highlights that dyssynergic defecation is frequently accompanied by additional anorectal dysfunctions. Applying the London classification enhances the recognition of coexisting abnormalities, which may have therapeutic implications. Future research should investigate whether addressing these additional dysfunctions improves treatment outcomes in FDD.
Aim:
Functional defecatory disorder (FDD) is associated with impaired defecation not only due to abnormalities in recto-anal coordination, but also due to abnormalities in anal tone and rectal sensation. This study aimed to characterize the spectrum of anorectal motor and sensory abnormalities in patients with FDD using the London classification.
Methods:
In this single-center prospective study, 100 consecutive patients fulfilling Rome IV criteria for FDD were included. Secondary causes of constipation were excluded. High-resolution anorectal manometry, rectal sensory testing, and a balloon expulsion test were performed as per the International Anorectal Physiology Working Group (IAPWG) protocol. Patients were classified using the London classification, which identifies multiple subtypes of anorectal dysfunction.
Results:
The median age was 34 years (range: 18–78), with 64% males and 36% females. Among the cohort, 85% had abnormal expulsion with dyssynergia, while 15% had abnormal expulsion with poor propulsion and dyssynergia. Anal hypotension with normal contractility was seen in 9%, while 4% had anal normotension with hypocontractility. Rectal hyposensitivity and borderline rectal hyposensitivity were noted in 4% and 5% of patients, respectively.
Conclusions:
This study highlights that dyssynergic defecation is frequently accompanied by additional anorectal dysfunctions. Applying the London classification enhances the recognition of coexisting abnormalities, which may have therapeutic implications. Future research should investigate whether addressing these additional dysfunctions improves treatment outcomes in FDD.
DOI: https://doi.org/10.37349/edd.2025.100596

Primary liver cancer, particularly hepatocellular carcinoma, remains a major global health challenge due to its multifactorial etiology, late-stage detection, and high mortality. This review proposes a precision prevention framework that (i) categorizes risk factors into biological (e.g., HBV/HCV, aflatoxins), environmental (e.g., air pollution, occupational/waterborne toxins), and host-related domains (e.g., obesity, diabetes, genetic susceptibility); and (ii) aligns interventions to three complementary strategies: elimination of dominant risk (HBV vaccination, aflatoxin control, alcohol/tobacco reduction), early warning and targeted management (life-course immunization, MAFLD screening and control, metformin in diabetics), and chemoprevention (e.g., oltipraz, chlorophyllin, sulforaphane). We further articulate “green” prevention as a scalable, diet-centered approach that can be tailored to risk tiers and local food systems. Advances in multi-omics, microbiome science, and AI-enabled risk models—together with cohort evidence from East Asia, sub-Saharan Africa, and Western populations—support stratified surveillance and earlier interventions. Finally, we discuss generalizability and implementation challenges (regional dietary diversity, resource access) and outline pragmatic solutions to improve uptake across diverse settings.
Primary liver cancer, particularly hepatocellular carcinoma, remains a major global health challenge due to its multifactorial etiology, late-stage detection, and high mortality. This review proposes a precision prevention framework that (i) categorizes risk factors into biological (e.g., HBV/HCV, aflatoxins), environmental (e.g., air pollution, occupational/waterborne toxins), and host-related domains (e.g., obesity, diabetes, genetic susceptibility); and (ii) aligns interventions to three complementary strategies: elimination of dominant risk (HBV vaccination, aflatoxin control, alcohol/tobacco reduction), early warning and targeted management (life-course immunization, MAFLD screening and control, metformin in diabetics), and chemoprevention (e.g., oltipraz, chlorophyllin, sulforaphane). We further articulate “green” prevention as a scalable, diet-centered approach that can be tailored to risk tiers and local food systems. Advances in multi-omics, microbiome science, and AI-enabled risk models—together with cohort evidence from East Asia, sub-Saharan Africa, and Western populations—support stratified surveillance and earlier interventions. Finally, we discuss generalizability and implementation challenges (regional dietary diversity, resource access) and outline pragmatic solutions to improve uptake across diverse settings.
DOI: https://doi.org/10.37349/edd.2025.100595
This article belongs to the special issue Prevention, Screening and Diagnosis for Primary Liver Cancer

Aim:
Hepatocellular carcinoma (HCC) poses a significant global health threat. The pregnane X receptor (PXR) is a central regulator of xenobiotic metabolism and plays a key role in mediating cellular resistance to anti-tumor drugs in HCC. Indeed, the precise role of PXR in HCC pathogenesis remains unclear. This study aimed to investigate blood and hepatic PXR levels and their association with inflammation in HCC patients. Additionally, we assessed the diagnostic potential of PXR in HCC patients compared to control subjects.
Methods:
Following approval by the Institute Ethical Committee, 40 HCC patients and 40 healthy volunteers were enrolled in this study. Baseline patient characteristics, serum alpha-fetoprotein (AFP), and biochemical parameters were analyzed. Serum levels of PXR, tumor necrosis factor alpha (TNF-α), and interleukin (IL)-1β were measured using ELISA. The hepatic expression of phosphorylated nuclear factor kappa B (NFκB) and PXR proteins was analyzed by western blotting.
Results:
When compared to control subjects, serum PXR levels were increased in HCC cases (1.34 ± 0.16 vs. 4.09 ± 0.33; P < 0.0001). Similarly, hepatic PXR expression was increased in HCC tissues. Moreover, HCC patients exhibited elevated inflammatory cytokines and a deranged hepatobiliary profile compared to controls.
Conclusions:
Elevated serum PXR levels in HCC patients were positively correlated with inflammation. Notably, serum PXR demonstrated greater sensitivity and specificity in diagnosing HCC. These findings suggest that PXR may serve as a plausible biomarker in the diagnosis of HCC.
Aim:
Hepatocellular carcinoma (HCC) poses a significant global health threat. The pregnane X receptor (PXR) is a central regulator of xenobiotic metabolism and plays a key role in mediating cellular resistance to anti-tumor drugs in HCC. Indeed, the precise role of PXR in HCC pathogenesis remains unclear. This study aimed to investigate blood and hepatic PXR levels and their association with inflammation in HCC patients. Additionally, we assessed the diagnostic potential of PXR in HCC patients compared to control subjects.
Methods:
Following approval by the Institute Ethical Committee, 40 HCC patients and 40 healthy volunteers were enrolled in this study. Baseline patient characteristics, serum alpha-fetoprotein (AFP), and biochemical parameters were analyzed. Serum levels of PXR, tumor necrosis factor alpha (TNF-α), and interleukin (IL)-1β were measured using ELISA. The hepatic expression of phosphorylated nuclear factor kappa B (NFκB) and PXR proteins was analyzed by western blotting.
Results:
When compared to control subjects, serum PXR levels were increased in HCC cases (1.34 ± 0.16 vs. 4.09 ± 0.33; P < 0.0001). Similarly, hepatic PXR expression was increased in HCC tissues. Moreover, HCC patients exhibited elevated inflammatory cytokines and a deranged hepatobiliary profile compared to controls.
Conclusions:
Elevated serum PXR levels in HCC patients were positively correlated with inflammation. Notably, serum PXR demonstrated greater sensitivity and specificity in diagnosing HCC. These findings suggest that PXR may serve as a plausible biomarker in the diagnosis of HCC.
DOI: https://doi.org/10.37349/edd.2025.100594
This article belongs to the special issue Prevention, Screening and Diagnosis for Primary Liver Cancer

Hepatitis A virus (HAV) is a spherical, non-enveloped, linear-positive single-stranded RNA virus that belongs to the Picornaviridae family. The virus attacks the liver, which leads to inflammation and the onset of jaundice. It represents a disease of the pediatric population and, in most cases, it causes an acute self-limited illness, but rarely a fulminant condition. HAV spreads from person to person through the fecal-oral route and ingestion of contaminated food or drink. It is highly endemic in large geographical areas of the world, including the Indian subcontinent, where most of the population is exposed to the virus in childhood. Most of the viral infections at this age cause asymptomatic disease that provides lifelong protection against HAV. However, our recent study showed an increased incidence of HAV infection in the adult population. This signifies a change in the pattern of age-specific seroprevalence of antibodies for hepatitis A and a huge number of non-immune susceptible individuals. Molecular epidemiological studies define various aspects of viral infection and transmission. Sequence characterization based on the VP1/P2A junction region confirmed IIIA and IA as the predominant genotypes circulating in the Indian subcontinent. The duration of the viremia is dependent on the host, and viral genotypes have no role in the severity of the disease. A mutational study confirmed the lack of genetic variations among Indian strains. Due to the high endemicity of this disease in the Indian subcontinent, vaccination is not recommended. However, individuals who are susceptible and seronegative for HAV-IgG should be targeted for vaccination. It will be a rational and cost-effective approach.
Hepatitis A virus (HAV) is a spherical, non-enveloped, linear-positive single-stranded RNA virus that belongs to the Picornaviridae family. The virus attacks the liver, which leads to inflammation and the onset of jaundice. It represents a disease of the pediatric population and, in most cases, it causes an acute self-limited illness, but rarely a fulminant condition. HAV spreads from person to person through the fecal-oral route and ingestion of contaminated food or drink. It is highly endemic in large geographical areas of the world, including the Indian subcontinent, where most of the population is exposed to the virus in childhood. Most of the viral infections at this age cause asymptomatic disease that provides lifelong protection against HAV. However, our recent study showed an increased incidence of HAV infection in the adult population. This signifies a change in the pattern of age-specific seroprevalence of antibodies for hepatitis A and a huge number of non-immune susceptible individuals. Molecular epidemiological studies define various aspects of viral infection and transmission. Sequence characterization based on the VP1/P2A junction region confirmed IIIA and IA as the predominant genotypes circulating in the Indian subcontinent. The duration of the viremia is dependent on the host, and viral genotypes have no role in the severity of the disease. A mutational study confirmed the lack of genetic variations among Indian strains. Due to the high endemicity of this disease in the Indian subcontinent, vaccination is not recommended. However, individuals who are susceptible and seronegative for HAV-IgG should be targeted for vaccination. It will be a rational and cost-effective approach.
DOI: https://doi.org/10.37349/edd.2025.100593
This article belongs to the special issue Viral Hepatitis
 Previous
Previous