Affiliation:
Qidong People’s Hospital/Qidong Liver Cancer Institute/Affiliated Qidong Hospital of Nantong University, Qidong 226200, Jiangsu, China
Email: chenjg@ntu.edu.cn
ORCID: https://orcid.org/0000-0003-4102-8934
Explor Dig Dis. 2025;4:100595 DOI: https://doi.org/10.37349/edd.2025.100595
Received: August 04, 2025 Accepted: September 16, 2025 Published: September 29, 2025
Academic Editor: Jose Carlos Fernandez-Checa, Institute of Biomedical Research August Pi i Sunyer (IDIBAPS), Spain
The article belongs to the special issue Prevention, Screening and Diagnosis for Primary Liver Cancer
Primary liver cancer, particularly hepatocellular carcinoma, remains a major global health challenge due to its multifactorial etiology, late-stage detection, and high mortality. This review proposes a precision prevention framework that (i) categorizes risk factors into biological (e.g., HBV/HCV, aflatoxins), environmental (e.g., air pollution, occupational/waterborne toxins), and host-related domains (e.g., obesity, diabetes, genetic susceptibility); and (ii) aligns interventions to three complementary strategies: elimination of dominant risk (HBV vaccination, aflatoxin control, alcohol/tobacco reduction), early warning and targeted management (life-course immunization, MAFLD screening and control, metformin in diabetics), and chemoprevention (e.g., oltipraz, chlorophyllin, sulforaphane). We further articulate “green” prevention as a scalable, diet-centered approach that can be tailored to risk tiers and local food systems. Advances in multi-omics, microbiome science, and AI-enabled risk models—together with cohort evidence from East Asia, sub-Saharan Africa, and Western populations—support stratified surveillance and earlier interventions. Finally, we discuss generalizability and implementation challenges (regional dietary diversity, resource access) and outline pragmatic solutions to improve uptake across diverse settings.
Primary liver cancer (PLC), particularly hepatocellular carcinoma (HCC), remains one of the most lethal malignancies worldwide. In 2024, it ranked sixth in incidence and third in cancer mortality, with an estimated 865,000 new cases and approximately 758,000 deaths annually [1]. Lethality reflects asymptomatic onset, late diagnosis, and limited curative options, and the burden is unevenly distributed—highest in East Asia and sub-Saharan Africa—where hepatitis B virus (HBV), hepatitis C virus (HCV), aflatoxin B1 (AFB1), and related exposures are prevalent [1–4]. These geographic disparities highlight the limits of uniform, population-wide strategies.
As a shift toward “precision prevention”—a personalized approach that integrates molecular, epidemiologic, and environmental data to stratify populations by risk and tailor interventions accordingly [5–8]. Chronic HBV or HCV infection accounts for over 75% of global PLC [9]. HBV promotes carcinogenesis via genome integration, oncogenic proteins (e.g., HBx), inflammation, and chromosomal instability [10]. In synergy with HBV, AFB1—a potent mycotoxin produced by Aspergillus flavus—induces the TP53 R249S mutation, a molecular hallmark found in endemic regions such as Qidong (China) and The Gambia [11].
Host-related metabolic risks—obesity, type 2 diabetes mellitus (T2DM), and non-alcoholic fatty liver disease (NAFLD) / metabolic-associated fatty liver disease (MAFLD)—are now driving a growing fraction of HCC in low-incidence countries, especially in Western populations [12–14]. Affecting nearly one-quarter of adults worldwide, MAFLD is a leading cause of liver cancer events in some non-cirrhotic patients.
Advances in multi-omics deepen mechanistic understanding and enable risk tools. Genome-wide association studies (GWAS) have identified multiple susceptibility loci for HBV-related HCC (e.g., FZD4, PCDH9, PRMT6, LHX1, KIF2B, and L3MBTL4) [15]; combined homozygous risk at PNPLA3 and TERT confers a 6.5-fold increase in non-viral HCC risk [16]. Metabolomic profiling reveals early alterations in bile acids, amino acids, and lipid signatures that can precede diagnosis [17]. The gut–liver axis also contributes to hepatic inflammation and carcinogenesis, motivating microbiota-targeted approaches.
This review synthesizes risk factors across biological, environmental, and host-related domains and discusses contemporary strategies in risk elimination, early warning/targeted intervention, and chemoprevention. We further examine cohort-based evidence and the potential of environmentally sustainable (“green”) and data-driven prevention models to inform public health policy.
Liver cancer arises from a diverse set of risk factors that can be broadly categorized into three domains: biological, environmental, and host-related (Figure 1). Each category encompasses distinct yet overlapping mechanisms that contribute to hepatocarcinogenesis, and their interplay varies by population, geography, and exposure history. Understanding these sources of risk is critical for designing precision prevention strategies.
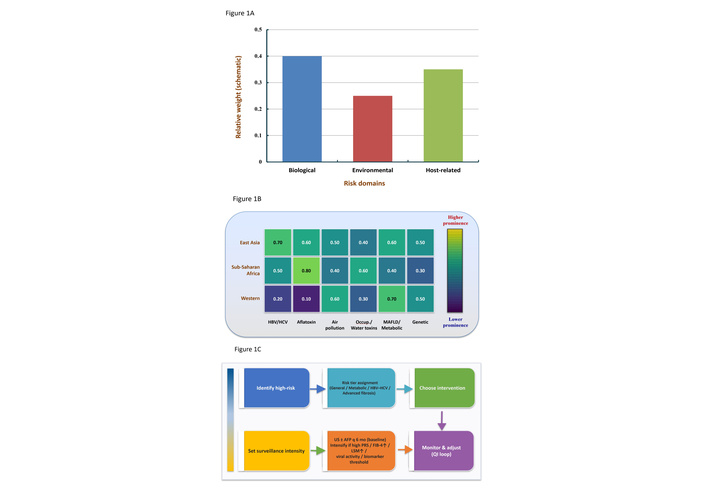
Framework for precision prevention of primary liver cancer: risk domains, regional context, and decision pathway. (A) Domain-level contribution of major risk categories—biological (e.g., HBV/HCV, aflatoxins), environmental (e.g., air pollution, occupational/waterborne toxins), and host-related (e.g., MAFLD/metabolic, genetics)—displayed as relative weights (schematic). (B) Cross-regional heatmap (schematic) illustrating the prominence of specific risk factors across East Asia, sub-Saharan Africa, and Western settings (HBV/HCV, aflatoxin, air pollution, occupational/water toxins, MAFLD/metabolic, genetic). (C) Prevention-strategy flow chart outlining the decision path from high-risk identification and risk-tier assignment (general/metabolic/HBV–HCV/advanced fibrosis) to choice of intervention (risk elimination; early warning & targeted management; chemoprevention), setting surveillance intensity (baseline US ± AFP every 6 months; intensify if high PRS, elevated FIB-4 or LSM, viral activity, or biomarker thresholds), and monitor-and-adjust (QI loop). Values in panels A and B are illustrative placeholders to be replaced with cohort-specific estimates as available. AFP: alpha-fetoprotein; FIB-4: fibrosis-4 index (also called the fibrosis-4 score); HBV: hepatitis B virus; HCV: hepatitis C virus; LSM: liver stiffness measurement; MAFLD: metabolic dysfunction–associated fatty liver disease (i.e., NAFLD: non-alcoholic fatty liver disease); PRS: polygenic risk score; QI: quality improvement; US: ultrasound.
The most well-established biological drivers of liver cancer include chronic infections with HBV, HCV, and liver flukes (e.g., Clonorchis sinensis) [18]. While aflatoxins are biologically derived, their classification often falls under environmental exposures due to the route of ingestion via contaminated food [19, 20].
Genomic studies have established a causal relationship between HBV mutations and HCC development, particularly in high-incidence regions [21]. In Qidong, China, analysis of the HBV basal core promoter (BCP) and overlapping X regions from 58 HCC patients and 71 chronic hepatitis patients revealed a high prevalence of T1762/A1764 dual mutations among HCC cases. Additional mutations at T1766/A1768 significantly elevated HCC risk [22]. Furthermore, triple mutations in liver tissues were significantly more common in HCC patients than in those with only chronic hepatitis. Longitudinal data suggest that these BCP mutations accumulate progressively during hepatocarcinogenesis [23].
A prospective cohort study in Taiwan (China) involving 12,008 men demonstrated that anti-HCV seropositivity was associated with a 20-fold increased risk of HCC, independent of HBV status [24]. Supporting this, a Japanese study of 15,597 individuals found that prior blood transfusion—a proxy for HCV exposure—significantly increased HCC risk for both men (RR (relative risk) = 1.86, 95% CI: 1.05–3.29) and women (RR = 4.20, 95% CI: 1.83–9.61) [25]. A nested case-control study in two prospective Chinese cohorts (n = 108 and 55 matched pairs) used liquid chromatography–mass spectrometry (LC-MS) to identify plasma metabolites associated with future HCC risk. Using orthogonal partial least squares discriminant analysis (OPLS-DA) and weighted gene co-expression network analysis (WGCNA), 44 differential metabolites were identified, including 12 steroid hormones, 8 bile acids, 10 amino acids, and 6 phospholipids. Odds ratios (OR) ranged from 0.19 (95% CI: 0.11–0.35) to 5.09 (95% CI: 2.73–9.50), indicating strong predictive associations [26].
Host–exposure interactions may also modulate biological risk. A multivariate Cox regression analysis in Taiwan (China) found that advanced age (≥ 60 years old), male sex, high body mass index (BMI), alpha-fetoprotein (AFP) ≥ 6 ng/mL, elevated fibrosis-4 index (FIB-4, also called the fibrosis-4 score), and advanced chronic liver disease (ACLD) status were independent predictors of HCC [4].
Environmental exposures play a critical role in liver carcinogenesis, particularly in high-risk regions where dietary and occupational contaminants are prevalent. Among these, aflatoxins—secondary metabolites produced by Aspergillus flavus and A. parasiticus—are the most extensively studied. AFB1 is a potent hepatocarcinogen and has been classified as Group 1 by the International Agency for Research on Cancer (IARC).
AFB1 contamination occurs predominantly in grains and nuts stored under warm, humid conditions. Ingestion of AFB1-contaminated food is strongly associated with the development of HCC, especially when combined with HBV infection [5, 27, 28]. This synergism is exemplified by the TP53 R249S mutation, a hallmark of aflatoxin-related DNA damage.
Occupational exposure to airborne aflatoxins also contributes to liver cancer risk. A case-control study in Guangxi Province, China, demonstrated that workers in sugarcane storage and paper milling facilities who inhaled aflatoxin-contaminated dust had significantly elevated levels of serum AFB1-albumin adducts. The study reported an OR of 5.24 (95% CI: 2.77–9.88) for HCC among exposed workers compared to unexposed controls [29]. Emerging research has identified air pollution as a possible risk factor for liver cancer. A pooled analysis of six European adult cohorts (n = 330,064 cancer-free individuals) followed over 18.1 years identified 512 liver cancer cases. Adjusted Cox models showed that exposure to nitrogen dioxide (NO₂) was associated with increased liver cancer risk (HR (hazard ratio) = 1.17, 95% CI: 1.02–1.35), as were fine particulate matter (PM2.5: HR = 1.12, 95% CI: 0.92–1.36) and black carbon (HR = 1.15, 95% CI: 1.00–1.33) [30]. A Thai observational study and other studies provided supporting evidence, demonstrating that long-term ambient air pollution exposure correlated with increased liver cancer mortality risk [31, 32]. Given increasing urbanization and climate-driven environmental changes, these associations warrant further investigation in both high- and low-incidence settings.
Other environmental carcinogens include inorganic arsenic, vinyl chloride, polychlorinated biphenyls (PCBs), and microcystins from water contamination [33–35]. However, the epidemiological evidence for these agents remains limited.
Genetics of MAFLD and progression to HCC: replicated loci—including PNPLA3 (rs738409), TM6SF2 (rs58542926), MBOAT7 (rs641738), GCKR (rs780094/rs1260326), and HSD17B13 (rs72613567, protective)—modulate hepatic lipid handling and inflammation, thereby influencing transition from steatosis to cirrhosis and HCC. Polygenic risk scores (PRS) that combine these variants, optionally adjusted for HSD17B13, enhance risk stratification for NAFLD/MASLD‑related HCC, including in non‑cirrhotic individuals [36, 37]. Integrating PRS with clinical features (age, sex, obesity, T2DM, non‑invasive fibrosis scores) can prioritize intensified surveillance and early metabolic intervention. Mechanistically, oxidative‑stress and mitochondrial‑dysfunction pathways (e.g., heme oxygenase‑1/HO‑1) have been implicated in the transition from steatohepatitis to HCC [38]. Host-related susceptibility factors significantly influence liver cancer risk and may explain observed disparities across racial, ethnic, and geographic populations [39]. These factors include metabolic disorders, obesity, diabetes, and heritable genetic variations that affect liver physiology and immune function. In a large U.S.-based cohort study, T2DM was associated with a significantly increased risk of HCC (HR = 2.61, 95% CI: 2.34–2.91). A 5 kg/m² increase in BMI was linked to a 26% increase in liver cancer risk, with HRs of 1.38 for men (95% CI: 1.30–1.46) and 1.25 for women (95% CI: 1.17–1.35). Waist circumference was also positively correlated with HCC risk (HR per 5 cm = 1.08, 95% CI: 1.04–1.13) [40].
A retrospective analysis of U.S. Veterans Health Administration records from 2003 to 2011 revealed a dramatic rise in NAFLD prevalence, increasing from 6.3% to 17.6%—a 2.8-fold surge over less than a decade. Among the 9.78 million individuals analyzed, 1.33 million (13.6%) had NAFLD, and many later developed liver-related complications, including HCC [41]. The combination of hepatic fat accumulation, systemic inflammation, and oxidative stress creates a carcinogenic microenvironment in NAFLD patients. Genetic predisposition plays a crucial role in modulating liver cancer risk. A U.S. GWAS identified three major loci associated with HCC and cirrhosis, including polymorphisms in the major histocompatibility complex (MHC) class II region—key to antigen presentation and immune surveillance [42]. These findings suggest that impaired T-cell function due to germline variants may underlie susceptibility to hepatocarcinogenesis.
In a large GWAS involving 715 HBV-positive carriers in Guangxi, researchers genotyped 440,794 SNPs and validated 45 significant loci across multiple Chinese populations. One key variant, rs17401966 at 1p36.22 (encompassing UBE4B, KIF1B, and PGD), was consistently associated with HCC in replication cohorts from Guangdong, Shanghai, Jiangsu, and Beijing (China) [43]. The same study also examined long non-coding RNAs and found that the MALAT1 variant rs7763881 (AC/CC vs. AA) was significantly associated with reduced HCC risk (OR = 0.81, 95% CI: 0.68–0.97), while the HULC variant rs619586 (AG/GG vs. AA) demonstrated a borderline protective effect (OR = 0.81, 95% CI: 0.65–1.01) [44].
A single-center cohort study from the Michigan Genomics Initiative evaluated the effect of genetic variants in cirrhotic patients. The PNPLA3 rs738409-GG genotype was independently associated with increased HCC risk compared to the CC genotype (adjusted subhazard ratio: 2.42, 95% CI: 1.40–4.17) [45]. These findings support the incorporation of PNPLA3 and other high-risk variants into HCC surveillance algorithms, particularly in MAFLD patients with compensated cirrhosis.
Although many susceptibility-related factors have been categorized here, other potential contributors remain outside this classification or are still under investigation. Due to space limitations, additional emerging risk factors can be found in recent literature [46, 47].
Effective prevention of liver cancer requires strategies that are not only evidence-based but also adapted to the individual’s biological, environmental, and behavioral risk profile. Precision prevention in this context involves three main approaches: eliminating modifiable risk exposures, implementing early warning and targeted interventions, and applying chemoprevention in high-risk populations (Figure 2). Each approach is rooted in strong mechanistic rationale and supported by evidence from clinical and population-based studies.
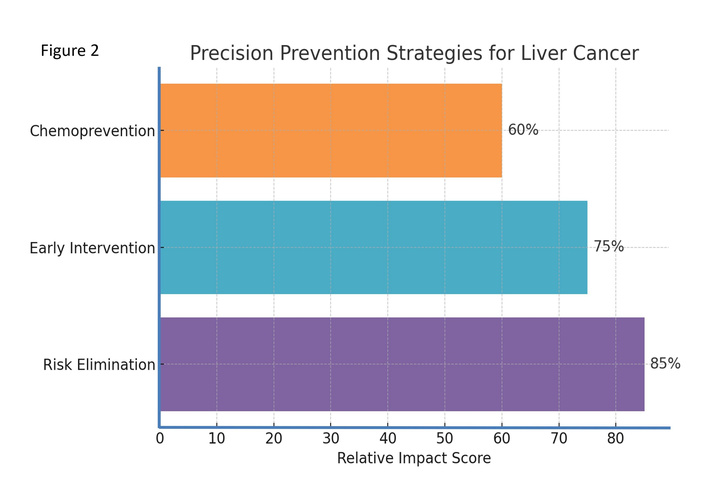
Precision prevention strategies for liver cancer. Percentages represent the relative impact score of each strategy.
Elimination of key etiological agents is the cornerstone of primary prevention. Among modifiable factors, AFB1 is a priority target given its potent hepatocarcinogenic potential. AFB1 contamination is prevalent in regions with warm, humid climates, where corn and peanuts serve as dietary staples. Public health measures that reduce exposure—through proper crop drying, safe storage, dietary shifts, and regulatory limits—have been shown to substantially lower liver cancer incidence [5].
In Qidong, China, corn was historically the main dietary staple, with aflatoxin contamination found in 35%–98% of samples collected between 1973 and 1982. Rice, by contrast, was free of contamination. A regional dietary transition from corn to rice beginning in the mid-1980s led to a marked decrease in AFB1 exposure, paralleled by a substantial reduction in PLC incidence [8, 20].
Elimination of environmental risk factors should also address occupational safety, water pollution control, and viral transmission prevention. This includes reducing arsenic exposure, strengthening blood screening for HBV/HCV, and improving hygienic practices [3]. At the individual level, reducing alcohol and tobacco use, avoiding moldy foods, and increasing intake of fruits and vegetables are key lifestyle modifications.
With the rising global burden of NAFLD, elimination efforts must also focus on its early detection and management. Estimated to affect one in four people globally, NAFLD—especially in its metabolic form MAFLD—has emerged as a leading cause of liver cancer in both developed and developing nations [48, 49].
A biomarker‑guided approach improves sensitivity for early‑stage HCC and high‑grade dysplastic lesions. Key serum markers include AFP, AFP‑L3%, and des‑gamma‑carboxy prothrombin (DCP/PIVKA‑II). Combined algorithms such as GALAD (gender, age, AFP, AFP‑L3, DCP) outperform single markers and can be layered onto ultrasound for surveillance [50]. Imaging biomarkers—ultrasound (± contrast), multiphasic CT, and gadoxetate‑enhanced MRI—complement blood tests; elastography provides a non‑invasive fibrosis surrogate to trigger intensified surveillance. Emerging molecular tools include cell‑free DNA methylation/fragmentomics classifiers, 5‑hydroxymethylcytosine (5hmC) signatures, circulating microRNA panels (e.g., miR‑122, miR‑21), and metabolomic fingerprints; in aflatoxin‑exposed regions, urinary aflatoxin‑DNA adducts enable exposure monitoring and risk tracking [51]. Risk‑stratified algorithms that integrate clinical factors (age, sex, MAFLD, diabetes), non‑invasive fibrosis scores (e.g., FIB‑4), polygenic scores, and biomarker panels can define who should receive 6‑monthly versus more intensive surveillance, and when cross‑sectional imaging is warranted [52].
Early intervention strategies target individuals at the precancerous or high-risk stage to prevent progression to liver cancer. Among these, universal childhood HBV vaccination remains the most impactful intervention globally, especially in endemic regions. Vaccination at birth prevents vertical transmission from HBsAg (+) or HBeAg (+) mothers and drastically reduces chronic HBV infection and HCC incidence later in life [53].
In Gambia, a national randomized controlled trial enrolled 124,577 children into HBV vaccination and control groups. The trial included a national cancer registry and long-term surveillance, providing compelling evidence for vaccine efficacy in HCC prevention over a planned 30 to 35–year follow-up [54]. A similar study in Qidong, China, enrolled 80,000 children in immunized and control groups between 1985 and 1990 [55], allowing a long-term follow-up to assess vaccination impact.
The immunization program in Taiwan (China) began in 1984, introducing universal screening of pregnant women and targeted vaccination of high-risk newborns. The program achieved significant reductions in HBV carrier rates [56]. Singapore and Hong Kong (China) implemented similar phased programs, with Hong Kong (China) observing a reduction in HBsAg prevalence from 14.3% (1970 cohort) to 6.7% (1988 cohort) [57].
Apart from vaccination, early-life metabolic interventions are gaining prominence. Obesity and diabetes prevention through dietary management, exercise, and public health education are essential for reducing MAFLD incidence. Among diabetic patients, the antidiabetic drug metformin has been identified as a potential chemopreventive agent. A meta-analysis of 19 studies involving over 550,000 individuals found that metformin use was associated with a 48% reduced risk of HCC (OR = 0.52, 95% CI: 0.40–0.68) [58].
These findings support a life-course approach to liver cancer prevention—starting with early immunization and continuing with metabolic risk management into adulthood.
Chemoprevention involves the use of natural or synthetic agents to prevent, delay, or reverse carcinogenesis in high-risk individuals. For liver cancer, this approach has been studied primarily in regions with high aflatoxin exposure and HBV prevalence, with a focus on interrupting the initiation and promotion phases of hepatocarcinogenesis.
In Qidong, China, a series of landmark trials have evaluated various chemopreventive agents. Oltipraz, a synthetic dithiolethione compound, has demonstrated efficacy in reducing aflatoxin-induced DNA damage. It enhances phase II detoxification pathways (e.g., glutathione S-transferase), suppresses phase I activation enzymes (e.g., cytochrome P450), and promotes the excretion of aflatoxin metabolites. Human trials in Qidong showed oltipraz increased urinary aflatoxin-mercapturic acid excretion [59, 60]. Chlorophyllin, a semi-synthetic water-soluble derivative of chlorophyll, functions by binding aflatoxins in the gut and reducing their systemic absorption. In a double-blind randomized trial in Qidong, 180 adults were assigned to receive either chlorophyllin (100 mg three times daily for 4 months) or placebo. The chlorophyllin group exhibited a 55% reduction in urinary aflatoxin-DNA adduct excretion [61]. Broccoli sprout extract, rich in sulforaphane—a potent inducer of phase II enzymes—has also shown promise. In a pilot trial in Qidong, daily consumption of a broccoli beverage was associated with reduced excretion of both aflatoxin and benzene metabolites, suggesting dual chemopreventive effects [62].
Beyond aflatoxin-associated HCC, plant-derived compounds such as polyphenols and isothiocyanates may inhibit liver carcinogenesis by modulating inflammation, oxidative stress, apoptosis, and cell cycle control [63]. Vitamin E has also been linked to reduced liver cancer risk in a large cohort study (n = 132,837), where higher intake was associated with a significantly lower HCC risk (HR = 0.52, 95% CI: 0.30–0.90) [64].
Pharmacologic agents used for other indications may also offer chemopreventive benefits. A population-based study from a NHIRD program of Taiwan (China) found that selective serotonin reuptake inhibitors (SSRIs) reduced HCC risk in alcohol use disorder patients. After adjusting for comorbidities, SSRI use was associated with a 69% reduction in HCC risk (adjusted HR = 0.31, 95% CI: 0.24–0.39), with evidence of a dose–response relationship [65].
In chemoprevention, it is essential to consider dosing regimens, oral bioavailability, and long-term safety of preventive agents. Plant-derived compounds are promising, but their practical use requires careful attention to pharmacologic properties. For example, a double-blind randomized controlled trial (RCT) showed that participants who received chlorophyllin 100 mg three times daily for four months exhibited a significant reduction in urinary aflatoxin–DNA adduct excretion; community-based trials have further demonstrated that broccoli-sprout–based sulforaphane beverages enhance detoxication biomarkers [61, 66]. Nevertheless, oral bioavailability varies by formulation, food matrix, and adherence, and the dose–response relationship and durability beyond several months remain incompletely defined. Although short-term studies have indicated a favorable safety profile, large-scale deployment will require long-term surveillance and rigorous quality control, including standardization of active constituents and contaminant testing. Practical guidance should emphasize locally appropriate sourcing of plant-derived agents, the establishment of realistic dosing ranges, and monitoring for drug–nutrient interactions in high-risk populations [67].
Some chemopreventive agents used for liver cancer prevention are summarized in Table 1.
Some chemopreventive agents for liver cancer prevention in populations, with supporting evidence (references).
| Agent | Source | Proposed Mechanism | Evidence | Reference |
|---|---|---|---|---|
| Oltipraz | Synthetic dithiolethione | Enhances phase II detox enzymes, inhibits phase I activation | Human RCT in Qidong reduced aflatoxin biomarkers | Jacobson et al., 1997 [59] |
| Chlorophyllin | Semi-synthetic chlorophyll | Binds aflatoxins, reduces absorption | Human RCT in Qidong reduced aflatoxin-DNA adducts by 55% | Egner et al., 2001 [61] |
| Sulforaphane | Broccoli sprouts | Induces phase II enzymes, antioxidant activity | Pilot trial reduced aflatoxin & benzene metabolites | Kensler et al., 2005 [62] |
| Metformin | Antidiabetic drug | Improves insulin sensitivity, reduces inflammation | Meta-analysis: 48% reduced HCC risk in diabetics | Ma et al., 2017 [58] |
| SSRI | Antidepressant | Modulates serotonin pathways, reduces inflammation | NHIRD study: 69% reduced HCC risk in alcohol use disorder | Chen et al., 2021 [65] |
RCT: randomized controlled trial; SSRI: selective serotonin reuptake inhibitors; HCC: hepatocellular carcinoma.
Liver cancer typically follows a multistep and multifactorial carcinogenic process involving genetic, viral, and environmental triggers. A focus on dominant risk factors—such as chronic HBV infection or aflatoxin exposure—can yield outsized public health benefits if tackled early.
Community-based prevention targets common exposures shared by people living in the same ecological environment. For example, Qidong’s success in reducing aflatoxin exposure through staple food transition is a model of locally-informed intervention [20]. Similarly, the WHO’s recommendation of universal HBV vaccination in infants exemplifies proactive prevention at scale [68].
In a Shanghai (China) men’s cohort, liver cancer risk was significantly associated with aflatoxin metabolites, with a relative risk of 2.4 (95% CI: 1.0–5.9). The strongest risk was observed for aflatoxin P1 (RR = 6.2, 95% CI: 1.8–21.5). After adjusting for HBsAg, smoking, education, and alcohol, aflatoxin remained an independent risk factor (RR = 3.8, 95% CI: 1.2–12.2) [27]. A community-based education initiative for liver cancer prevention among the African, Asian, and Hispanic populations within the Greater Philadelphia and metropolitan New York City areas. This initiative generated different impacts in the three populations, highlighting the needs for more targeted, culturally tailored efforts in health promotion among these communities [69].
Precision prevention must be grounded in robust, reproducible, and context-specific evidence. Many liver cancer risk factors are well-established through cohort-based epidemiological studies—particularly viral hepatitis, aflatoxins, and metabolic disorders—but others remain inconclusive.
The relationship between coffee consumption and liver cancer is a prime example. Once thought to increase cancer risk, coffee has since been shown in multiple large cohorts (e.g., Japan Public Health Center, European Prospective Investigation into Cancer and Nutrition) to reduce HCC risk, likely via its antioxidant compounds, including caffeine and chlorogenic acid [47, 70, 71].
Novel agents are also under investigation. A cohort study in Taiwan (China) found that dipeptidyl peptidase-4 inhibitors (DPP-4i) used in diabetic patients with HCV infection were associated with a reduced risk of HCC [72]. Likewise, SGLT2 inhibitors have shown promise in lowering HCC risk in HBV-infected diabetics (HR = 0.54, 95% CI: 0.33–0.88) [73].
It is worth noting that in Asian, large Asian cohort datasets underpin several of our conclusions—for example, prospective study in Taiwan (China) on HCV and HCC risk [24], Japanese cohorts on transfusion‑linked risk and coffee’s protective association [70], mainland Chinese cohorts on vitamins/antioxidants [64] and prospective metabolomics [26], Hong Kong (China) data on SGLT2 inhibitors in HBV‑diabetes [73], and analyses from Taiwan (China) on DPP‑4 inhibitors in HCV‑diabetes [72]. These findings complement East Asian evidence already synthesized here and further support tailoring precision prevention for Asian populations.
Green prevention emphasizes the use of natural, plant-derived compounds and dietary modifications that are both effective and environmentally sustainable [74]. This is especially relevant in regions where pharmaceutical access is limited but dietary risks are high.
Prospective data from the Shanghai Women’s and Men’s Health Studies revealed an inverse association between dietary manganese intake and liver cancer risk. A 15.2-year follow-up in women and 9.3 years in men showed HRs of 0.51 and 0.38, respectively [75]. Broccoli-derived sulforaphane, green tea catechins (GTCs), and vitamin-rich diets have demonstrated chemoprotective effects via antioxidation, detoxification, and modulation of gene expression [61, 76].
A Japanese cohort study involving 14,517 men and 21,583 women found that higher dietary manganese intake was associated with reduced liver cancer risk in men without liver disease (HR = 0.56, 95% CI: 0.32–0.99) but not in women [77]. A recent meta-analysis of 32 studies (n = 2,492,625) found significant HCC risk reductions for high coffee intake (RR = 0.53, 95% CI: 0.47–0.59) and green tea (RR = 0.80, 95% CI: 0.67–0.95) [78].
In the field of “green” prevention, research on medicinal‑food homologous herbs may represent a highly promising direction for development. Diet patterns and cruciferous/tea‑based bioactives, several traditional Chinese medicinal‑food homologous materials may support liver health and risk reduction—e.g., turmeric/curcumin, licorice/glycyrrhizin, schisandra lignans, goji polysaccharides, astragalus saponins, and berberine‑containing botanicals [79–84]. Evidence ranges from mechanistic and animal studies to small human trials; standardization of active constituents, quality control, and pharmacovigilance are prerequisites for population‑level use [85, 86]. Given potential herb–drug interactions (e.g., with antivirals, antidiabetics, anticoagulants), individualized use under clinical supervision is recommended. These agents can be positioned as adjuncts within risk‑tiered “green” strategies, prioritizing locally available, safe, and affordable options while building higher‑quality randomized evidence. What needs to be considered is, adoption of diet‑centered “green” strategies varies with regional cuisines, affordability, and supply chains. Programs should be culturally tailored (leveraging local crucifers/legumes/fish/tea/coffee) [78, 87–89], include cost‑sensitive meal plans, and partner with community kitchens and primary care to deliver MAFLD‑friendly menus, weight‑management support, and brief alcohol‑/tobacco‑cessation counseling.
Liver cancer prevention is increasingly informed by integrative models that connect molecular data, population surveillance, and environmental monitoring. This transdisciplinary approach allows for both targeted risk prediction and scalable public health action.
Many liver cancer risk factors are shared with other diseases. For instance, aflatoxin, alcohol, and tobacco raise risk across multiple cancer types. Conversely, protective factors such as green tea and coffee provide cross-cancer benefits [90]. Thus, interventions can be designed for broad application while maintaining specificity.
The rise of big data—including genomics, transcriptomics, and exposomics—facilitates the identification of novel biomarkers and pathways. A Mendelian randomization study incorporating microbiome GWAS data from the UK Biobank and Chinese cohorts found inverse associations between certain gut bacterial families (e.g., Ruminococcaceae, Porphyromonadaceae) and HCC risk [91].
Moving forward, precision prevention will benefit from interoperable databases, AI-assisted modeling, and population stratification based on polygenic and exposomic risk scores [92]. This will enable earlier detection and smarter allocation of resources for at-risk individuals [93, 94].
To translate multi-omics data into actionable screening protocols, we recommend: (i) Deriving polygenic and metabolite risk scores from large biobanks (e.g., UK Biobank) and regional cohorts [95–98]; (ii) Integrating these scores with demographic and simple clinical characteristics (age, sex, NAFLD, fibrosis markers) to form calibrated risk models [95, 98]; (iii) Prospective validation of thresholds triggering high-frequency ultrasound ± AFP or elastography testing [99]; (iv) Ensuring cross-population applicability through external validation to prevent calibration drift [100, 101]. For example, microbiome-based Mendelian randomization studies have linked specific bacterial families to HCC risk, providing a mechanistic basis for biomarker design [91, 102]. This translational pathway facilitates the conversion of multi-omics signals into clinically actionable monitoring tools while maintaining cost-effectiveness and population equity.
The current evidence on precision prevention for HCC is concentrated in specific regions and populations (e.g., East Asia, sub-Saharan Africa, and selected Western countries). Differences in etiologic profiles (HBV/HCV, MAFLD/metabolic risk, aflatoxin exposure) and healthcare access may limit transportability of thresholds, workflows, and cost-effectiveness across settings [50, 103].
Composite blood-based models such as GALAD can outperform single markers, yet performance may vary in obesity/NAFLD and other contexts; robust prospective evaluation across ancestries and integration with imaging (ultrasound ± contrast, multiphasic CT, gadoxetate-enhanced MRI) and elastography are required to avoid sensitivity loss or threshold drift [50, 103].
Emerging tools—including cell-free DNA methylation/fragmentomics signatures, 5hmC profiles, circulating microRNA panels, and metabolomic fingerprints—show promise for early detection. However, larger, prospective, multi-ancestry validation and pragmatic integration with simple clinical features (age, sex, MAFLD, non-invasive fibrosis scores) are needed to produce calibrated risk triggers for intensified surveillance [51].
PRS often transfer poorly across genetic ancestries and socio-environmental contexts. Uncritical deployment could exacerbate health inequities; ancestry-aware recalibration on diverse datasets and combination with non-genetic risk factors are necessary to reduce bias [101].
Plant-derived or diet-centered chemopreventive strategies face uncertainties in dose standardization, oral bioavailability (preparation, food matrix, adherence), and long-term safety. Randomized evidence—for example, chlorophyllin 100 mg three times daily for 4 months reducing aflatoxin-DNA adduct excretion—indicates potential, but population-level deployment will require extended follow-up, quality control/standardization of active constituents, and pharmacovigilance [61].
This narrative synthesis may reflect selection bias related to search scope, language, and study geography. Some summarized strategies (multi-omics biomarkers, PRS, plant bioactives) aggregate heterogeneous designs and endpoints. We indicate evidence levels where possible and emphasize local adaptation along a dual axis of risk and resources when formulating recommendations.
Liver cancer remains a formidable public health challenge due to its multifactorial etiology, late diagnosis, and high mortality. Precision prevention offers a forward-looking, integrative framework to address this burden by tailoring interventions to individual and population-level risk profiles.
This review categorized liver cancer risk factors into three domains—biological (e.g., HBV, HCV, aflatoxins), environmental (e.g., pollution, occupational exposure), and host-related (e.g., diabetes, MAFLD/NAFLD, genetic polymorphisms). Each domain presents opportunities for targeted prevention based on mechanistic understanding and epidemiological validation.
Three major precision prevention strategies were explored: (i) risk elimination, including HBV vaccination, aflatoxin control, and metabolic syndrome management; (ii) early warning and life-course interventions, especially for high-risk infants and diabetic patients; and (iii) chemoprevention, using natural and pharmacological agents such as oltipraz, chlorophyllin, metformin, and SSRIs.
The outlook for liver cancer control lies in the integration of community-based actions, sustainable “green” dietary strategies, evidence-based pharmacologic innovation, and big data analytics. Cohort studies from high-risk areas, and elsewhere demonstrate the long-term feasibility and impact of well-designed interventions.
In the era of AI and multi-omics, the fusion of clinical, molecular, and environmental data empowers early identification of high-risk individuals and the development of actionable preventive strategies. However, successful implementation depends on health system capacity, interdisciplinary collaboration, and continued policy support. Future efforts must harmonize traditional public health frameworks with data-driven precision approaches to reduce the global burden of liver cancer in an equitable, effective, and sustainable manner.
5hmC: 5-hydroxymethylcytosine
AFB1: aflatoxin B1
AFP: alpha-fetoprotein
BCP: basal core promoter
BMI: body mass index
FIB-4: fibrosis-4 index
GALAD: gender, age, AFP, AFP-L3, DCP
GWAS: genome-wide association study
HBV: hepatitis B virus
HCC: hepatocellular carcinoma
HCV: hepatitis C virus
HR: hazard ratio
MAFLD: metabolic-associated fatty liver disease
NAFLD: non-alcoholic fatty liver disease
OR: odds ratio
PLC: primary liver cancer
PRS: polygenic risk scores
RR: relative risk
SSRI: selective serotonin reuptake inhibitor
T2DM: type 2 diabetes mellitus
JGC: Conceptualization, Writing—original draft, Writing—review & editing. The author has read and approved the final version of the manuscript.
Jian-Guo Chen, who is the Editorial Board Member and Guest Editor of Exploration of Digestive Diseases, had no involvement in the decision-making or the review process of this manuscript.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2557
Download: 22
Times Cited: 0
Balasubramaniyan Vairappan ... Biju Pottakkat
