Affiliation:
1Azienda Ospedaliero-Universitaria di Modena (–2023), 41100 Modena, Italy
†
Email: a.lonardo@libero.it
ORCID: https://orcid.org/0000-0001-9886-0698
Affiliation:
2MAFLD Research Center, Department of Hepatology, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang, China
3Key Laboratory of Diagnosis and Treatment for the Development of Chronic Liver Disease in Zhejiang Province, Wenzhou 325000, Zhejiang, China
4Institute of Hepatology, Wenzhou Medical University, Wenzhou 325000, Zhejiang, China
†
ORCID: https://orcid.org/0000-0003-4984-2631
Affiliation:
5Storr Liver Centre, Westmead Institute for Medical Research, Westmead Hospital and University of Sydney, Westmead, NSW 2145, Australia
†
Explor Dig Dis. 2025;4:100586 DOI: https://doi.org/10.37349/edd.2025.100586
Received: May 21, 2025 Accepted: July 29, 2025 Published: August 14, 2025
Academic Editor: Han Moshage, University of Groningen, The Netherlands
Here, the history of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis nomenclatures is summarized. Metabolic dysfunction-associated fatty liver disease (MAFLD) was coined in 2020, and metabolic dysfunction-associated steatotic liver disease (MASLD) was proposed in 2023. With this backset, the present article aims at reviewing the similarities and differences between MAFLD and MASLD through a systematic analysis of published comparative studies. MAFLD and MASLD have a complex disease spectrum comprising, further to all-cause mortality, hepatic (fibrosis, cirrhosis, and primary liver cancer) and extrahepatic outcomes (major adverse cardiovascular events, chronic kidney disease, extrahepatic cancers, type 2 diabetes, and vascular dementia). Comparative studies document that—due to its superior ability to identify liver fibrosis—MAFLD better captures mortality owing to all-causes, hepatic and extrahepatic outcomes, which are strongly associated with the severity of liver fibrosis. Moreover, MASLD is inappropriate in pediatric care, lacks specificity, tends to overdiagnosis, does not consider coexistent viral hepatitis or lean subjects, and amplifies disease heterogeneity. Collectively, the evidence presented in this narrative review supports an urgent need for the development of evidence-based guideline statements. This novel developmental process should involve not only a systematic review of the evidence, with equal contribution from all the world’s regions of stakeholders and clinical panelists, but also should use quantitative data to identify an objective-level consensus to guarantee wide adoption of the process outcomes.
What’s in a name? That which we call a rose by any other name would smell as sweet. —William Shakespeare, Romeo and Juliet, 1594.
The liver exerts innumerable physiological functions that span the whole spectrum of metabolism and immunology, as well as the detoxification of endogenous and exogenous harmful substances [1]. However, the normal liver is not responsible for storing significant amounts of fatty substrates [2], and excess fat within the hepatocytes, defined as steatosis, has the potential for triggering progressive liver injury and is also associated with systemic adverse outcomes [3, 4].
Medical scientists have been struggling for centuries to understand fatty liver disease [5], and various definitions have been utilized over time. Metabolic dysfunction-associated steatotic liver disease (MASLD) describes hepatic steatosis (detected with either imaging techniques or histologically) which, in the absence of any other competing causes of steatotic liver disease (SLD), is associated with at least one among the cardiometabolic risk factors (CMRFs) that comprise the metabolic syndrome (MetS) among obesity, impaired glucose tolerance, arterial hypertension, and dyslipidemia [6]. However, what we now define as MASLD, or metabolic dysfunction-associated steatohepatitis (MASH), has variably been called in the past, when other definitions, such as nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), and metabolic dysfunction-associated fatty liver disease (MAFLD) have rather been used [7]. MASLD is a global health concern, raising awareness of which is essential in every country, especially where its incidence and prevalence are increasing at a rapid pace [8].
Although all these various disease nomenclatures broadly allude to steatosis, their definitions and scope differ significantly, potentially identifying variable clinical outcomes and different risk trajectories. With this rapidly evolving scenario, the present review article aims to systematically analyze the spectrum of differences between MAFLD and MASLD, given that they represent the two most recent proposals and are still in competition among them to supplant the old NAFLD/NASH nomenclature. To this end, the relevant history of medicine is summarized first. Next, the natural course of SLD is analyzed. Moreover, the published comparative studies addressing MAFLD and NAFLD are discussed. Finally, the research agenda is examined.
Firstly, a comprehensive bibliographic research strategy was conducted to identify the relevant published articles addressing the history of SLD.
Next, those original studies comparing MAFLD to MASLD published from inception till the 20th of May 2025 were retrieved in PubMed, Web of Science, Scopus, and Google Scholar, combining the keywords ‘MAFLD’ and ‘MASLD’. All original articles that combined the research keywords ‘MAFLD’ and ‘MASLD’ were included, and no published article matching this criterion was excluded.
While clinical manifestations that can be conducive to the MetS and its complications were already identified by Hippocrates (460–370 BC) and Avicenna (981 AD), the first description of histopathological features of “fatty liver” is attributed to Addison in 1863 [9].
In 1838, Rokitansky was the first to report that the accumulation of intrahepatic fat might cause cirrhosis, while Pepper in 1884, and Bartholow in 1885 observed the associations of “fatty infiltration of the liver” with diabetes and obesity, respectively [9].
In 1938, Connor reported the clinical-pathological correlations of cirrhosis with severe portal hypertension developing in patients with diabetes and fibrosing SLD [9].
In 1958, Westwater and Feiner published on fatty infiltration of the liver in obese patients, and, in 1962, Thaler added a more accurate clinical-pathological description of the disease, which was further documented by several reports from the 1950s to the 1970s of fatty liver disease occurring among individuals with obesity and diabetes [9].
In 1980, Ludwig et al. [10] described liver histology of 20 patients with “nonalcoholic steatohepatitis of unknown cause”, which he defined as a “previously unnamed disease”. Liver changes comprised “striking fatty changes with evidence of lobular hepatitis, focal necroses with mixed inflammatory infiltrates, and, in most instances, Mallory bodies; evidence of fibrosis was found in most specimens, and cirrhosis was diagnosed in biopsy tissue from three patients”. Clinically, the disease was more common in women with moderate obesity, diabetes, and gallstones exhibiting enlarged livers and mildly abnormal liver tests.
In 1986, Schaffner and Thaler [11] expanded the disease spectrum of NASH to NAFLD, which also includes uncomplicated fatty liver, i.e., simple steatosis.
The adjective “nonalcoholic” may be defined as a “non-association” [12]. Therefore, in 2020, to turn a “negative” definition into a condition with a positive diagnostic criterion, notably pinpointing the pathogenic dysmetabolic origin, international experts led by Eslam and George [13] proposed renaming NAFLD to MAFLD.
MAFLD definition met universal stakeholder endorsement [14] and, although not totally devoid of ambiguity and even though it intensifies NAFLD’s heterogeneity, it has major significance for hepatological research and practice [15–17].
In 2023, to address various challenges inherent in the existing disease nomenclatures, a modified Delphi process led by Hepatological Scientific Societies culminated in the proposal to remove the adjective ‘nonalcoholic’ and replace ‘fatty’ with ‘steatotic’ [6]. This Delphi consensus coined the nomenclature of MASLD that defines those individuals with steatosis (assessed either histologically or based on imaging techniques) and at ≥ 1 out of 5 CMRFs (Figure 1). According to this novel nomenclature, NASH was supplanted by MASH. Additionally, to identify those individuals with MASLD with a daily alcohol intake of 20–50/30–60 g for females/males, respectively, the term MASLD and increased alcohol intake (MetALD) was introduced.
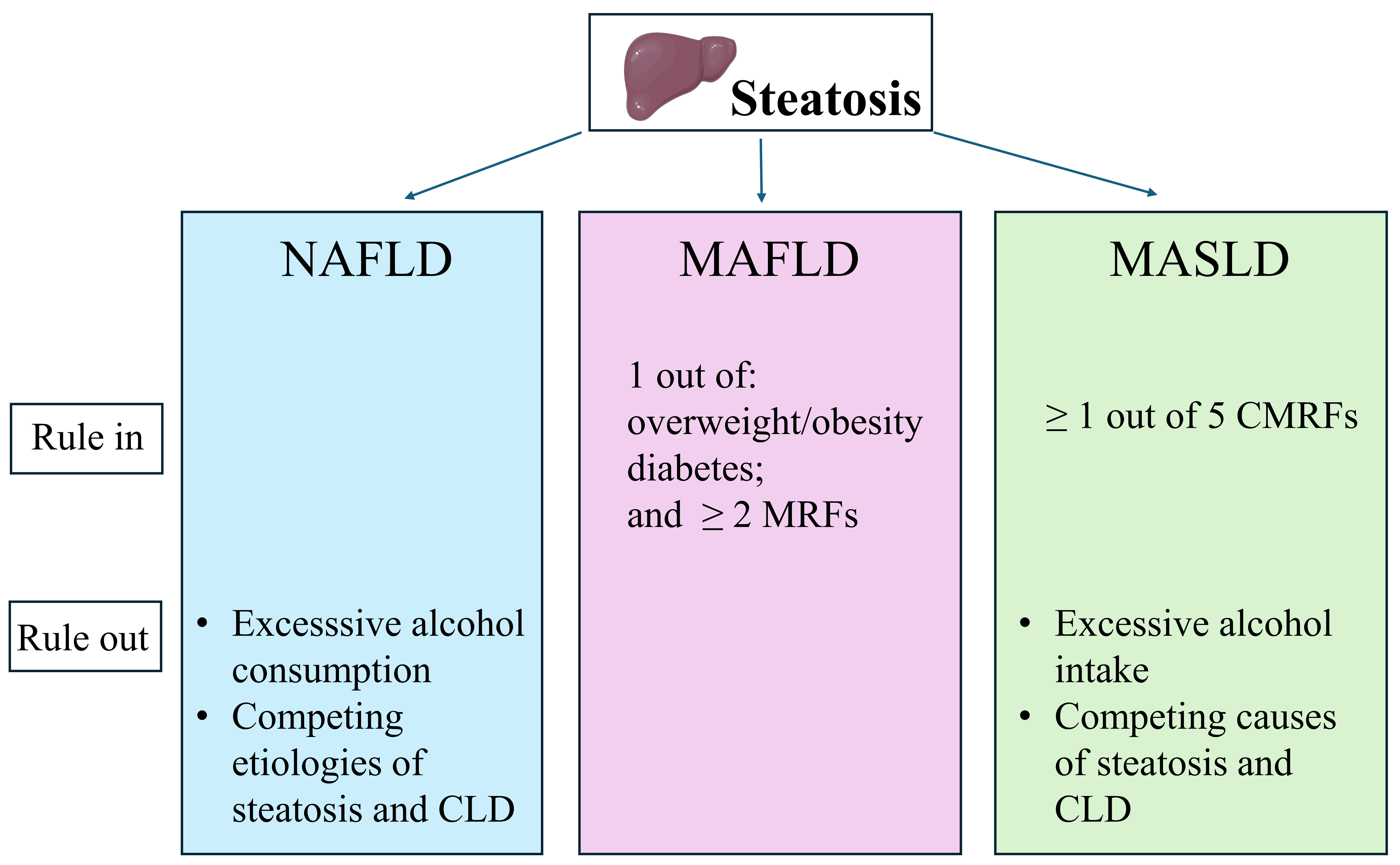
Differences in the criteria to define NAFLD, MAFLD, and MASLD. NAFLD, MAFLD, and MASLD have in common steatosis. This is typically defined histologically in NAFLD, while in MAFLD and MASLD, liver steatosis may also be identified with imaging techniques or serum-based biomarkers, not only with liver biopsy. Moreover, the exclusion of excessive alcohol consumption and other competing causes of steatosis is requested for NAFLD and MASLD, not for MAFLD [18]. CLD: chronic liver disease; MAFLD: metabolic dysfunction-associated fatty liver disease; MASLD: metabolic dysfunction-associated; MRFs: metabolic risk factors; NAFLD: nonalcoholic fatty liver disease. Original image drawn with Servier Medical Art, licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/)
To sum up, Figure 2 graphically depicts some key steps in the evolution of NAFLD/MAFLD/MASLD nomenclatures over time.
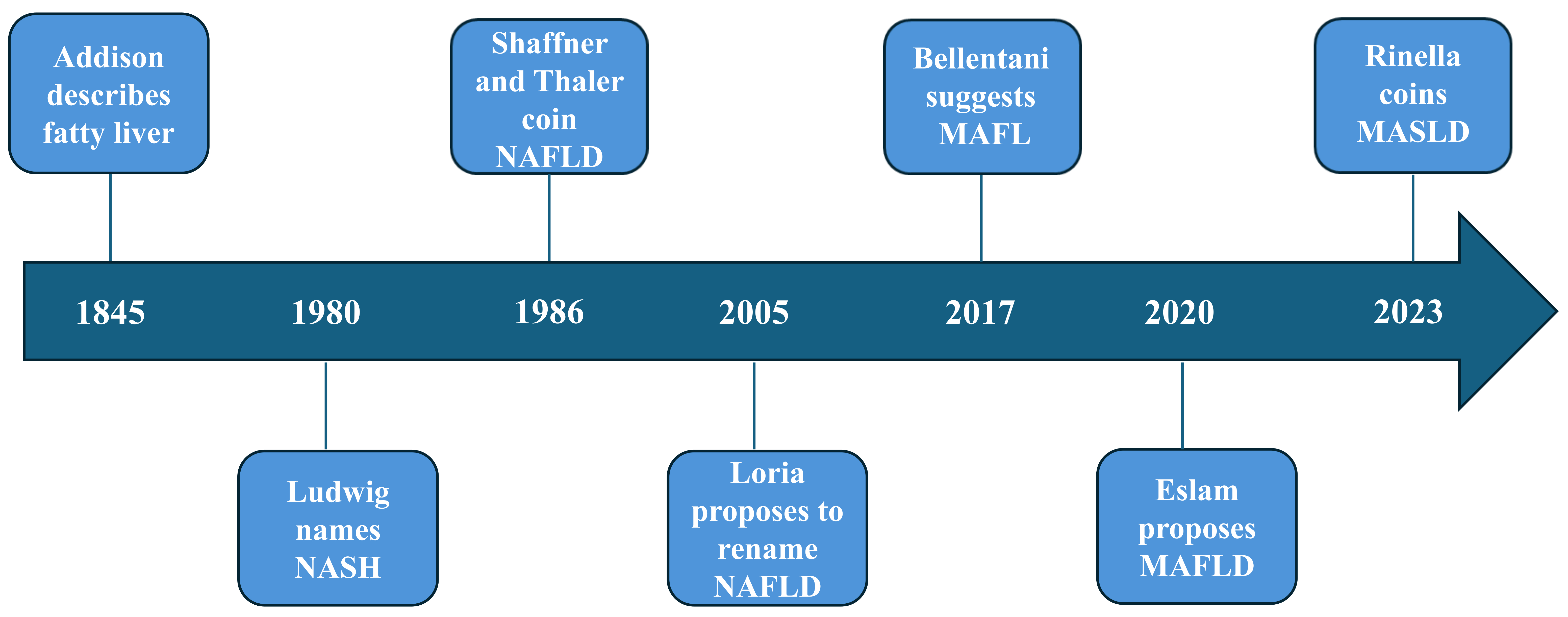
Graphical summary of some of the most relevant advances in the field of SLD nomenclatures [6, 10, 11, 13, 19–21]. Arbitrary time scale illustrating the steps leading from the early description of fatty liver in 1845 [19] through MASLD in 2023 [6]. Intermediate nomenclatures comprise NASH [10], NAFLD [11], insulin-resistant (or metabolic) fatty liver disease [20], MAFL [21], and MAFLD [13]. MAFL: metabolic-associated fatty liver; MAFLD: metabolic dysfunction-associated fatty liver disease; MASLD: metabolic dysfunction-associated; NAFLD: nonalcoholic fatty liver disease; NASH: nonalcoholic steatohepatitis
NAFLD is the prototypic disease entity in the SLD spectrum, and most of what is presently known regarding the natural history of SLD is based on analysis of NAFLD patient populations. The spectrum of hepatic and extrahepatic NAFLD outcomes serves as a reminder of the outcomes that also MAFLD and MASLD should be able to capture.
As a systemic disorder, NAFLD affects not only the liver, but also various extra-hepatic organ systems, and the spectrum of extra-hepatic associations is ever-expanding. Indeed, NAFLD carries an increased risk of MetS and its individual components, adverse cardiovascular events, chronic kidney disease (CKD), extra-hepatic cancers, and possibly, certain types of dementia [22–27].
It is widely acknowledged that cardiovascular disease (CVD) is the most common cause of mortality in NAFLD patients, followed by malignancies, while liver-related complications are a distant third [28]. Indeed, NAFLD (compared to individuals without NAFLD) is associated with higher odds of fatal and non-fatal CVD events [hazard ratio (HR): 1.45, 95% confidence interval (CI): 1.31 to 1.61] [29]. Additionally, compared to NAFLD-free controls, individuals with NAFLD exhibit a higher risk of all-cause mortality (HR: 1.34, 95% CI: 1.17 to 1.54) and cardiovascular mortality (HR: 1.30, 95% CI: 1.08 to 1.56) [29].
According to Fu et al. [30], who conducted a systematic review of 15 published studies totaling 10,286,490 individuals, NAFLD was associated with higher odds of all-cause mortality (HR: 1.32, 95% CI: 1.09 to 1.59), CVD-related mortality (HR: 1.22, 95% CI: 1.06 to 1.41), and cancer-related mortality (HR: 1.67, 95% CI: 1.15 to 2.41). However, no significant association was found between liver-related mortality and NAFLD, probably because fibrosis has a low prevalence in the community.
A study of 957 subjects with NAFLD found age (HR: 1.070), hypertension (HR: 4.361), and decompensated cirrhosis (HR: 15.036) to independently predict poor outcomes at multivariate Cox proportional hazard analysis. Additionally, liver stiffness measurement, bilirubin, compensated and decompensated cirrhosis independently predicted liver-related events [28].
Xiao et al. [29] have confirmed that NAFLD increased the risks of CVD, systemic malignancies, diabetes, and CKD. As regards liver-related outcomes, the incidence of hepatocellular carcinoma (HCC) in NAFLD is 2.39 per 100 person years (95% CI: 1.40 to 4.08) and NAFLD—compared to individuals without NAFLD—is also associated with higher odds of cholangiocarcinoma, the second most common type of primary liver cancer after HCC [odds ratio (OR): 1.88, 95% CI: 1.25 to 2.83] [29, 31].
Liver fibrosis is a major determinant of liver-related outcomes among NAFLD individuals. For example, subjects with NAFLD living with fibrosis stage 3 exhibit a five-fold increased risk of liver-related events (i.e., end-stage cirrhosis and HCC) compared to those patients with NAFLD with no or little fibrosis [32]. Consistently, a cohort study of 1,260 Swedish with non-cirrhotic NAFLD submitted to long-term follow-up has shown that the 20-year cumulative incidence of major adverse liver outcomes were in the same magnitude for the reference population as among subjects with F0 fibrosis (2% vs. 3%, respectively) while it was 35% for those with F3 liver fibrosis [33]. Fibrosis progression may also occur among those with uncomplicated steatosis [34]. However, the speed of progression of fibrosis is two-fold higher in NASH than in simple steatosis (14.3 years vs. 7.1 years, respectively) [35].
Additionally, the severity of fibrosis modulates the risks of cause-specific mortality in NAFLD. A seminal study by Vilar-Gomez et al. [36] found that individuals with NAFLD-cirrhosis experience predominantly liver-related events, whereas those with bridging fibrosis predominantly have extra-hepatic cancers and adverse cardiovascular events.
At variance with the prominent role of fibrosis, the inflammatory component of NAFLD (NASH) has no proven association with adverse clinical outcomes [37]. In NAFLD, the putative factors that are associated with poor liver events comprise genetic variants, intestinal dysbiosis, body weight gain, increased insulin resistance, and exacerbation of liver steatosis [37].
Among these aggravating factors, type 2 diabetes (T2D) undoubtedly plays a major role. A systematic review and meta-analysis of population-based cohort studies reported that T2D was associated with an increased risk of incident severe liver disease events (adjusted HR: 2.25, 95% CI: 1.83 to 2.76) [38]. This is relevant given that NAFLD subjects (and particularly those with more severe fibrosis) are at risk of developing incident T2D [39]. This mutual and bi-directional relationship generates a cause-and-effect vicious circle [40].
CKD participates in a similar vicious circle. On the one hand, NAFLD is a risk factor for incident CKD [25]; on the other hand, the coexistence of NAFLD with CKD negatively affects the outcomes of either condition separately and amplifies the risk of cardiovascular events and mortality [41–43].
Finally, the role of sex and liver fibrosis as modifiers of the risks of HCC and extra-hepatic outcomes in MASLD has been reviewed elsewhere [44].
Mendelian randomization analysis suggests causal associations between biopsy-proven MASLD with vascular dementia, whereas no evidence was found of a causal nexus between MASLD and any dementia, Alzheimer’s disease, dementia with Lewy bodies, or frontotemporal dementia [27].
To sum up, Figure 3 illustrates the spectrum of the main hepatic and extra-hepatic MASLD outcomes such as described in this paragraph. In conclusion, owing to the more abundant literature and long-lasting observational studies available, we have used the NAFLD paradigm to recapitulate the natural course of SLD. There is no reason to believe that MAFLD and MASLD have different disease outcomes compared to NAFLD, and rather these two nomenclatures might differ regarding their variable ability to capture these hepatic and extra-hepatic disease manifestations and complications.
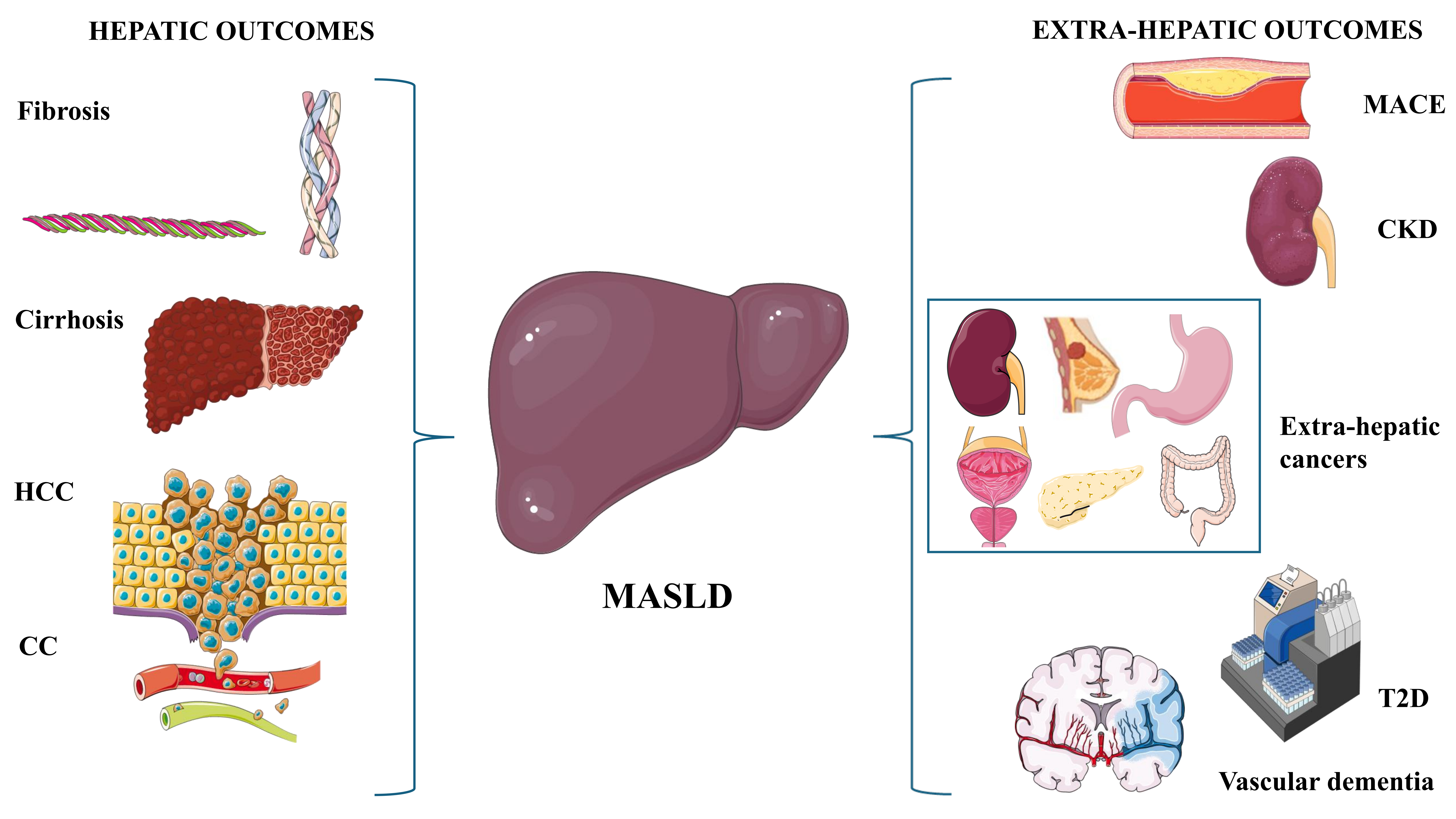
Spectrum of the MASLD outcomes. Figure 3 graphically depicts the notions illustrated in The natural course of NAFLD. The left-hand part of the illustration depicts hepatic outcomes, whereas the extra-hepatic outcomes are shown in the right-hand part of the figure. CC: cholangiocarcinoma; CKD: chronic kidney disease; HCC: hepatocellular carcinoma; MACE: major adverse cardiovascular events; MASLD: metabolic dysfunction-associated steatotic liver disease; T2D: type 2 diabetes. Original image drawn with Servier Medical Art, licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/)
Multiple published original articles have compared the scope of the novel MASLD nomenclature to the previous MAFLD definition in a few months [45–63], indicating strong interest in this topic. These original published studies are summarized in Table 1.
Synopsis of published comparative studies, ordered chronologically
| Author, year [Ref] | Method | Results | Conclusion |
|---|---|---|---|
| Zhao and Deng, 2024 [45] | 13,856 adults were identified using the NHANES III database and followed for 23.7 ± 7.62 years. Of these, 10,940 had non-MASLD/MAFLD, 855 MAFLD-only, 93 MASLD-only, and 1,968 had overlapping MASLD/MAFLD. | Subjects with MAFLD-only (aHR: 1.151, 95% CI: 1.030–1.285) and overlapping MASLD/MAFLD (aHR: 1.109, 95% CI: 1.033–1.292) had a higher risk of all-cause mortality than individuals with non-MASLD/MAFLD. However, MASLD-only individuals were not at increased risk of all-cause mortality. | It is MAFLD (not MASLD) that determines the natural course of liver disease. |
| Ramírez-Mejía et al., 2024 [46] | Cross-sectional analysis of 500 participants. | 59.4% of participants were diagnosed as MAFLD+MASLD+, 10.2% as MASLD+ and 30.4% as MAFLD-MASLD-. These variable prevalence rates depended on the detection of individuals with a BMI < 25 kg/m2, where MASLD identified the highest number (p < 0.001). | MASLD captures lean individuals better than MAFLD, whereas MAFLD better identifies subjects at a higher risk of liver fibrosis and disease progression. |
| Zhou et al., 2024 [47] | Retrospective analysis of 92,177 participants with SLD ascertained with USG. | MAFLD-MASLD+ subjects did not exhibit a greater MACE risk than MAFLD-MASLD- individuals, and their risk was significantly lower than that of MAFLD+MASLD+ people (16.2% vs. 5.3%, p < 0.001). Among lean SLD subjects, approximately 10% of cases have MASLD and are not at as high a risk of MACE as those who are MAFLD+MASLD+ (17.7% vs. 5.8%, p < 0.001). | The MASLD definition captures a greater number of individuals. Conversely, the MAFLD nomenclature is more selective in identifying those individuals who are at increased risk of adverse outcomes. |
| Park et al., 2024 [48] | Among 844 participants, SLD was defined as MRI-PDFF ≥ 5%. | The prevalence rates were as follows: NAFLD 25.9%, MAFLD 29.5%, and MASLD 25.2%. | The prevalences of NAFLD and MASLD assessed using MRI-PDFF were similar, with MASLD accounting for 97.3% of the patients with NAFLD. |
| Mori et al., 2024 [49] | Among 15,788 recruited Japanese, clustering analyses were used to investigate the NAFLD and MAFLD nomenclatures. | MASLD and NAFLD shared three clusters: (i) low alcohol consumption with low-grade obesity; (ii) obesity with dyslipidemia; and (iii) dysfunctional glucose metabolism. Both MetALD and ALD displayed one distinct cluster intertwined with alcohol consumption. MAFLD was widely shared across all five clusters. | The various SLD nomenclatures provide variable insights into predictive factors and the dynamic disease interplay. |
| Pan et al., 2024 [50] | 6,096 individuals from the 2017 to 2020 NHANES cohort. | Compared to the absence of each condition, MAFLD (OR: 2.14, 95% CI: 1.78–2.57, p < 0.001) was more strongly associated with high ASCVD risk than MASLD (OR: 1.82, 95% CI: 1.52–2.18, p < 0.001). At MLR, MAFLD alone was significantly more strongly associated with a high risk of ASCVD (OR: 2.82, 95% CI: 1.13–7.01, p < 0.03) than MASLD alone. | MAFLD and MASLD are both associated with ASCVD risks. However, MAFLD predicts ASCVD risk better than MASLD. |
| Kim et al., 2024 [51] | Analysis of 7,811 participants of the 3rd NHANES database and linked mortality through 2019. SLD was identified with USG. | During a median 27.1-year follow-up, the risk of all-cause mortality was HR: 1.23, 95% CI: 1.09–1.38 for MAFLD; HR: 1.13, 95% CI: 1.01–1.27 for MASLD. | MAFLD nomenclature identifies a patient population at higher risk of all-cause mortality than MASLD. |
| Wu et al., 2024 [52] | Population-based cross-sectional survey of 7,388 Chinese participants. | Cryptogenic SLD and MASLD+MAFLD- patients exhibited milder SLD and a lower frequency of liver injury than NAFLD, MAFLD, or MASLD patients (all p < 0.05). | MAFLD identifies subjects with more severe SLD and a higher frequency of liver injury. |
| Pan et al., 2024 [53] | 5,492 participants from the 2017–2020 NHANES database were enrolled. Albuminuria was defined as a urinary albumin-to-creatinine ratio ≥ 3 mg/mmol. | MAFLD+MASLD- individuals exhibited a greater prevalence of CKD (24.7% vs. 8.3%, p < 0.006) and albuminuria (18.6% vs. 5%, p < 0.01) than did those with MAFLD-MASLD+. After adjustment for confounders, MAFLD+MASLD- subjects had a 4.73-fold higher risk of having prevalent CKD than those in the MASLD+MAFLD- group (p < 0.03). | MAFLD nomenclature captures CKD patients better than MASLD. |
| Pan et al., 2024 [54] | 8,317 subjects from the 2017–2020 NHANES database were included. Liver fibrosis ≥ F2 (i.e., “significant fibrosis”) was determined by a median LSM ≥ 8.0 kPa. | Compared to MASLD (OR: 2.63, 95% CI: 2.22–3.11, p < 0.0001), MAFLD (OR: 3.44, 95% CI: 2.88–4.12, p < 0.0001) tended to be more strongly associated with significant fibrosis. Compared to MAFLD-MASLD+ individuals, those with MASLD-MAFLD+ had a 14.28-fold higher risk of significant fibrosis. | The MAFLD nomenclature identifies significant fibrosis more precisely among individuals with SLD. |
| Bao et al., 2024 [55] | Prospective study enrolling 403,506 participants from the UK Biobank. | MAFLD was associated with a higher risk of vascular dementia (HR: 1.32, 95% CI: 1.18–1.48) but a reduced risk of Alzheimer’s disease. MASLD was associated with an increased risk of vascular dementia (HR: 1.24, 95% CI: 1.1–1.39) but not Alzheimer’s disease. While the effect of MAFLD on vascular dementia was consistent regardless of MASLD presence, Alzheimer’s disease risk was only present in those without MASLD (HR: 0.78, 95% CI: 0.67–0.91). | MAFLD is associated with vascular dementia irrespective of the presence of MASLD, whereas the risks of Alzheimer’s disease were only evident in MASLD- individuals. |
| Mayén et al., 2024 [56] | Data from 15,784 EPIC (a prospective cohort study of more than 521,324 European participants) were used. SLD was assessed with FLI. | Compared to those without the condition, MAFLD was positively associated with all-cause mortality (HR: 1.39, 95% CI: 1.25–1.55) and so was MASLD (HR: 1.40, 95% CI: 1.26−1.56). | Both MAFLD and MASLD are associated with a higher risk of all-cause mortality compared to non-MAFLD and non-MASLD, respectively. However, this study fails to compare MAFLD vs. MASLD directly. |
| Pennisi et al., 2024 [57] | Meta-analysis of 21 eligible cohort studies. | Compared to those with NAFLD, MAFLD individuals had significantly higher rates of overall mortality (random-effect OR: 1.12, 95% CI: 1.04–1.21, p = 0.004) and CV mortality (random-effect OR: 1.15, 95% CI: 1.04–1.26, p = 0.004), and a marginal trend towards higher rates of developing CKD (random-effect OR: 1.06, 95% CI: 1.00–1.12, p = 0.058) and EHCs (random-effect OR: 1.11, 95% CI: 1.00–1.23, p = 0.052). In meta-regression analyses, male sex and metabolic comorbidities were the strongest risk factors associated with adverse outcomes in MAFLD vs. NAFLD. | Individuals with MAFLD have higher rates of overall and CV mortality and higher risks of developing CKD and EHC events than those with NAFLD. |
| Jiang et al., 2024 [58] | Data from 7,791 adults participating in the NHANES III study were analyzed. | Compared to those without any SLD and to MASLD-MAFLD+ individuals, MASLD+MAFLD- subjects were younger and had better metabolic profiles and lower fibrosis stages. Consistently, MASLD+MAFLD- people tended to have lower cumulative incidence of all-cause and CV. Clustering analysis showed that MAFLD-MASLD+ clustered differently from individuals with MASLD-MAFLD+. | MAFLD-MASLD+ individuals exhibit a distinct, clinically heterogeneous phenotype compared to those identified by the MAFLD definition. |
| Kang et al., 2024 [59] | Cross-sectional, multicenter, retrospective study of 2,906 Koreans who underwent abdominal USG and cardiac CT (2017–2021). | In an ASCVD risk score-adjusted model, both MASLD (aOR: 1.21, 95% CI: 1.02–1.44, p = 0.033) and MAFLD (aOR: 1.20, 95% CI: 1.01–1.42, p = 0.034) were associated with CAC. However, only MASLD was associated with severe CAC (aOR: 1.38, 95% CI: 1.01–1.89, p = 0.041). | MASLD may predict higher ASCVD risk better than MAFLD. |
| Pan et al., 2024 [60] | 1,359 participants aged 12 to 17 years from the 2017–2020 NHANES. | 25% of patients who were missed based on the MASLD nomenclature and who met the MAFLD criteria had significant liver fibrosis. MAFLD participants tended to have significantly elevated LSMs compared to those without MAFLD (5.66 ± 2.28 vs. 4.94 ± 1.86, p = 0.06). However, this was not the case for MASLD individuals compared to those without MASLD (5.27 ± 3.10 vs. 5.62 ± 2.12, p = 0.3). | The MASLD criteria overlook several adolescents with severe SLD. |
| Vaz et al., 2025 [61] | Longitudinal study of a cohort of 1,454 randomly selected community-dwelling adult Australians between 2001 and 2003 and followed for a median of 19.7 years (IQR: 19.1–20.1). | MAFLD remained as a risk factor for all-cause mortality on multivariable models adjusted for lifestyle and socioeconomic variables, but not when adjusted for metabolic risk factors. MALOs were increased in MAFLD (IRR: 3.03, 95% CI: 1.22–8.18) and MASLD (IRR: 2.80, 95% CI: 1.05–7.90). Metabolic risk factors were associated with an increased risk of overall mortality and MALO, and cancer (34.3–34.6%) and CVD (30.1–33.7%) were the most common causes of death in SLD. | MAFLD, but not MASLD, is associated with an increased risk of overall mortality, with the components of the MetS playing a major role in increasing the mortality risk. |
| Peng et al., 2025 [62] | 1,862 eligible individuals from the NHANES 2017–2018 cycle. | The risk of HTN was positively associated with MASLD (OR: 2.892, 95% CI: 2.226–3.756), MetALD (OR: 1.802, 95% CI: 1.355–2.398), MAFLD (OR: 3.455, 95% CI: 2.741–4.354) and NAFLD (OR: 1.983, 95% CI: 1.584–2.484) and the risk of T2DM was positively associated with MASLD (OR: 6.360, 95% CI: 4.440–9.109), MAFLD (OR: 7.026, 95% CI: 4.893–10.090) and NAFLD (OR: 3.372, 95% CI: 2.511–4.528). Similar findings were identified for hyperlipidemia. | MAFLD more effectively identifies subjects who are at increased risk of components of the MetS, such as HTN and hyperlipidemia. |
| Jin et al., 2025 [63] | 340,998 participants of the UK Biobank study were followed over a median of 13.5 years. | MAFLD diabetes subtype (HR: 2.26, 95% CI: 2.17–2.35), regardless of the presence of MASLD and ALD (HR: 1.65, 95% CI: 1.55–1.76), showed the highest risk of CVDs. | Irrespective of the presence of MASLD, MAFLD is associated with CVD outcomes. |
aHR: adjusted hazard ratio; ALD: alcohol-related liver disease; aOR: adjusted odds ratio; ASCVD: atherosclerotic cardiovascular disease; CAC: coronary artery calcification; CKD: chronic kidney disease; CI: confidence interval; CT: computerized tomography; CVD: cardiovascular disease; EHCs: extra-hepatic cancers; EPIC: European Prospective Investigation into Cancer and Nutrition; FLI: fatty liver index; HR: hazard ratio; HTN: arterial hypertension; IQR: interquartile range; IRR: incidence rate ratio; LSM: liver stiffness measurement; MACE: major adverse cardiovascular events; MAFLD: metabolic dysfunction-associated fatty liver disease; MALOs: major adverse liver outcomes; MASLD: metabolic dysfunction-associated; MetALD: MASLD and increased alcohol intake; MetS: metabolic syndrome; MLR: Multiple logistic regression; MRI-PDFF: magnetic resonance imaging-proton density fat fraction; NAFLD: nonalcoholic fatty liver disease; NHANES: national health and nutrition examination survey; OR: odds ratio; SLD: steatotic liver disease; T2DM: type 2 diabetes mellitus; USG: ultrasonography
As far as mortality is concerned, studies agree in demonstrating that MAFLD predicts all-cause and mortality owing to CVD in SLD better than MASLD [45, 51, 57, 61]. Other studies explain the mechanisms underlying this finding. For example, the papers by Ramírez-Mejía et al. [46] and Pan et al. [54] both concur in indicating that MAFLD is better than MASLD at identifying those subjects who are at a higher risk of liver fibrosis, the main driver of the natural course of liver disease. Indeed, in early NAFLD, metabolic low-grade inflammation (“metaflammation”) typically serves as the primary driver of disease, promoting the fibrotic progression [64]. However, once established in more advanced stages of disease, liver fibrosis emerges as the dominant driver shaping liver-related outcomes (e.g., cirrhosis, HCC) and extra-hepatic events (such as metabolic dysfunction, extrahepatic cancers, and cardio-nephro-vascular disease) [24–26, 36, 65].
It is noteworthy in this regard that liver fibrosis is also associated with extra-hepatic outcomes and, in agreement, studies have shown that, compared to MASLD, the MAFLD nomenclature more selectively identifies those subjects who have higher odds of the MetS components [62], CVD events [47, 51, 59, 63]; more severe SLD and higher rates of liver injury in the general population [52]; increased prevalence of CKD [53].
Finally, a single study has found that independent of MASLD, MAFLD is associated with vascular dementia; however, the associations with Alzheimer’s disease were found only among MASLD- individuals [55].
Robust evidence supports the practicality and usefulness of the MAFLD definition, which, compared to MASLD, achieves an optimal balance between sensitivity and specificity. In agreement, over 80 professional societies representing multiple stakeholders across the global liver health community have recently reaffirmed their endorsement of the MAFLD nomenclature as an appropriate definition, which predictably has major implications transcending the change of nomenclature to include fields such as cost-effectiveness and health policy [66]. Additionally, the Asian Pacific Association for the Study of the Liver has issued clinical practice guidelines focused on MAFLD [67], suggesting that a large hepatological community is going to continue using the MAFLD nomenclature.
Analysis of studies summarized in Table 1. Supports the conclusion that MAFLD and MASLD are both associated with a higher risk of all-cause mortality compared to non-MAFLD and non-MASLD controls, respectively [50].
MAFLD has the following advantages over MASLD: a) the MAFLD nomenclature more selectively identifies those individuals who are at increased risk of adverse outcomes; b) MAFLD (not MASLD) determines the natural course of liver disease [46] and, specifically, identifies a patient population at higher risk of all-cause mortality than MASLD [51, 61], identifies individuals with more severe SLD and higher frequency of liver injury [52], and significant liver fibrosis [46, 54]. Additionally, c) MAFLD nomenclature captures cardiovascular outcomes [50, 63] and CKD better than MASLD [53, 57]. However, MAFLD’s “dual etiology” model for viral hepatitis lacks solid clinical data or relevance.
Conversely, MASLD has the following advantages over MAFLD: a) The MASLD definition is more inclusive and captures a greater number of individuals [47]; b) MASLD identifies lean individuals better than MAFLD [46]. However, MASLD is considered less suitable for pediatrics [68], and there is minimal evidence regarding its overlap with viral hepatitis, supporting a growing body of research exploring the interplay between MASLD and viral hepatitis, particularly in the context of dual etiologies [69, 70].
Collectively, the MASLD vs. MAFLD comparison has triggered a scientific debate that transcends the strict arena of clinical practice and research and enters the wider scope of philosophy of science.
Based on comparative analysis of the yields of the MASLD and MAFLD nomenclatures, several limitations have been identified. Collectively, these explain why, rather than providing any conceptual improvement compared to the MAFLD nomenclature, MASLD criteria tend to perform worse than MAFLD [71].
Limitations of MASLD nomenclature compared to the MAFLD nosography include the following: it is deemed to be inappropriate in pediatric care; it lacks specificity, has a lower performance, and tends to overdiagnosis. Additionally, it fails to consider a global perspective; does not consider coexistent viral hepatitis; lacks consideration of lean subjects; and amplifies disease heterogeneity.
For example, Cusi et al. [72] have pinpointed that the middle-aged general population has a very high prevalence of at least one CMRF irrespective of the presence or absence of steatosis and that the majority of overweight MASLD are indeed insulin resistant, suggesting a significant discordance and relatively low specificity for these clinical comorbidities as surrogates for insulin resistance.
Henry et al. [73] impute the finding that MAFLD captures patients at high risk for adverse outcomes better than MASLD, owing to the MAFLD definition including more liberal use of alcohol, which often negatively impacts clinical endpoints. In this context, these authors pinpoint the more granular sub-classification of SLD categories, such as MetALD and alcohol-related liver disease (ALD). Bringing these observations further, Ciardullo [74] has pinpointed the important notion that the higher the number of CMRFs that concur in the same individual, the higher the overall cardiovascular risk, and that MASLD and MetALD, as a whole, probably encompass the whole MAFLD spectrum. Scrutiny has shown that the MetALD entity contains some elements of strong heterogeneity, if not clear ambiguity, since MetALD patients share some characteristics with MASLD while resembling ALD subjects more, especially after adjusting for BMI [75]. Moreover, the current classification of patients may be made challenging by the finding that alcohol consumption dissociates insulin resistance from certain CMFRs, such as blood pressure and HDL-cholesterol [75].
MAFLD has been adopted by scientific societies due to its concise diagnostic criterion, removal of the requirement to exclude concomitant liver diseases, and reduction in the stigma associated with this condition [76]. Furthermore, it has been endorsed by a collaborative panel of 890 international experts in various fields from 61 countries who agreed that MAFLD should be coded in the World Health Organization’s International Classification of Diseases (ICD)-11 [77].
The advent of the novel MASLD nomenclature has generated a debate about the essential requirements that disease names should have in medicine. Sanal et al. [78] have clearly identified such requirements, which include the following: accuracy, uniqueness, consistency, objectivity, granularity, comprehensiveness, adaptability, accessibility, and global applicability.
The MAFLD nomenclature has abundantly met its promise which was inspired by four robust foundational principles comprising: a) applicability to all age groups; b) focus on removing alcohol from the diagnostic criteria; c) emphasis on evaluating (rather than excluding) concurrent etiologies of chronic liver disease (CLD); d) universal applicability of clinically relevant diagnostic criteria [79].
Whether or not it is appropriate to change “fatty” with “steatotic” represents a topic of major significance for the philosophy of science. In this regard, Alboraie et al. [80] have pinpointed that the adjective “fatty” is commonly used across various medical specialties, indicating that it is largely accepted. Moreover, systematic analysis of 17 non-latin languages, collectively spoken by more than 5 billion people globally, has indicated that these languages either had a translation for the word fatty” while lacking a specific translation for the word “steatotic,” or had translations for both “steatotic” and “fatty” with similar meanings, or had similar meanings and writing patterns for both adjectives [81]. Additionally, in Chinese, the adjective “fatty” ha a neutral qualification, encompassing a variety of situations from daily use (e.g., fatty meal) to academic expressions (e.g., fatty liver) [82]. This implies that the old NAFLD definition has negligible stigma impact when translated into Chinese [82]. These considerations are particularly important when considering the substantial disproportion between the abundant fraction of patients from China confronted with the small minority of Asian participants in the Delphi consensus [82].
Regarding the relationship between democracy and science, it has been nicely pinpointed that the scientific method has nothing to do with democracy [83]. We cannot decide by consensus whether the world is flat and whether the evolutionary theories are correct [78]. However, it is true that the rules of democracy must be applied whenever a consensus is sought in the scientific community in front of insufficient evidence to support shared conclusions [83]. Although debates cannot be avoided in medical research, the only method to resolve them is by relying on scientific evidence, not using an eminence-based approach [84, 85].
The development of a novel medical terminology to receive global acceptance involves a complicated workflow, and the consensus panel that began to promote the MASLD concept of MAFLD deserves great credit [86]. However, the ultimate drivers of this initiative remain controversial according to some investigators [87, 88]. Although it met with wide acceptance, the MAFLD definition has also been criticized based on several points of weakness [5]. According to Anand, albeit pathogenically linked with the MetS, MAFLD was unresponsive to those classes of drugs used to treat the individual components of the MetS [5]. Secondly, the excess focus on metabolic components eventually led to neglecting major players in MAFLD pathophysiology (e.g., genetics, gut dysbiosis, and sarcopenia) [5]. Thirdly, MAFLD failed to completely account for the additive/synergistic effects on the risks of onset and worsening of fatty liver disease owing to concurrent viral hepatitis and alcohol abuse [5]. Finally, among individuals with “lean NAFLD”, MetS may not be prominent [5].
On these grounds, the rationale for developing the MASLD nomenclature was based on three major points [5]: a) the adjective “fatty” was deemed to be stigmatizing; b) scholars wanted to recognize he concurrence of multiple contributing factors that in most individuals with SLD; c) the potentially negative impact that changes in diagnostic criteria could have on the development of biomarkers and therapeutics. However, only a weak majority of 53% of the panelists (vs. 47% supporting no change) sustained the decision to change the definition of MASLD and this vote underscores the intricate nature of this matter, while simultaneously highlighting the existence of contentions which would have benefited from a more in-depth debate of controversial issues before adopting the new MASLD definition [72, 89].
MAFLD and MASLD definitions mainly differ in the number of metabolic derangements needed to define “metabolic dysfunction” in normal-weight individuals and in alcohol consumption [18]. A key difference between MAFLD and MASLD is how they consider SLD as related to other coexistent liver disorders. While MAFLD embraces the notion of “dual etiology”, which generates a unified classification system that is consistent with the needs of epidemiological and therapeutic research, MASLD, by introducing the newly coined MetALD definition, describes individuals exhibiting both metabolic dysfunction and excessive alcohol consumption [90]. Additionally, the MAFLD nomenclature may contribute to decreasing the heterogeneity of other disease definitions [91].
Based on the evidence shown in Table 1 and discussed in-depth in this narrative review, there is an urgent need for more studies that should include participants from different regions, ethnicities, and socioeconomic backgrounds to better understand the global impact of MASLD and MAFLD. Future research should focus on elucidating the pathophysiological pathways linking metabolic dysfunction to liver disease and other comorbidities to develop targeted therapies. With the rapid advancements in omics technologies, there is a growing interest in identifying novel biomarkers that can improve the accuracy of disease detection and prognosis. Moreover, a true consensus process that allows for the development of evidence-based guideline statement should involve a systematic review of the evidence, with equal contribution from all regions of the world, patients, research, and clinical panelists, and using quantifiable data to identify an objective-level consensus to ensure that the outcomes will be adopted globally [71].
ALD: alcohol-related liver disease
CI: confidence interval
CKD: chronic kidney disease
CMRFs: cardiometabolic risk factors
CVD: cardiovascular disease
HCC: hepatocellular carcinoma
HR: hazard ratio
MAFLD: metabolic dysfunction-associated fatty liver disease
MASH: metabolic dysfunction-associated steatohepatitis
MASLD: metabolic dysfunction-associated steatotic liver disease
MetALD: metabolic dysfunction-associated and increased alcohol intake
MetS: metabolic syndrome
NAFLD: nonalcoholic fatty liver disease
NASH: nonalcoholic steatohepatitis
OR: odds ratio
SLD: steatotic liver disease
T2D: type 2 diabetes
AL, MHZ, and ME: Conceptualization, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
Amedeo Lonardo who is an Editorial Board Member of Exploration of Digestive Diseases had no involvement in the decision-making or the review process of this manuscript. Other authors declare that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
