Affiliation:
1Faculdades Pequeno Príncipe, Curitiba 80230-020, Brazil
ORCID: https://orcid.org/0000-0002-1046-0655
Affiliation:
1Faculdades Pequeno Príncipe, Curitiba 80230-020, Brazil
ORCID: https://orcid.org/0000-0002-9584-5174
Affiliation:
1Faculdades Pequeno Príncipe, Curitiba 80230-020, Brazil
ORCID: https://orcid.org/0000-0003-3778-2264
Affiliation:
1Faculdades Pequeno Príncipe, Curitiba 80230-020, Brazil
ORCID: https://orcid.org/0000-0003-0632-8915
Affiliation:
1Faculdades Pequeno Príncipe, Curitiba 80230-020, Brazil
2Hospital Pequeno Príncipe, Curitiba 80250-060, Brazil
3Santa Casa de Curitiba, Curitiba 80310-030, Brazil
ORCID: https://orcid.org/0000-0003-2863-2184
Affiliation:
1Faculdades Pequeno Príncipe, Curitiba 80230-020, Brazil
2Hospital Pequeno Príncipe, Curitiba 80250-060, Brazil
ORCID: https://orcid.org/0000-0002-4904-0746
Affiliation:
1Faculdades Pequeno Príncipe, Curitiba 80230-020, Brazil
Email: camilam14@gmail.com
ORCID: https://orcid.org/0000-0001-5121-922X
Explor Dig Dis. 2025;4:100587 DOI: https://doi.org/10.37349/edd.2025.100587
Received: April 25, 2025 Accepted: August 03, 2025 Published: August 19, 2025
Academic Editor: Maria Carlota Londoño, University of Barcelona, Spain
Brazil ranks second globally in absolute liver transplants and leads pediatric transplantation in Latin America. This scoping review aims to map the results and perspectives of pediatric liver transplantation in Brazil from 2000 to 2022. A scoping review was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews Checklist (PRISMA-ScR) guidelines, using PubMed, Virtual Health Library (VHL), and ScienceDirect. From 293 records, 26 studies were included based on predefined criteria. The review focused on clinical indications, techniques, outcomes, and regional disparities. Results: Of the 26 included studies, 10 (38%) reported survival rates, showing 1-, 5-, and 10-year survival of 89.3%, 78.1%, and 68.5% for deceased donors and 93.1%, 85.7%, and 67.5% for living donors, respectively. Eleven studies (42%) discussed living donor liver transplantation (LDLT), which accounts for 53.4% of pediatric transplants. Eight studies (31%) detailed postoperative complications, such as vascular (up to 19%) and biliary (15.7%) issues, rejection (~ 50%), and infections. The COVID-19 pandemic led to a 20.6% reduction in transplant activity and increased waiting list mortality from 8.4% to 11.9%. Despite Brazil’s leadership in pediatric liver transplants, challenges persist, including donor shortages, diagnostic delays, geographic concentration in São Paulo (66%), and limited data systematization. These factors hinder equitable access and optimal outcomes across the country. To improve pediatric liver transplantation in Brazil, actions are needed to strengthen donor registration systems, decentralize services, enhance team training, and adopt techniques like split liver transplantation. Expanding national databases and prognostic tools will help address disparities and improve care.
Liver transplantation was introduced into medical practice in the 1960s by Thomas Starzl in Denver, United States [1]. In the early 2000s, around 10,000 liver transplants (LT) were carried out every year worldwide [2]. In Brazil, this procedure began experimentally at the Hospital de Clínicas of the University of São Paulo (HC-USP) in 1968, and was recognized as a medical therapy in the 1980s [3]. In Latin America, only eight countries carry out paediatric liver transplantation: Brazil, Argentina, Mexico, Chile, Colombia, Peru, Venezuela, and Cuba. Factors such as low Human Development Index, low government investment in health, and low health indices interfere with the growth of this practice in Latin America [4]. In 2022, Brazil ranked third in the world in terms of the number of LT performed, behind only the United States and China, which ranked first and second, respectively. However, in that same year, Brazil ranked 21st in the number of LT per million people (pmp) in a ranking of 44 countries. According to data from the Brazilian Transplant Registry published by the Brazilian Organ Transplant Association, during the first half of 2010, more than 3,200 organ transplants were carried out, of which around 700 were LT. This shows an increase of almost 100% in relation to the number of operations carried out in the 2000s [5]. According to reports in the literature, the first patient to undergo liver transplantation, carried out almost six decades ago, was a 3-year-old child with biliary atresia. Currently, the main groups of indications for liver transplantation in the pediatric population include extrahepatic and intrahepatic cholestasis, metabolic disorders, acute liver failure, and primary liver malignancy [6, 7].
In Latin America, the practice of pediatric liver transplantation began in the 1990s and had some limitations in terms of surgical techniques, which hindered the selection of donors for this specific population. They also had more post-transplant complications, such as primary graft dysfunction, acute cellular rejection, and recurrences of hepatitis B and C [6]. However, with the development and improvement of surgical techniques, transplants in the pediatric population can be performed with portions of the liver from adult donors [8]. Among the techniques used to perform liver transplantation described in the literature are orthotopic transplantation and its variations. The original orthotopic transplant is often not viable for children, due to the need for graft weight and size matching and the lack of infant cadaveric donors [9]. In Brazil, 236 pediatric LT were performed in 2019, an increase of 4.7% compared to the previous year. In December of the same year, the Brazilian Transplant Registry counted more than 400 children on the waiting list for an organ, of which around 40 were waiting for a liver [10].
The main complications related to pediatric liver transplantation include primary graft dysfunction, acute cellular rejection, recurrence of hepatitis B and C, as well as infectious, vascular, and biliary complications [6]. However, improvements in surgical techniques and post-operative care, nutritional support, and the introduction of living-donor transplants have had an impact on the increase of children's survival after liver transplantation, reducing complications and reducing their time on the waiting list [11]. To make the waiting list for liver transplantation more objective, transparent, and to prioritize candidates according to the severity of the disease, scores such as the PELD (pediatric end-stage liver disease) have been developed [12]. With the improvement of surgical techniques and post-operative care, and making the waiting list more effective, there has been an improvement in the post-procedure prognosis as well as an increase in the electivity of donors for the pediatric population [11]. Donors are evaluated according to clinical information, biochemical tests, and organ function [7]. In Brazil, living donors are recommended, corresponding to 53.4% of all pediatric LT performed in the country. Living-donor transplantation is more viable, since it is less dependent on the infrastructure of the public health system. Despite countless advances in medicine, invasive liver transplantation therapy remains the only viable curative option for various pathologies that culminate in terminal liver failure [13, 14]. Thus, the aim of this scoping review is to map the existing data in the literature to answer the question: “What are the results and perspectives of pediatric liver transplantation in Brazil from 2000 to 2022?”.
This study is a scoping review of the literature. The scoping review is a significant method in the health area, as it enables the mapping of different scientific materials to analyze the available evidence on a given topic of interest. Thus, this method is appropriate for covering broad topics of discussion, bringing together different methodological designs, tracking the different evidence available, aiming to guide the researchers, managers, and health professionals’ decision-making [15].
In addition to synthesizing the data available in the literature, the scoping review allows the identification of concepts, sources, and knowledge gaps, as well as the determination of the extent, scope and nature of the available evidence, to predict the demand for a systematic review on the subject and to guide possible new studies in the area of interest [16].
The development of this scoping review was based on the guidelines of the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews Checklist (PRISMA-ScR), which consists of a roadmap to guide the production of a scoping review. PRISMA-ScR has 22 items, 20 of which are essential and 2 optional, containing the following topics: Title, Abstract, Introduction, Method, Results, Discussion, and Funding [15]. This checklist was developed in accordance with the guidelines published by the EQUATOR Network (Enhancing the QUALity and Transparency Of Health Research) and aims to help readers, researchers, editors, and managers understand the basic concepts and essential items for producing a scoping review [16].
In addition to the PRISMA-ScR protocol, this study was based on the document created by the International Research Organization Joanna Briggs Institute (JBI). This organization was created in 1996 and focuses on the development of evidence-based information, through software and training to improve methodological practice [17]. Thus, the model developed seeks to guide the steps to be taken in a scoping review.
The research protocol for this review was registered with the Open Science Framework on July 11, 2022 (https://osf.io/yq8hr/). To formulate the guiding question for this study, the research question was devised using the PCC mnemonic, where P is the population, the first C refers to concept, and the second C represents context. The population selected in this study is pediatric patients, the concept is liver transplantation, and the delimited context is Brazil, between the years 2000 to 2022. Thus, the question developed was: “What are the results and perspectives on liver transplantation in pediatric patients in Brazil between 2000 and 2022?”.
Research sources were found based on an initial search in the following databases: PubMed, Virtual Health Library (VHL), and ScienceDirect. The descriptors used were [LIVER TRANSPLANTATION] AND [PEDIATRIC LIVER TRANSPLANTATION] AND [BRAZIL]. During the search, a date restriction filter was applied and articles published between 2000 and 2022 were included. In the PubMed database, to exclude results including “books and documents”, the following search filters were applied: “Clinical Trial, Meta-Analysis, Randomized Controlled Trial, Review, and Systematic Review”. In the VHL database, one thesis was excluded and all other types of scientific studies were included. Finally, the ScienceDirect database excluded “book chapters and conference abstracts” and included “research articles”. Repeated articles in the databases used were also excluded.
A total of 293 articles were found, from which, using the filters explained in the methodology of this study, 208 were excluded—204 from the PubMed database, 1 from the VHL, and 3 from ScienceDirect. This left 85 articles, 25 of which were excluded because they were repeated on the data platforms used, leaving 60 articles for titles and abstracts reading.
Following titles and abstracts reading of the 60 articles, 22 were excluded because they did not address the specific theme of pediatric transplantation in Brazil. These excluded studies typically focused on topics such as adult transplantation, pediatric transplantation in other countries, general immunosuppression protocols, bioethical or legal aspects without contextualization to the Brazilian context, or broader discussions on organ donation not specifically related to pediatric populations. As a result, 38 articles were considered eligible for full-text reading. After full-text analysis, 12 additional articles were excluded for not meeting the inclusion criteria. These exclusions were due to the absence of relevant data on pediatric patients, lack of focus on transplantation outcomes or policies, or because they addressed theoretical or conceptual aspects without empirical data or practical application. Consequently, 26 articles were selected to comprise the final version of this review. Summary of the article selection process included in this review is shown below in the form of a flowchart (Figure 1).
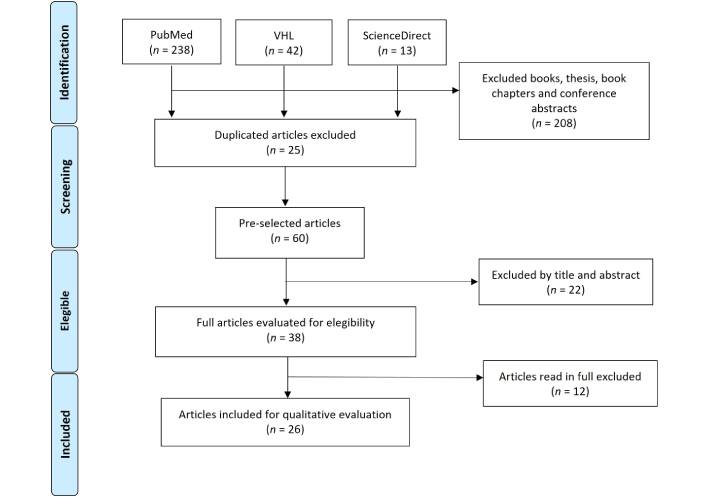
Study selection process. Modified from [43]. VHL: Virtual Health Library. © 2009 Moher et al. CC BY
Of the 26 studies selected for this review, 31% were cohort studies, 27% were reviews, 12% were case series, 8% were cross-sectional studies, and 8% were editorials. Fifteen percent were classified as other because they did not specifically define the category of study in their methodology, representing a total of 4 articles in absolute numbers (Figure 2).
For the year of publication, articles published between 2000 and 2022 were included, of which the most prevalent year was 2014, which totaled 4 articles, representing 15.4% of the articles that made up the final sample.
With regard to the countries of publication, 20 articles were published in Brazil, representing 76.9% of the articles selected. Of the articles selected, 4 (15.4%) were multicenter studies, 2 of which involved countries in Latin America, 1 in South America, and 1 that was produced in both South America and North America at the same time. In addition, 2 articles (7.8%) did not state the location of the study in their methodology.
Below are all the articles selected for this review, with their respective titles, year of publication, journal, and main recommendations (Table 1).
Summary of selected articles
| Reference No. | Title | Year | Journal | Main recommendations |
|---|---|---|---|---|
| [4] | Pediatric liver transplantation in Latin America: Where do we stand? | 2016 | Pediatr Transplant | Promote regional cooperation and investment in training and infrastructure for pediatric transplantation across Latin America. |
| [17] | Liver transplantation in Latin America: the state-of-the-art and future trends | 2014 | Transplantation | Encourage data sharing and development of public policies for transplant access and equity in Latin American countries. |
| [18] | Current status of liver transplantation in children | 2003 | Pediatr Clin North Am | Highlight the need for multidisciplinary care and ongoing research to improve pediatric transplant outcomes. |
| [19] | Model for end-stage liver disease: impact of the new deceased donor liver allocation policy in São Paulo, Brazil | 2009 | Transplant Proc | Monitor the impact of allocation models on pediatric outcomes and propose adjustments as necessary. |
| [21] | Postoperative care in pediatric liver transplantation | 2014 | Clinics (Sao Paulo) | Structure specialized units for pediatric postoperative care; ensure intensive support and systematic follow-up. |
| [22] | Frequency of and factors associated with vascular complications after pediatric liver transplantation | 2014 | J Pediatr (Rio J) | Identify and mitigate risk factors for vascular complications through surgical precision and intensive postoperative monitoring. |
| [23] | Pediatric liver transplantation: 10 years of experience at a single center in Brazil | 2008 | J Pediatr (Rio J) | Value centers of excellence and accumulated experience as key factors for positive outcomes; standardize pre- and post-operative care. |
| [24] | Development of a prognostic model for pediatric acute liver failure in a Brazilian center | 2022 | J Pediatr (Rio J) | Use national prognostic models to better identify transplant candidates; personalize clinical decision-making. |
| [25] | Pediatric Liver Transplant: Techniques and Complications | 2017 | Radiographics | Prioritize surgical training and postoperative monitoring to reduce complications; invest in experienced multidisciplinary teams. |
| [26] | Modified pediatric end-stage liver disease scoring system and pediatric liver transplantation in Brazil | 2010 | Liver Transpl | Adapt and validate severity scoring systems (such as modified PELD) to the Brazilian reality for better patient prioritization. |
| [27] | Pediatric Liver Transplantation Program at the Instituto da Criança do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo | 2016 | Clinics (Sao Paulo) | Strengthen specialized programs with long-term institutional experience to improve outcomes and ensure sustainability. |
| [28] | Pediatric liver transplantation: fourteen years of experience at the children institute in São Paulo, Brazil | 2004 | Transplant Proc | Emphasize longitudinal follow-up and standardized clinical approaches for sustainable long-term outcomes. |
| [29] | Consequences of the implementation of the Model for End-stage Liver Disease system for liver allocation in Brazil | 2013 | Transplant Proc | Review and adapt liver allocation policies to ensure fairness and better reflect pediatric needs within national systems. |
| [30] | Opinion of hepatic transplant waiting list patients regarding split liver transplantation | 2008 | Transplant Proc | Consider patient perspectives to improve acceptance and implementation of split liver transplantation strategies. |
| [31] | The HMS Birkenhead docks in Brazil: pediatric end-stage liver disease times three | 2010 | Liver Transpl | Adapt international models for pediatric liver disease to the Brazilian context, considering regional challenges. |
| [32] | Nutritional aspects of liver transplantation | 2002 | Curr Opin Clin Nutr Metab Care | Integrate specialized nutrition teams in pre- and post-transplant care; monitor nutritional status as a prognostic factor. |
| [33] | Severity of Ascites Is Associated with Increased Mortality in Patients with Cirrhosis Secondary to Biliary Atresia | 2020 | Dig Dis Sci | Closely monitor signs of decompensation, such as ascites, in biliary atresia patients; consider early transplantation in severe cases. |
| [34] | Functional capacity after pediatric liver transplantation: a pilot study | 2014 | Pediatr Transplant | Incorporate assessments of physical rehabilitation and long-term functionality into post-transplant care plans. |
| [35] | Biliary complications after pediatric liver transplantation: Risk factors, diagnosis and management | 2015 | World J Hepatol | Implement early screening protocols for biliary complications; train teams for rapid and effective intervention. |
| [36] | Vascular complications after living donor liver transplantation: a Brazilian, single-center experience | 2011 | Transplant Proc | Enhance surgical techniques and early detection strategies to reduce vascular complications. |
| [37] | Selection of donors for living donor liver transplantation in a single center of a developing country: lessons learned from the first 100 cases | 2006 | Pediatr Transplant | Establish rigorous donor selection criteria to maximize safety and long-term success in living donor liver transplantation. |
| [38] | Surgical complications in 100 donor hepatectomies for living donor liver transplantation in a single Brazilian center | 2010 | Transplant Proc | Improve donor safety through refined surgical protocols and comprehensive preoperative assessment. |
| [39] | Pediatric liver transplantation activity in a high-volume program during the COVID-19 pandemic in Brazil | 2021 | Pediatr Transplant | Maintain strict biosafety protocols to ensure continuity of pediatric transplants even during pandemics; invest in adapted care flows for emergency scenarios. |
| [40] | Impact of COVID-19 Infection on Children and Adolescents after Liver Transplantation in a Latin American Reference Center | 2022 | Microorganisms | Reinforce vaccination and epidemiological monitoring in transplant patients; continuously assess post-COVID impacts. |
| [41] | Extrahepatic biliary atresia: current concepts and future directions | 2007 | J Pediatr (Rio J) | Encourage early diagnosis and quick referral to transplant centers; promote research on new therapies. |
| [42] | Four hundred thirty consecutive pediatric living donor liver transplants: variables associated with posttransplant patient and graft survival | 2012 | Liver Transpl | Focus on donor-recipient matching and perioperative management to optimize survival outcomes. |
PELD: pediatric end-stage liver disease
According to data from the Transplant Society of Latin America and the Caribbean and national organizations and societies, by 2014, Latin American countries had performed more than 2,500 LT per year, representing 17% of the world’s transplant activity, thus resulting in a ratio of 4.4 LT pmp per year [18]. Of the top twenty countries with the highest rates of LT per million inhabitants, in Latin America, there are only Brazil and Argentina, even though they are countries with lower investment in health compared to those in North America and Europe. However, only eight of the twelve countries performing LT in Latin America do it on pediatric patients, and in 2016, a total of 4,593 pediatric LT were performed [19].
Specifically in Brazil, liver transplantation began in the 1960s with deceased donors, and in 1989, it was the first country in the world to perform the transplant procedure with a living donor. From 1989 to 2014, the LT procedure with deceased donors was performed 1,615 times, and from 1995 to 2014, 1,209 were performed with living donors. The implementation of living donor transplantation has mainly benefited the pediatric population, as this technique accounts for 53.4% of LT performed on children in Brazil [18, 19].
With the creation of the National Transplant System (SNT) in 1997, organ transplants in Brazil are regulated by a federal law that seeks to guarantee equal access to treatment on a national scale, and its control is carried out by the Health Departments of each state in the country, allowing for regional allocation [20]. It is recognized worldwide as one of the largest transplant systems in the world. Around 42.3% of LT are carried out in the South West region, due to the great economic disparity present in the country. With regard to pediatric LT, this difference is even greater, with 66% of them performed in the city of São Paulo [19].
Brazil is the second country in absolute numbers for LT. In the last two decades, there has been an increase in the donation rate: in 2006, it was 6.5 pmp, and in 2014 it was 13.1 pmp [19]. Although the annual donation rate has doubled in the past few years, it still cannot be compared to that seen in European countries (15 pmp) and the United States (26 pmp). This scenario can be explained by the lack of notification of potential brain-dead donors, family recurrence, and donors who are not viable due to the lack of adequate donor care in the region [18].
In general terms, Latin America started practicing LT early, but it has shown slow growth, mainly due to the prevalence of countries in a medium human development index zone, low government investment in health, and low health indices. Compared to more developed countries, Latin America invests around 10% to 15% of its gross domestic index in health, while countries in Europe, North America, and Oceania spend more than 20%. In addition, countries such as Japan, the United States, and Canada have better national registries of transplants performed, thus allowing an adequate assessment of pediatric LT activity. These evaluations allow the improvement of transplant centers’ quality, and thus have better results compared to Latin American countries. Although countries such as Brazil and Argentina have a volume of procedures comparable to European and North American countries, there is a great lack of information on pediatric transplants carried out throughout Latin America [19].
The main pathology leading to the indication for liver transplantation in children and adolescents in Brazil today is extrahepatic biliary atresia (EHBA), which can be divided into two etiopathogeneses: intrauterine, which accounts for 20% of cases, and postnatal, which accounts for 80%, and can be caused by toxic or infectious processes [21]. Other important causes include fulminant hepatitis of viral or drug etiology, autoimmune hepatitis, biliary hypoplasia, alpha-1-antitrypsin deficiency, Wilson’s disease, and primary sclerosing cholangitis [22]. In a retrospective quantitative study that analyzed indications and complications related to pediatric liver transplantation, of the 128 transplants analyzed, biliary atresia represented 52.5% of the indications for transplantation, followed by hepatitis of unknown etiology (17.2%) and autoimmune hepatitis (7%) [23]. When comparing Brazil and Latin America with developed countries, there is a difference in the etiology of biliary tract obstruction, with viral pathogens predominating in Brazil and Latin America [21, 24].
The symptoms and signs of EHBA consist of jaundice, acholic feces, choluria, and hepatomegaly, which characterize the disease in pediatric patients, both in the embryonic and perinatal forms. Other manifestations can occur concomitantly, such as steatorrhea, which can lead to complications such as protein and vitamin malnutrition. As far as laboratory tests are concerned, these patients show an increase in total bilirubin (BT), with a predominance of the conjugated fraction (direct bilirubin), as well as an increase in liver enzymes (ALT and AST), canalicular enzymes, gamma-glutamyltransferase (GGT), alkaline phosphatase (AF), and also bile acids. An altered INR may be present, due to a possible vitamin K deficiency [21].
Some indicators corroborate transplant indication: cholestasis with repercussions on structural development, pruritus or intractable ascites, portal hypertension with variceal bleeding, hepatic encephalopathy, repeated spontaneous bacterial cholangitis or peritonitis, deficient liver synthesis, or a condition with repercussions on the child’s weight and/or structural development [25].
Pre- and post-operative imaging tests are of great importance in pediatric patients who are candidates for liver transplantation. The most commonly used in medical practice are computed tomography, magnetic resonance imaging, ultrasound, scintigraphy, angiography, and cholangiography, as they are important tools for analyzing possible suitable donors and recipients, for planning the best surgical technique to be used, and for helping to predict intra- and post-operative complications in these patients. Moreover, with regard to tests useful for diagnosing this pathology, laparoscopy with intra-operative cholangiography and liver biopsy are used for diagnostic confirmation, especially in infants [21, 26].
The PELD score is a formula developed to assess the risk of death in pediatric patients waiting for a transplant and thus allocate the most severely ill patients as a priority on the waiting list. Until July 2006, the transplant waiting list in Brazil was organized in chronological order, i.e., the first person on the list was the one who was registered first, which was not an accurate representation of the degree of illness and of the patients’ risk of death [27]. Currently, the calculated PELD score is multiplied by three, and the result is used to include patients under twelve years of age on the waiting list. The parameters used for evaluation include serum albumin levels, INR, and bilirubin levels [28, 29]. Since then, pediatric transplants have increased from 6.5% to 9.3% [30]. Despite the establishment of priority on the waiting list according to the patient’s condition severity, data have shown that the reduction in time on the waiting list has not fallen significantly (from 23% to 15% in two years). In this scenario, living donor transplantation has become an alternative, especially for the pediatric population in Brazil [19].
Liver transplantation has emerged for pediatric and adult patients as a therapeutic option for diseases that previously had no prospect of improvement. However, the waiting list for an organ remains a problem for transplants in general, especially in children, due to the lack of compatibility between the donor organ and the recipient patient’s body. In paediatric transplants, the volume of grafts has to be reduced, and in addition, the patients’ clinical conditions are usually severe and make the choice of deceased donors even more selective [28].
In order to reduce the waiting time for an organ, the PELD score was implemented, which prioritizes LT. However, there was still the problem of compatibility between donors and recipients, which is why the technique of living donor liver transplantation (LDLT) was introduced, especially favoring the pediatric population [18, 19]. This technique benefits from good graft quality, early treatment of children, extensively evaluated grafts with optimal function, shorter cold ischemia time, and elective scheduling of the operation. These advantages outweigh the difficulties related to some possible complications, such as biliary reconstruction, organ size incompatibility, and the smaller caliber of vascular anastomoses [27–30].
Besides the option between deceased and living donors, there are also reduced, partial, double, or domino LT techniques. Split liver transplantation (SLT) increases the supply of organs for liver transplantation, as it divides the donated liver into two grafts that are normally transplanted into an adult and a child. Both the orthotopic transplant technique and SLT have equivalent patient survival and complication rates [18, 31].
The Brazilian policy aimed at encouraging the acceptance of split grafts by adult transplant centers ensures that all potentially divisible livers are offered first to pediatric patients with higher PELD scores and then the remaining grafts are offered secondarily to adult candidates [32].
The nutritional aspects of patients who are candidates for liver transplantation are directly influenced by their residual liver function, which causes metabolic changes that lead to a state of protein-energy malnutrition. This condition is characterized by an increase in glycolysis, lipid oxidation and protein catabolism, resulting in a loss of adipose tissue, with a depletion of essential fatty acids (linoleic and alpha-linolenic) and long-chain polyunsaturated fatty acids, as well as muscle tissue [33].
The high incidence of malnutrition in children who are candidates for transplantation, in addition to liver dysfunction, can also be attributed to the delay in their referral and the long time they remain on waiting lists [27]. The degree of nutrition before and after liver transplantation is an important predictor of the success of this therapy, both in the pediatric and adult population, since episodes of acute graft rejection are more associated with a lower percentage of total body fat [33]. According to a cohort study that analyzed variables related to the survival of paediatric patients and the post-transplant graft, children with low body weight or with pre-transplant malnutrition had a higher rate of late post-transplant mortality, as well as growth retardation, delayed neuropsychomotor development, and increased hospital admissions, consequently generating higher costs related to their treatment [21, 27]. Thus, malnutrition should be considered as important a parameter for predicting the success of transplant therapy as the other clinical manifestations usually analyzed for this. In addition to the organ failure resulting from the progression of the liver disease, there are other factors contributing to the persistence of the pediatric patient’s catabolic state in the post-transplant period, including the stress of the surgical intervention, the use of immunosuppressive therapy, the occurrence of new liver dysfunction, or the appearance of other concomitant conditions, such as sepsis, renal failure and, in pediatric patients, the worsening of the catabolic state due to physiological growth. Therefore, providing aggressive nutritional support in the post-operative period is essential for a better recovery and acceptance of the transplant [33].
Currently, the main therapeutic option for children with EHBA is hepatoportoenterostomy (HPE) surgery [34]. The Kasai technique and its variations consist of an anastomosis performed in an intestinal conduit to the surface of the hepatic hilum, of the Y-de-Roux type, to enable biliary drainage [21]. Despite the technical options for the LT procedure, there are currently two types of transplant: whole-liver and split deceased-donor and living-donor transplant, which correspond, respectively, to whole organ transplant and transplant of only part of the liver, which can come from a living or a deceased donor [26].
Several factors have a direct impact on the success of this surgical procedure, including the age of the patient. The outcome of liver transplantation in children is better when performed as early as possible after diagnosis. According to a review study on biliary atresia, the process of draining the bile ducts via portoenterostomy is satisfactory in up to 80% of children when performed in children under two months of age, a rate which drops to 10% to 20% in infants operated on at four months of age [21].
With regard to the risk factors influencing the success of the surgical intervention, the discrepancy between the arterial and portal diameters of the donor and recipient, the recipient’s low weight, and the smaller diameter of the portal vein, in addition to the surgical skills and experience of the transplant team, are important variables to be considered in the pediatric population. This is because liver transplantation in children poses a challenge in terms of size discrepancies between the portal vein and the hepatic artery between donor and recipient, due to the small size of the vasculature and biliary tree in children [23].
Thus, there are three possible outcomes for pediatric patients undergoing surgical treatment for EHBA: satisfactory response, partial response, or therapeutic failure. A satisfactory response is a patient with a good clinical evolution, while a partial response is a patient who has good bile duct drainage but progresses to liver fibrosis. Finally, therapeutic failure is a clinical evolution that is the same or even worse than that of untreated patients [21].
Although liver transplantation is one of the safest solid organ transplant procedures, with satisfactory success rates, it is considered a complex procedure and has high morbidity and mortality rates, with several consequences for the transplant patients’ quality of life.
Hepatic artery thrombosis, which compromises the blood supply to the bile ducts, is one of the most serious complications. In a specialized center, 19% of 99 patients had vascular complications, with arterial events being predominant and often associated with graft loss and mortality. Biliary complications affected 15.7% of 134 children, with a higher risk in patients aged > 2 years, with arterial thrombosis, or previous Kasai surgery. In general, arterial complications are more common, occur early in the postoperative period, and are associated with high rates of graft loss and patient mortality. In contrast, venous complications are less frequent, occur late in the postoperative period, and have no significant effect on graft loss or mortality rates [23].
Acute cellular rejection occurs in approximately 50% of patients within the first 3 months and, although often asymptomatic initially, can progress to chronic rejection and require retransplantation if left untreated. Early retransplantation, usually due to vascular or graft failure, has significantly reduced survival rates: 42.4% at 1 year and 33.9% at 5 years [35].
Infectious complications are common, with 21% of patients presenting with severe sepsis and requiring intensive care, mainly due to bloodstream bacterial infections. Acute kidney injury (AKI) is also common, with an estimated incidence of up to 40.8% in adults and 7% requiring replacement therapy; in children, it is associated with multiple factors such as prolonged surgery, bleeding, use of nephrotoxic drugs, and hemodynamic instability. These complications usually occur three months after transplantation and are classified as biliary stricture, bile leakage, obstruction, stones, and Oddi sphincter dysfunction.They were more prevalent in LT with small grafts, with risk factors including ischemia time, hepatic artery thrombosis or stenosis, cytomegalovirus (CMV) infection, and chronic rejection. Knowledge of this complication is necessary to differentiate it from rejection, infection, or recurrence of the primary disease, since the initial phases of these conditions can be confusing [26, 36].
Massive postoperative bleeding occurred in 43% of 68 children in a United States hospital, defined by hemoglobin < 8.5 g/dL, INR > 1.5, thrombocytopenia, and surgical duration > 10 h. Most bleeding episodes require reoperation or transfusions, especially in patients with an arterial duct or previous abdominal surgery [35].
Malnutrition in pre-transplant patients also influences the success of the procedure. Several studies also report functional consequences of liver transplantation, such as patients’ intolerance to physical exercise. It has been observed that the recipient’s exercise capacity is significantly impaired in the postoperative period, regardless of the type of organ transplant. Another decisive factor in the prognosis of transplant patients is the timing of referral to transplant centers. The LDLT technique has enabled transplant teams to recommend liver transplantation as a therapeutic option early in the disease, but late referral is still a problem and can compromise the success of the procedure [27]. In a study conducted at a transplant center in Brazil, vascular complications were more frequent in patients who underwent LDLT than in deceased donor transplants. However, one of the advantages of the LDLT technique is that it has a markedly reduced ischemia time [36–38].
Post-transplant mortality is mainly linked to sepsis, multiple organ failure, recurrent neoplasms, respiratory failure, and arterial thrombosis, highlighting management complexity and the importance of intensive monitoring in the first weeks after transplantation. Pediatric liver transplantation is challenging due to the small size of the vasculature and biliary tree, and the incidence of vascular complications reported in the literature varies widely, but is always higher than in samples analyzing LT in adults [35, 36].
Since the first pediatric LT was performed in 1963, survival rates have improved due to advances in surgical techniques, organ preservation, pediatric intensive care, and immunosuppressive therapy. Currently, liver transplantation is considered a long-lasting procedure that gives patients a good long-term survival rate [26, 29].
In a 2013 study, the survival rates at 1, 5, and 10 years in patients who received a cadaveric donor transplant were 89.3%, 78.1%, and 68.5%. In the case of living donors, it was 93.1%, 85.7%, and 67.5% [26]. It is known that multiple factors are associated with the survival rates of pediatric patients after transplantation. In the 2012 cohort study, which analyzed 430 pediatric transplant procedures with living donors, it was concluded that factors such as low body weight (≤ 10 kg) and hepatic artery thrombosis were determinants of worse patient and graft survival [27].
In a 2018 study, in which 84 children and adolescents were evaluated, 40 of whom were transplanted, the probability of survival at 180 days was 70%, and 67.2% at the end of 5 years after transplantation. In addition, in the evaluation of the group transplanted for chronic liver diseases, survival after 5 years was within the results obtained by the main transplant centers, with a rate that can reach 90% in the first year after transplantation and 64.3% to 83.3% in 5 years [24].
The variables related to the worst outcome were: late referral for treatment, decreased albumin and sodium, increased serum bilirubin, increased INR (higher PELD score), extent of liver fibrosis, degree of destruction of intrahepatic bile ducts, previous episodes of cholangitis, site of bile duct obliteration (distal obliteration, type I, has a better prognosis), the form of atresia (perinatal form has a better prognosis compared to embryonic form) and presence of cirrhosis [21].
The presence or absence of ascites, according to a study conducted in 2020, plays an important role in the prognosis of pediatric patients with indications for liver transplantation. According to the study, which assessed 106 children with cirrhosis secondary to biliary atresia, the presence of ascites was associated with a one-year decrease in the survival rate of these patients. Furthermore, the one-year survival rate for patients without ascites was 97.1%, compared to only 56.9% in the presence of ascites. This is attributable to the fact that pediatric patients who develop ascites have a more exacerbated state of systemic inflammation and, consequently, are more likely to develop organ dysfunction [34].
Improvements in surgical techniques have resulted in a significant improvement in the survival rate of patients with biliary atresia, corresponding to values between 80% and 90% [21]. Despite data showing improvements in the survival of pediatric patients, liver transplantation remains a complex surgery with considerable morbidity and mortality rates. Approximately 40% of pediatric patients can present post-surgical complications, and early diagnosis of these complications is a crucial factor for survival rates, considering that the 5-year survival rate for patients who underwent their first transplant is 76.4% compared to a rate of 64.5% for patients who required re-transplantation after complications [26].
The COVID-19 pandemic is one of the main challenges currently faced by the LT program in Brazil and around the world. Meanwhile, indications for liver transplantation in children have been restricted, due to the risk of contamination of these patients and donors by the SARS-COV-2 virus. In addition, other limitations, such as the significant increase in intensive care unit (ICU) bed occupancy and the reorganization of health services to meet the demand from COVID-positive patients, have also affected the organ transplant service. Furthermore, there was a significant reduction in the number of allograft donors in Brazil during this period [39, 40].
According to data provided by a retrospective cohort study carried out at a pediatric LT center in Brazil, 97 LT were carried out in 2019 before the arrival of the SARS-COV-2 virus in Brazil, compared to 77 in the same time interval after the start of the pandemic, which represented a 20.6% reduction in the activity of this service [40]. This scenario had a negative impact on the mortality rate on the waiting list who were candidates for transplant therapy, which went from 8.4% to 11.9% before and after the pandemic, respectively. In addition to changes in the number of pediatric LT performed during the pandemic, there were also changes in the epidemiological profile of the children transplanted. A study conducted in 2022 showed that patients referred for transplantation during the pandemic period had higher PELD/MELD scores, a higher prevalence of complications such as ascites, and a higher frequency of pre-transplant hospitalizations, all of which combined with a younger age when compared to the same profile of pre-pandemic patients [41, 42].
Also, in the study in question, 66.2% of transplanted children who acquired the SARS-COV-2 virus were symptomatic, with respiratory symptoms (runny nose and cough) being the most prevalent. With regard to the management of these patients, 87.8% were treated on an outpatient basis, 8.4% needed to be hospitalized, and only 5.4% of the total number of infected patients developed a serious condition and required monitoring in the ICU. Among the children who required hospitalization, 33% also needed to have their immunosuppressive therapy adjusted due to COVID-19 infection. Thus, based on an analysis of the epidemiological profile of these patients with major complications, it was concluded that an interval of less than six months between the transplant and COVID-19 infection is a predictor of severity in pediatric patients [41, 42].
Therefore, various preventive measures have been employed to reduce the possibility of infection in pediatric patients before and after transplantation. These include the implementation of epidemiological and clinical screening, RT-PCR testing, and intensified quarantine measures for donors and recipients. In addition, some transplant programs have restricted pre-, intra-, and post-operative care to the same medical team [40].
In Latin America, liver transplantation has experienced slow growth due to a lack of financial and educational support, limiting its reach and the availability of donors [4]. However, recent advances in the field of pediatric liver transplantation have improved outcomes. A 2022 study in Brazil developed a new prognostic model for children on the transplant waiting list, based on INR and ALT values, aimed at supporting clinical decision-making for children with pediatric acute liver failure (PALF). This new prognostic model is considered to be the first formulated in Latin America [25].
Although Brazil is the second country in absolute number of LT worldwide and the largest pediatric transplant center in Latin America, the outcomes of pediatric liver transplantation in the country still reveal significant challenges when compared with other middle-income countries. In terms of transplants per million population, Brazil still lags behind many developed countries and even some upper-middle-income nations. While Brazil doubled its donation rate from 6.5 pmp in 2006 to 13.1 pmp in 2014, this figure remains lower than that seen in European countries (15 pmp) and far below the United States (26 pmp) [19].
Moreover, there is a marked geographic concentration: 66% of pediatric LT are performed solely in the city of São Paulo. This indicates unequal access, with less developed regions facing logistical barriers, a shortage of trained teams, and lower notification rates of potential donors. Compared with other middle-income countries (such as Argentina or Mexico), Brazil has a comparable or even greater transplant volume, but important gaps remain in the systematic national registry and outcome evaluation. In other countries, more robust national databases allow real-time analyses of survival, complications, and regional flows, supporting fairer and more effective policies [4, 19].
Technically, Brazil has made significant advances with the use of LDLT, accounting for 53.4% of pediatric transplants [19]. This strategy partially offsets the shortage of deceased pediatric donors but also brings specific challenges, such as a higher incidence of vascular complications in LDLT compared with deceased donor transplants [37].
Regarding survival, Brazilian data are comparable to international centers: 1-, 5-, and 10-year survival of 89.3%, 78.1%, and 68.5% for deceased donors, and 93.1%, 85.7%, and 67.5% for living donors [26]. However, there is a strong influence from factors such as delayed diagnosis, poor nutritional status, and the complexity of postoperative care, all of which directly impact outcomes, especially among more vulnerable populations [21, 24, 27, 32].
Brazil is currently the largest pediatric transplant center in Latin America and has a significant absolute number of transplants performed worldwide. The main pediatric indication for liver transplantation in the country is biliary atresia, and over the years, many factors have been improved to achieve more satisfactory results in this therapy. These include the development of new surgical techniques, the establishment of the PELD score for prioritizing patients on the waiting list, and the increased use of living donors for pediatric liver transplantation.
However, this scoping review shows that there are still important points to be addressed to optimize this practice nationwide. Delays in diagnosis, long waiting times, and a reduced number of allograft donors directly influence the prognosis of pediatric patients. Additional key factors include the child’s nutritional status and other laboratory parameters before transplantation, the experience of the transplant team with the chosen technique, and rigorous control of post-transplant complications and infections. The COVID-19 pandemic has further aggravated many of these problems, increasing waiting list mortality and complicating service provision.
Moreover, there remains a marked geographic concentration of transplant activity in a few high-volume centers, creating inequalities in access for children in less developed regions. This highlights the need for broader decentralization, improved logistics, and targeted investment in training for multidisciplinary teams across the country.
Given these challenges, concrete recommendations to strengthen pediatric liver transplantation in Brazil include:
Improvement of the SNT: Invest in a more detailed and updated national registry with stratified pediatric data on complications, survival, and waiting times. This would help identify regional bottlenecks and improve allocation.
Strengthening of donation campaigns: Promote targeted educational efforts to increase notification of potential donors and reduce family refusals, especially in less developed regions.
Multiprofessional training: Invest in training for teams in lower-volume centers to decentralize care, with standardized protocols for pre- and post-operative management to reduce inequalities in access and outcomes.
Research on local prognostic models: Support multicenter studies to validate and improve tools like the modified PELD score, tailored to the Brazilian context and local epidemiology.
Adoption of alternative techniques: Promote wider use of SLT through better integration between pediatric and adult teams, ensuring priority allocation to children with high PELD scores.
Integrated nutritional care: Embed nutrition teams into pre- and post-transplant management, recognizing that nutritional status is critical for survival and recovery.
Finally, while improvements such as new prognostic models are being developed in Brazilian reference centers, there is a clear need for greater systematization of records in official agencies. This would enable continuous evaluation of services and help identify gaps to be addressed, ultimately optimizing outcomes and expanding equitable access to life-saving liver transplantation for children across Brazil.
EHBA: extrahepatic biliary atresia
ICU: intensive care unit
LDLT: living donor liver transplantation
LT: liver transplants
PELD: pediatric end-stage liver disease
pmp: per million people
PRISMA-ScR: Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews Checklist
SLT: split liver transplantation
SNT: National Transplant System
VHL: Virtual Health Library
JRK, KR, and MMA: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. APdSL: Writing—original draft, Writing—review & editing. JSN: Conceptualization, Investigation. ICMMC: Conceptualization, Investigation. CAMM: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Supervisor. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This study did not receive any financial support, either for its development or in the form of scholarships granted to the researchers.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2571
Download: 28
Times Cited: 0
