










DOI: https://doi.org/10.37349/en.2025.1006125

Aim:
Tissue transglutaminase [transglutaminase 2 (TG2)] is implicated in central neuronal apoptosis and is expressed in the peripheral nervous system; however, its role in sensory neuron survival and neuropathic pain after nerve injury remains poorly defined. This study examined whether TG2 knockout (KO) affects dorsal root ganglion (DRG) neuron survival and pain-related behaviors following sciatic nerve injury.
Methods:
TG2 KO mice and wild-type (WT) controls underwent complete sciatic nerve transection (axotomy). Pain-related behavior was evaluated using detailed autotomy scoring over 14 days. DRG neuron survival was assessed using unbiased stereological counts.
Results:
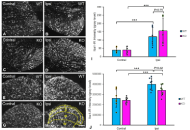
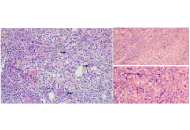
TG2 KO resulted in a distinct, previously unreported “atypical autotomy” pattern, with lesions localized mainly to the midplantar paw region. In contrast, WT mice exhibited typical autotomy directed primarily at the toes. Despite this clear difference in pain phenotype, stereological analysis revealed that TG2 KO did not alter neuronal counts in intact or axotomized DRGs, with both groups showing comparable, significant neuronal loss after injury.
Conclusions:
These findings indicate that TG2 functions as an important modulator of neuropathic pain but is not required for neuronal survival in the adult DRG following nerve injury.
Aim:
Tissue transglutaminase [transglutaminase 2 (TG2)] is implicated in central neuronal apoptosis and is expressed in the peripheral nervous system; however, its role in sensory neuron survival and neuropathic pain after nerve injury remains poorly defined. This study examined whether TG2 knockout (KO) affects dorsal root ganglion (DRG) neuron survival and pain-related behaviors following sciatic nerve injury.
Methods:
TG2 KO mice and wild-type (WT) controls underwent complete sciatic nerve transection (axotomy). Pain-related behavior was evaluated using detailed autotomy scoring over 14 days. DRG neuron survival was assessed using unbiased stereological counts.
Results:
TG2 KO resulted in a distinct, previously unreported “atypical autotomy” pattern, with lesions localized mainly to the midplantar paw region. In contrast, WT mice exhibited typical autotomy directed primarily at the toes. Despite this clear difference in pain phenotype, stereological analysis revealed that TG2 KO did not alter neuronal counts in intact or axotomized DRGs, with both groups showing comparable, significant neuronal loss after injury.
Conclusions:
These findings indicate that TG2 functions as an important modulator of neuropathic pain but is not required for neuronal survival in the adult DRG following nerve injury.
DOI: https://doi.org/10.37349/en.2026.1006124

Aim:
Male infertility resulting from neurological disorders, oxidative stress, and hormonal imbalance is a growing health concern. This study, therefore, investigated the effects of Aframomum melegueta and Aframomum danielli-supplemented diets on sperm quality and testicular oxidative damage in a scopolamine-induced rat model.
Methods:
Adult male rats were randomly allocated into seven groups: normal group; scopolamine-induced group; donepezil-treated scopolamine group and four treatment groups receiving 4% or 8% dietary supplementation of Aframomum melegueta or Aframomum danielli, respectively. Sperm motility, count, and morphology were evaluated. In addition, serum testosterone and follicle stimulating hormone levels, testicular oxidative stress markers, inflammatory cytokines, and antioxidant activities were assessed to determine reproductive and biochemical responses. High performance liquid chromatography (HPLC) profiling was also conducted to identify the major phenolic compounds in both seeds.
Results:
Scopolamine administration impaired sperm quality, decreased hormonal levels, promoted oxidative stress, and altered inflammatory responses. These alterations were, however, reversed by diets supplemented with Aframomum melegueta and Aframomum danielli in a dose-dependent manner. The 8% supplementation produced better outcomes than 4% supplementation and donepezil treatment in most parameters, indicating protective effects on sperm quality and other reproduction-related indices. HPLC profiling revealed bioactive compounds that may collectively account for the observed restorative effects of the seeds.
Conclusions:
These findings demonstrate that Aframomum melegueta and Aframomum danielli seeds effectively reversed the adverse reproductive alterations caused by scopolamine-induced neurotoxicity. Both species significantly improved sperm quality and testicular function, which may suggest their possible development as plant-based nutraceuticals for protecting male reproductive health in future studies. Their phytochemical abundance further supports their potential as plant-based nutraceuticals.
Aim:
Male infertility resulting from neurological disorders, oxidative stress, and hormonal imbalance is a growing health concern. This study, therefore, investigated the effects of Aframomum melegueta and Aframomum danielli-supplemented diets on sperm quality and testicular oxidative damage in a scopolamine-induced rat model.
Methods:
Adult male rats were randomly allocated into seven groups: normal group; scopolamine-induced group; donepezil-treated scopolamine group and four treatment groups receiving 4% or 8% dietary supplementation of Aframomum melegueta or Aframomum danielli, respectively. Sperm motility, count, and morphology were evaluated. In addition, serum testosterone and follicle stimulating hormone levels, testicular oxidative stress markers, inflammatory cytokines, and antioxidant activities were assessed to determine reproductive and biochemical responses. High performance liquid chromatography (HPLC) profiling was also conducted to identify the major phenolic compounds in both seeds.
Results:
Scopolamine administration impaired sperm quality, decreased hormonal levels, promoted oxidative stress, and altered inflammatory responses. These alterations were, however, reversed by diets supplemented with Aframomum melegueta and Aframomum danielli in a dose-dependent manner. The 8% supplementation produced better outcomes than 4% supplementation and donepezil treatment in most parameters, indicating protective effects on sperm quality and other reproduction-related indices. HPLC profiling revealed bioactive compounds that may collectively account for the observed restorative effects of the seeds.
Conclusions:
These findings demonstrate that Aframomum melegueta and Aframomum danielli seeds effectively reversed the adverse reproductive alterations caused by scopolamine-induced neurotoxicity. Both species significantly improved sperm quality and testicular function, which may suggest their possible development as plant-based nutraceuticals for protecting male reproductive health in future studies. Their phytochemical abundance further supports their potential as plant-based nutraceuticals.
DOI: https://doi.org/10.37349/en.2026.1006123
This article belongs to the special issue Medicinal Plants and Bioactive Phytochemicals in Neuroprotection (Vol II)

Flavonoids are a large class of natural polyphenolic substances ubiquitously synthesized in the plant kingdom. When entering the human body, these compounds can exert a wide range of biological activities, including immunomodulatory, antiinflammatory, and anticancer effects. Over the recent years, the mechanisms underlying these actions have become increasingly clear, also indicating the important involvement of G protein-coupled receptors (GPCRs), such as adenosine receptors, in signal transduction networks. In this perspective article, the potential role of flavonoids as adenosine receptor antagonists on the development, progression, and spread of glioblastoma is discussed, blocking the tumor-promoting and immunosuppressive actions of elevated levels of endogenous adenosine. Therefore, flavonoids can be considered as structural leads for developing novel antiglioblastoma agents, applied either alone or as boosters of chemo- or immunotherapy to improve the quality of life and outcome of patients. The importance of these studies is, in turn, emphasized by the current lack of effective treatment strategies for this highly aggressive and fast-growing brain tumor, associated with poor prognosis.
Flavonoids are a large class of natural polyphenolic substances ubiquitously synthesized in the plant kingdom. When entering the human body, these compounds can exert a wide range of biological activities, including immunomodulatory, antiinflammatory, and anticancer effects. Over the recent years, the mechanisms underlying these actions have become increasingly clear, also indicating the important involvement of G protein-coupled receptors (GPCRs), such as adenosine receptors, in signal transduction networks. In this perspective article, the potential role of flavonoids as adenosine receptor antagonists on the development, progression, and spread of glioblastoma is discussed, blocking the tumor-promoting and immunosuppressive actions of elevated levels of endogenous adenosine. Therefore, flavonoids can be considered as structural leads for developing novel antiglioblastoma agents, applied either alone or as boosters of chemo- or immunotherapy to improve the quality of life and outcome of patients. The importance of these studies is, in turn, emphasized by the current lack of effective treatment strategies for this highly aggressive and fast-growing brain tumor, associated with poor prognosis.
DOI: https://doi.org/10.37349/en.2026.1006122
This article belongs to the special issue Current Approaches to Malignant Tumors of the Nervous System

Cerebral amyloid angiopathy (CAA), characterized by amyloid β deposition in cerebral vasculature, is increasingly recognized as a major contributor to both cognitive decline and lobar intracerebral hemorrhage (ICH) in older adults and often coexists with Alzheimer’s disease (AD). Understanding CAA is a crucial step for improving health outcomes and the development of effective therapies. However, significant gaps remain in our understanding of CAA’s pathophysiology, diagnostic approaches, biomarker development, and clinical management. A comprehensive review is therefore essential to synthesize existing knowledge and highlight key directions for future research. This review goes beyond prior summaries by critically synthesizing recent evidence on diagnostic innovations—including the Boston criteria v2.0 and emerging plasma biomarkers—and addressing pressing clinical dilemmas such as anticoagulation management in patients with coexisting atrial fibrillation and CAA. It also highlights ongoing research into multimodal diagnostic frameworks and precision treatment strategies aimed at bridging current diagnostic and therapeutic gaps. Together, these updates underscore how advancing biomarker validation, individualized risk stratification, and amyloid-targeted approaches may shape future CAA management and prevention.
Cerebral amyloid angiopathy (CAA), characterized by amyloid β deposition in cerebral vasculature, is increasingly recognized as a major contributor to both cognitive decline and lobar intracerebral hemorrhage (ICH) in older adults and often coexists with Alzheimer’s disease (AD). Understanding CAA is a crucial step for improving health outcomes and the development of effective therapies. However, significant gaps remain in our understanding of CAA’s pathophysiology, diagnostic approaches, biomarker development, and clinical management. A comprehensive review is therefore essential to synthesize existing knowledge and highlight key directions for future research. This review goes beyond prior summaries by critically synthesizing recent evidence on diagnostic innovations—including the Boston criteria v2.0 and emerging plasma biomarkers—and addressing pressing clinical dilemmas such as anticoagulation management in patients with coexisting atrial fibrillation and CAA. It also highlights ongoing research into multimodal diagnostic frameworks and precision treatment strategies aimed at bridging current diagnostic and therapeutic gaps. Together, these updates underscore how advancing biomarker validation, individualized risk stratification, and amyloid-targeted approaches may shape future CAA management and prevention.
DOI: https://doi.org/10.37349/en.2026.1006121

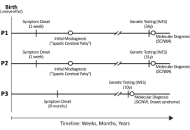
Neurogenetic disorders remain genetically uncharacterized in many populations, including Libya. We report three Libyan patients from two consanguineous families with pathogenic variants in sodium channel genes. Two adult sisters (Patients 1 & 2) presented with global developmental delay and progressive spastic paraparesis without epilepsy. Whole exome sequencing identified the same heterozygous SCN8A variant (c.142G>A; p.Asp48Asn) in both sisters, classified as a variant of uncertain significance (VUS). Its occurrence in two affected siblings with a consistent phenotype and the absence of other explanatory variants provide supporting evidence for its potential pathogenicity. These cases represent the first documented instances of a suspected SCN8A-related disorder in Libya. A third, unrelated 10-year-old boy (Patient 3) with a phenotype consistent with Dravet syndrome, including refractory seizures and neurodevelopmental regression, was found to harbor a likely pathogenic heterozygous SCN1A variant (c.2113del; p.Glu705Lysfs*10). This report expands the genetic and phenotypic spectrum of neurological disorders in Libya and underscores the critical role of genetic testing, while also highlighting the need for segregation studies to achieve a definitive molecular diagnosis.
Neurogenetic disorders remain genetically uncharacterized in many populations, including Libya. We report three Libyan patients from two consanguineous families with pathogenic variants in sodium channel genes. Two adult sisters (Patients 1 & 2) presented with global developmental delay and progressive spastic paraparesis without epilepsy. Whole exome sequencing identified the same heterozygous SCN8A variant (c.142G>A; p.Asp48Asn) in both sisters, classified as a variant of uncertain significance (VUS). Its occurrence in two affected siblings with a consistent phenotype and the absence of other explanatory variants provide supporting evidence for its potential pathogenicity. These cases represent the first documented instances of a suspected SCN8A-related disorder in Libya. A third, unrelated 10-year-old boy (Patient 3) with a phenotype consistent with Dravet syndrome, including refractory seizures and neurodevelopmental regression, was found to harbor a likely pathogenic heterozygous SCN1A variant (c.2113del; p.Glu705Lysfs*10). This report expands the genetic and phenotypic spectrum of neurological disorders in Libya and underscores the critical role of genetic testing, while also highlighting the need for segregation studies to achieve a definitive molecular diagnosis.
DOI: https://doi.org/10.37349/en.2025.1006120
This article belongs to the special issue Advances in Epilepsy Research

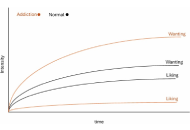
Although addiction is a complex and contextually embedded disorder that extends beyond individual pathology and neurobiological dysfunction, prevailing computational and clinical models often reduce addiction to a chronic brain disease. While such frameworks have shaped dominant approaches to treatment and theory, they remain poorly aligned with the lived experience and behavioral phenomena of addiction, ignoring its psychological, social, and systemic dimensions. This paper examines the limitations of various disease and compulsion models both critically and in-depth, highlighting their empirical and conceptual shortcomings. In doing so, it argues for the development of context-sensitive and psychologically grounded computational models, ones capable of capturing the nuanced realities of addiction and informing more effective, personalized interventions.
Although addiction is a complex and contextually embedded disorder that extends beyond individual pathology and neurobiological dysfunction, prevailing computational and clinical models often reduce addiction to a chronic brain disease. While such frameworks have shaped dominant approaches to treatment and theory, they remain poorly aligned with the lived experience and behavioral phenomena of addiction, ignoring its psychological, social, and systemic dimensions. This paper examines the limitations of various disease and compulsion models both critically and in-depth, highlighting their empirical and conceptual shortcomings. In doing so, it argues for the development of context-sensitive and psychologically grounded computational models, ones capable of capturing the nuanced realities of addiction and informing more effective, personalized interventions.
DOI: https://doi.org/10.37349/en.2025.1006119

Primary central nervous system lymphoma is a rare form of extranodal non-Hodgkin lymphoma that is confined to the brain, spinal cord, leptomeninges, or eyes, representing less than one percent of all non-Hodgkin lymphomas and approximately four percent of primary brain tumors. When the disease is truly isolated to the central nervous system, with no evidence of systemic spread, it poses unique diagnostic and therapeutic challenges, particularly in immunocompetent patients. We reviewed nine recently published cases from 2021 to 2024 that described isolated primary central nervous system lymphoma without extracranial involvement. Patients ranged in age from forty-four to eighty-five years, with both immunocompetent and immunosuppressed individuals represented. Presenting symptoms include focal neurological deficits, seizures, progressive confusion, cranial neuropathies, and neurolymphomatosis. Magnetic resonance imaging findings were diverse, including intra-axial masses, leptomeningeal and cranial nerve enhancement, and mass effect. Cerebrospinal fluid analysis was variably positive for lymphoma cells. Histopathological analysis confirmed diffuse large B-cell lymphoma in all cases, although initial biopsies were sometimes inconclusive, underscoring the importance of repeat tissue sampling and expert pathology review. Treatment strategies most often included high-dose methotrexate-based chemotherapy, monoclonal antibody therapy, and radiotherapy, with some patients undergoing surgical decompression or diagnostic craniotomy. Follow-up data revealed variable survival outcomes, with a subset of patients achieving disease-free survival beyond one year. These cases highlight the wide clinical spectrum and diagnostic complexity of isolated primary central nervous system lymphoma and reinforce the need for a high index of suspicion, timely advanced imaging, multidisciplinary discussion, and appropriate tissue diagnosis to guide individualized management.
Primary central nervous system lymphoma is a rare form of extranodal non-Hodgkin lymphoma that is confined to the brain, spinal cord, leptomeninges, or eyes, representing less than one percent of all non-Hodgkin lymphomas and approximately four percent of primary brain tumors. When the disease is truly isolated to the central nervous system, with no evidence of systemic spread, it poses unique diagnostic and therapeutic challenges, particularly in immunocompetent patients. We reviewed nine recently published cases from 2021 to 2024 that described isolated primary central nervous system lymphoma without extracranial involvement. Patients ranged in age from forty-four to eighty-five years, with both immunocompetent and immunosuppressed individuals represented. Presenting symptoms include focal neurological deficits, seizures, progressive confusion, cranial neuropathies, and neurolymphomatosis. Magnetic resonance imaging findings were diverse, including intra-axial masses, leptomeningeal and cranial nerve enhancement, and mass effect. Cerebrospinal fluid analysis was variably positive for lymphoma cells. Histopathological analysis confirmed diffuse large B-cell lymphoma in all cases, although initial biopsies were sometimes inconclusive, underscoring the importance of repeat tissue sampling and expert pathology review. Treatment strategies most often included high-dose methotrexate-based chemotherapy, monoclonal antibody therapy, and radiotherapy, with some patients undergoing surgical decompression or diagnostic craniotomy. Follow-up data revealed variable survival outcomes, with a subset of patients achieving disease-free survival beyond one year. These cases highlight the wide clinical spectrum and diagnostic complexity of isolated primary central nervous system lymphoma and reinforce the need for a high index of suspicion, timely advanced imaging, multidisciplinary discussion, and appropriate tissue diagnosis to guide individualized management.
DOI: https://doi.org/10.37349/en.2025.1006117

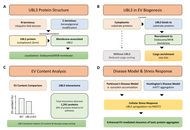
Neurological disorders constitute a major global health burden with limited effective treatments. Despite advances in molecular neuroscience, critical gaps persist in understanding intercellular communication systems underlying central nervous system homeostasis and neurodegeneration. Extracellular vesicles (EVs), nanoscale to microscale membrane-bound vesicles secreted by virtually all cell types, have emerged as pivotal mediators of intercellular communication in neurological pathologies. This review examines molecular mechanisms governing EV biogenesis, cargo selection, and pathological functions in neurological disorders, emphasizing the emerging role of ubiquitin-like protein 3 (UBL3) as a novel regulator of EV-mediated protein sorting. Neural cell populations produce specialized EV subtypes containing distinct molecular cargo reflecting their physiological states. UBL3, a membrane-anchored post-translational modifier, operates through geranylgeranylation-dependent mechanisms to promote selective protein incorporation into small EVs (sEVs), with knockout studies demonstrating approximately 60% reduction in EV protein content. Proteomic analyses reveal UBL3 interacts with over 1,200 proteins, with ~30% classified as EV cargo proteins. Critically, UBL3-mediated sorting influences disease-associated protein trafficking, including α-synuclein in Parkinson’s disease and mutant huntingtin in Huntington’s disease, suggesting involvement in prion-like spreading mechanisms. EVs’ dual nature as pathological mediators and therapeutic vehicles represents a paradigm shift in neurological medicine. EVs offer advantages as natural drug delivery systems capable of crossing the blood-brain barrier, accessible biomarkers for noninvasive disease monitoring via liquid biopsies (achieving diagnostic accuracies exceeding 0.88 ROC-AUC), and engineered therapeutic platforms for delivering CRISPR-Cas9 systems and neuroprotective factors. However, clinical translation requires addressing challenges, including standardizing isolation protocols, elucidating cell-type-specific cargo sorting mechanisms, and defining optimal administration routes. Understanding UBL3-mediated cargo sorting mechanisms presents promising therapeutic opportunities by selectively modulating pathogenic protein trafficking. EVs, positioned at the intersection of pathogenesis and therapy, represent attractive targets for precision medicine approaches in neurological conditions, with UBL3 emerging as a novel molecular handle for manipulating EV composition and function.
Neurological disorders constitute a major global health burden with limited effective treatments. Despite advances in molecular neuroscience, critical gaps persist in understanding intercellular communication systems underlying central nervous system homeostasis and neurodegeneration. Extracellular vesicles (EVs), nanoscale to microscale membrane-bound vesicles secreted by virtually all cell types, have emerged as pivotal mediators of intercellular communication in neurological pathologies. This review examines molecular mechanisms governing EV biogenesis, cargo selection, and pathological functions in neurological disorders, emphasizing the emerging role of ubiquitin-like protein 3 (UBL3) as a novel regulator of EV-mediated protein sorting. Neural cell populations produce specialized EV subtypes containing distinct molecular cargo reflecting their physiological states. UBL3, a membrane-anchored post-translational modifier, operates through geranylgeranylation-dependent mechanisms to promote selective protein incorporation into small EVs (sEVs), with knockout studies demonstrating approximately 60% reduction in EV protein content. Proteomic analyses reveal UBL3 interacts with over 1,200 proteins, with ~30% classified as EV cargo proteins. Critically, UBL3-mediated sorting influences disease-associated protein trafficking, including α-synuclein in Parkinson’s disease and mutant huntingtin in Huntington’s disease, suggesting involvement in prion-like spreading mechanisms. EVs’ dual nature as pathological mediators and therapeutic vehicles represents a paradigm shift in neurological medicine. EVs offer advantages as natural drug delivery systems capable of crossing the blood-brain barrier, accessible biomarkers for noninvasive disease monitoring via liquid biopsies (achieving diagnostic accuracies exceeding 0.88 ROC-AUC), and engineered therapeutic platforms for delivering CRISPR-Cas9 systems and neuroprotective factors. However, clinical translation requires addressing challenges, including standardizing isolation protocols, elucidating cell-type-specific cargo sorting mechanisms, and defining optimal administration routes. Understanding UBL3-mediated cargo sorting mechanisms presents promising therapeutic opportunities by selectively modulating pathogenic protein trafficking. EVs, positioned at the intersection of pathogenesis and therapy, represent attractive targets for precision medicine approaches in neurological conditions, with UBL3 emerging as a novel molecular handle for manipulating EV composition and function.
DOI: https://doi.org/10.37349/en.2025.1006118

Background:
Glioblastoma multiforme (GBM) is the most common primary malignant brain tumor in adults, with a poor prognosis despite advances in treatment options. T-cell-engager therapies, which have an antibody-based structure connecting immune cells to target cancer cells with high affinity, offer a promising strategy but face four key barriers: antigen heterogeneity, immune escape, the blood-brain barrier (BBB), and the immunosuppressive tumor microenvironment (TME). This systematic review synthesizes preclinical developments in bispecific T-cell engager (BiTE), tri-specific T-cell engager (TriTE), and multi-specific T-cell engagers for GBM over the last 10 years, evaluating their capacity to overcome these barriers.
Methods:
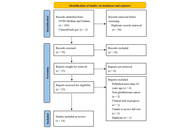
A systematic search was conducted in OVID Medline, Embase, and ClinicalTrials.gov for pre-clinical and clinical studies. A descriptive analysis without meta-analysis was formulated in which data were grouped thematically by the ability of treatments to overcome GBM-specific barriers.
Results:
Among the 14 studies meeting inclusion criteria, all studies were preclinical, with 12/14 (85.7%) utilizing an in vivo mouse model. BiTEs were used in 12/14 (85.7%) studies, while 4/14 (28.6%) studies targeted multiple antigens through either TriTEs or multivalent BiTEs. There was a range of antigen targets with the most common being interleukin 13 receptor alpha 2 (IL13Rα2) as well as epidermal growth factor receptor (EGFR) or EGFR variant III (EGFRvIII) in 7/14 (50.0%) studies. Most studies (85.7%) addressed two or more barriers, with 13/14 (92.9%) showing evidence of affecting the TME.
Discussion:
In the last decade, T-cell engager therapies have evolved in both antigenic targets and delivery vehicles used to overcome the key barriers. An emerging area within T-cell engager therapies is targeting multiple antigens through multi-specific T-cell engager therapies, such as the TriTEs. Studies have explored chimeric antigen receptor T-cells (CAR-Ts) as a potential delivery vehicle for BiTEs. A future clinical trial using multi-specific T-cell engager therapies or a CAR-T-secreting BiTE in adult patients is required to determine the potential clinical utility of T-cell engagers.
Background:
Glioblastoma multiforme (GBM) is the most common primary malignant brain tumor in adults, with a poor prognosis despite advances in treatment options. T-cell-engager therapies, which have an antibody-based structure connecting immune cells to target cancer cells with high affinity, offer a promising strategy but face four key barriers: antigen heterogeneity, immune escape, the blood-brain barrier (BBB), and the immunosuppressive tumor microenvironment (TME). This systematic review synthesizes preclinical developments in bispecific T-cell engager (BiTE), tri-specific T-cell engager (TriTE), and multi-specific T-cell engagers for GBM over the last 10 years, evaluating their capacity to overcome these barriers.
Methods:
A systematic search was conducted in OVID Medline, Embase, and ClinicalTrials.gov for pre-clinical and clinical studies. A descriptive analysis without meta-analysis was formulated in which data were grouped thematically by the ability of treatments to overcome GBM-specific barriers.
Results:
Among the 14 studies meeting inclusion criteria, all studies were preclinical, with 12/14 (85.7%) utilizing an in vivo mouse model. BiTEs were used in 12/14 (85.7%) studies, while 4/14 (28.6%) studies targeted multiple antigens through either TriTEs or multivalent BiTEs. There was a range of antigen targets with the most common being interleukin 13 receptor alpha 2 (IL13Rα2) as well as epidermal growth factor receptor (EGFR) or EGFR variant III (EGFRvIII) in 7/14 (50.0%) studies. Most studies (85.7%) addressed two or more barriers, with 13/14 (92.9%) showing evidence of affecting the TME.
Discussion:
In the last decade, T-cell engager therapies have evolved in both antigenic targets and delivery vehicles used to overcome the key barriers. An emerging area within T-cell engager therapies is targeting multiple antigens through multi-specific T-cell engager therapies, such as the TriTEs. Studies have explored chimeric antigen receptor T-cells (CAR-Ts) as a potential delivery vehicle for BiTEs. A future clinical trial using multi-specific T-cell engager therapies or a CAR-T-secreting BiTE in adult patients is required to determine the potential clinical utility of T-cell engagers.
DOI: https://doi.org/10.37349/en.2025.1006116
This article belongs to the special issue Current Approaches to Malignant Tumors of the Nervous System

Aim:
This study investigated the effect of brain-derived neurotrophic factor (BDNF) Val66Met polymorphism on post-stroke outcomes, including quality of life, physical fitness, cognitive function, depression, and overall disability.
Methods:
The difference between Met carriers and non-Met carriers was analyzed for the entire sample and in pair-matched analysis, using age, sex, time since stroke, and race.
Results:
We evaluated 89 stroke participants (mean age, 57 ± 10 years; 58% male; 54% White, and 49% Hispanic). Twelve participants (13%) had one copy of the BDNF Val66Met (Val/Met heterozygotes) and none had two copies (Met/Met homozygotes). Comparing Met (n = 12) and non-Met carriers (n = 77), no significant differences were observed in demographics or clinical characteristics, including motor or cognitive outcomes. In pair-matched analysis, a significant difference was observed for the Center for Epidemiological Studies Depression (CES-D) scale, where Met carriers had significantly greater CES-D scores than non-Met carriers (24 ± 16 vs. 9 ± 9, p = 0.011). Regardless of the chosen CES-D cut-off scores (≥ 16 vs. ≥ 20), more cases of depressive symptomatology were observed among those with the BDNF Val66Met polymorphism than those without it (p values < 0.05).
Conclusions:
The BDNF Val66Met polymorphism may be associated with post-stroke depression but not motor or cognitive recovery.
Aim:
This study investigated the effect of brain-derived neurotrophic factor (BDNF) Val66Met polymorphism on post-stroke outcomes, including quality of life, physical fitness, cognitive function, depression, and overall disability.
Methods:
The difference between Met carriers and non-Met carriers was analyzed for the entire sample and in pair-matched analysis, using age, sex, time since stroke, and race.
Results:
We evaluated 89 stroke participants (mean age, 57 ± 10 years; 58% male; 54% White, and 49% Hispanic). Twelve participants (13%) had one copy of the BDNF Val66Met (Val/Met heterozygotes) and none had two copies (Met/Met homozygotes). Comparing Met (n = 12) and non-Met carriers (n = 77), no significant differences were observed in demographics or clinical characteristics, including motor or cognitive outcomes. In pair-matched analysis, a significant difference was observed for the Center for Epidemiological Studies Depression (CES-D) scale, where Met carriers had significantly greater CES-D scores than non-Met carriers (24 ± 16 vs. 9 ± 9, p = 0.011). Regardless of the chosen CES-D cut-off scores (≥ 16 vs. ≥ 20), more cases of depressive symptomatology were observed among those with the BDNF Val66Met polymorphism than those without it (p values < 0.05).
Conclusions:
The BDNF Val66Met polymorphism may be associated with post-stroke depression but not motor or cognitive recovery.
DOI: https://doi.org/10.37349/en.2025.1006115

Guillain-Barré Syndrome (GBS) is a rare cause of acute, flaccid paralysis and affects populations around the world, usually in the setting of recent gastrointestinal infection. The myelin sheaths of affected patients are destroyed, and consequently, the disease can manifest variably with the most common complaints including weakness, disturbances in sensation, and pain. Multiple available pharmacotherapies are employed to address disease progression and promote the reversal of symptoms. However, there is no widely accepted guideline detailing tiers of pain management options, despite pain being a significant primary complaint during the acute phase of the disease. To address this, we searched the GBS literature for publications that specifically discussed patient pain, how the pain was managed by the clinician, and how patients responded to various modalities. We discuss the findings of the literature review we conducted, evaluate the expansive list of existing options for treating pain and how they fared in symptom resolution, and draw conclusions based on our observations of which interventions addressed patient pain effectively and which were less successful. While general management of GBS, including treatment and efforts towards symptom reversal, has been robustly discussed in the literature, our work stresses the lack of research towards pain management in GBS and emphasizes the need to fill the gap in patient care for patients with this disease.
Guillain-Barré Syndrome (GBS) is a rare cause of acute, flaccid paralysis and affects populations around the world, usually in the setting of recent gastrointestinal infection. The myelin sheaths of affected patients are destroyed, and consequently, the disease can manifest variably with the most common complaints including weakness, disturbances in sensation, and pain. Multiple available pharmacotherapies are employed to address disease progression and promote the reversal of symptoms. However, there is no widely accepted guideline detailing tiers of pain management options, despite pain being a significant primary complaint during the acute phase of the disease. To address this, we searched the GBS literature for publications that specifically discussed patient pain, how the pain was managed by the clinician, and how patients responded to various modalities. We discuss the findings of the literature review we conducted, evaluate the expansive list of existing options for treating pain and how they fared in symptom resolution, and draw conclusions based on our observations of which interventions addressed patient pain effectively and which were less successful. While general management of GBS, including treatment and efforts towards symptom reversal, has been robustly discussed in the literature, our work stresses the lack of research towards pain management in GBS and emphasizes the need to fill the gap in patient care for patients with this disease.
DOI: https://doi.org/10.37349/en.2025.1006114

Cyclic vomiting syndrome (CVS) is a rare disorder in which stereotypical periods of intermittent nausea and vomiting last between hours and over a week. The disorder overlaps with migraine, and the current treatment recommendations follow those of migraine management. The current patient had experienced vomiting periods lasting up to a week since the age of two. Prophylactic amitriptyline had led to probably slightly longer intervals between CVS periods, while several medications had proven ineffective. At the age of 17, there was an excellent response to peroral olanzapine, which eventually proved sufficient to abort the vomiting periods in a single dose when taken at the beginning of one. In light of these and previously reported cases, early administration of olanzapine is suggested to treat CVS periods.
Cyclic vomiting syndrome (CVS) is a rare disorder in which stereotypical periods of intermittent nausea and vomiting last between hours and over a week. The disorder overlaps with migraine, and the current treatment recommendations follow those of migraine management. The current patient had experienced vomiting periods lasting up to a week since the age of two. Prophylactic amitriptyline had led to probably slightly longer intervals between CVS periods, while several medications had proven ineffective. At the age of 17, there was an excellent response to peroral olanzapine, which eventually proved sufficient to abort the vomiting periods in a single dose when taken at the beginning of one. In light of these and previously reported cases, early administration of olanzapine is suggested to treat CVS periods.
DOI: https://doi.org/10.37349/en.2025.1006113

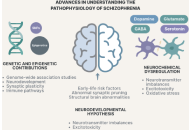
Schizophrenia (SZ) is a complex psychiatric disorder characterized by disruptions in cognition, perception, and behavior, contributing significantly to the global burden of psychiatric disorders and necessitating ongoing research into its pathophysiology, diagnosis, and treatment. This narrative review explores recent insights into SZ research, highlighting the genetic, neurochemical, and neurodevelopmental factors that contribute to the disorder. Emerging evidence underscores the dynamic interplay between neurotransmitter imbalances, particularly involving dopamine, glutamate, and gamma-aminobutyric acid (GABA), and neuroinflammation, oxidative stress, and immune dysregulation in the pathophysiology of SZ. Neuroimaging, clinical staging models, and multi-omics technologies have deepened our understanding of structural and functional brain abnormalities, identifying potential biomarkers for early detection and subtyping. This has refined diagnostic frameworks and informed precision psychiatry approaches. Advances in pharmacological treatments, including trace amine-associated receptor 1 agonists, glutamatergic modulators, psychedelics, and anti-inflammatory agents, offer new therapeutic possibilities beyond conventional dopamine antagonists. Novel targets, such as N-methyl-D-aspartate (NMDA) receptor modulation and neuroprotective strategies, are also being explored to address negative and cognitive symptoms. Additionally, non-pharmacological interventions, such as neuromodulation techniques, digital therapeutics, and psychosocial interventions, are promising complementary strategies. Digital phenotyping, machine learning (ML), and artificial intelligence (AI)-driven tools enable real-time symptom tracking, early risk prediction, and personalized care delivery. Despite these advancements, challenges remain in early diagnosis, treatment adherence, and equitable access to mental health care, particularly in low-resource settings. Therefore, addressing these barriers requires interdisciplinary collaboration, public health education, and the integration of scalable, culturally sensitive, and AI-based mental health innovations. Future research should prioritize multi-omics integration, longitudinal and transdiagnostic studies, biomarker validation, and the real-world implementation of personalized interventions to improve outcomes and quality of life for individuals living with SZ.
Schizophrenia (SZ) is a complex psychiatric disorder characterized by disruptions in cognition, perception, and behavior, contributing significantly to the global burden of psychiatric disorders and necessitating ongoing research into its pathophysiology, diagnosis, and treatment. This narrative review explores recent insights into SZ research, highlighting the genetic, neurochemical, and neurodevelopmental factors that contribute to the disorder. Emerging evidence underscores the dynamic interplay between neurotransmitter imbalances, particularly involving dopamine, glutamate, and gamma-aminobutyric acid (GABA), and neuroinflammation, oxidative stress, and immune dysregulation in the pathophysiology of SZ. Neuroimaging, clinical staging models, and multi-omics technologies have deepened our understanding of structural and functional brain abnormalities, identifying potential biomarkers for early detection and subtyping. This has refined diagnostic frameworks and informed precision psychiatry approaches. Advances in pharmacological treatments, including trace amine-associated receptor 1 agonists, glutamatergic modulators, psychedelics, and anti-inflammatory agents, offer new therapeutic possibilities beyond conventional dopamine antagonists. Novel targets, such as N-methyl-D-aspartate (NMDA) receptor modulation and neuroprotective strategies, are also being explored to address negative and cognitive symptoms. Additionally, non-pharmacological interventions, such as neuromodulation techniques, digital therapeutics, and psychosocial interventions, are promising complementary strategies. Digital phenotyping, machine learning (ML), and artificial intelligence (AI)-driven tools enable real-time symptom tracking, early risk prediction, and personalized care delivery. Despite these advancements, challenges remain in early diagnosis, treatment adherence, and equitable access to mental health care, particularly in low-resource settings. Therefore, addressing these barriers requires interdisciplinary collaboration, public health education, and the integration of scalable, culturally sensitive, and AI-based mental health innovations. Future research should prioritize multi-omics integration, longitudinal and transdiagnostic studies, biomarker validation, and the real-world implementation of personalized interventions to improve outcomes and quality of life for individuals living with SZ.
DOI: https://doi.org/10.37349/en.2025.1006112

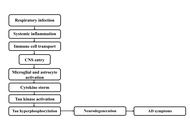
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder with declining memory and cognitive impairment, largely mediated by extracellular amyloid-beta (Aβ). Although the amyloid cascade and tau protein hypotheses have long served as established frameworks for AD pathology, recent evidence suggests that long-term infections, particularly with Chlamydia pneumoniae (C. pneumoniae), may contribute to disease progression. A systematic search strategy was used to identify relevant literature using PubMed, Scopus, Google Scholar, and Web of Science. Keywords and Boolean operators such as “Chlamydia pneumoniae and Alzheimer’s disease,” “neuroinflammation,” “amyloid-beta,” and “tau protein” were applied, with filters for peer-reviewed articles, human and experimental studies, and publications from the past 25 years. Epidemiological and background data were supplemented by official sources, including the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO). This review examines the potential relationship of C. pneumoniae infection with AD pathogenesis. Studies have identified DNA and antigens of C. pneumoniae in AD-infected brain regions, often co-localized within Aβ plaques and neurofibrillary tangles (NFTs). Proposed mechanisms of CNS invasion include olfactory, hematogenous, and immune cell-mediated routes, leading to persistent glial activation, neuroinflammation, altered amyloid precursor protein processing, and tau protein hyperphosphorylation. Experimental models support these associations, with infected animals developing AD-like pathology. Diagnostic challenges persist due to the limitations of PCR and immunohistochemistry, though advanced approaches such as next-generation sequencing and TSPO-PET imaging are emerging. Potential therapeutic approaches include antimicrobial and immunomodulatory strategies, although human trials have shown mixed results. While current evidence suggests a possible link, causality remains unproven. Future research must prioritize large-scale, longitudinal, and mechanistic studies to clarify these relationships. Establishing a definitive role for C. pneumoniae in AD pathogenesis could reshape current understanding of disease etiology and inform the development of novel preventive and therapeutic strategies.
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder with declining memory and cognitive impairment, largely mediated by extracellular amyloid-beta (Aβ). Although the amyloid cascade and tau protein hypotheses have long served as established frameworks for AD pathology, recent evidence suggests that long-term infections, particularly with Chlamydia pneumoniae (C. pneumoniae), may contribute to disease progression. A systematic search strategy was used to identify relevant literature using PubMed, Scopus, Google Scholar, and Web of Science. Keywords and Boolean operators such as “Chlamydia pneumoniae and Alzheimer’s disease,” “neuroinflammation,” “amyloid-beta,” and “tau protein” were applied, with filters for peer-reviewed articles, human and experimental studies, and publications from the past 25 years. Epidemiological and background data were supplemented by official sources, including the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO). This review examines the potential relationship of C. pneumoniae infection with AD pathogenesis. Studies have identified DNA and antigens of C. pneumoniae in AD-infected brain regions, often co-localized within Aβ plaques and neurofibrillary tangles (NFTs). Proposed mechanisms of CNS invasion include olfactory, hematogenous, and immune cell-mediated routes, leading to persistent glial activation, neuroinflammation, altered amyloid precursor protein processing, and tau protein hyperphosphorylation. Experimental models support these associations, with infected animals developing AD-like pathology. Diagnostic challenges persist due to the limitations of PCR and immunohistochemistry, though advanced approaches such as next-generation sequencing and TSPO-PET imaging are emerging. Potential therapeutic approaches include antimicrobial and immunomodulatory strategies, although human trials have shown mixed results. While current evidence suggests a possible link, causality remains unproven. Future research must prioritize large-scale, longitudinal, and mechanistic studies to clarify these relationships. Establishing a definitive role for C. pneumoniae in AD pathogenesis could reshape current understanding of disease etiology and inform the development of novel preventive and therapeutic strategies.
DOI: https://doi.org/10.37349/en.2025.1006111
This article belongs to the special issue Progress in Alzheimer's disease research: etiology, molecular mechanisms involved in disease progression, and advances in therapies aimed at slowing or reversing neurodegeneration

DOI: https://doi.org/10.37349/en.2025.1006110
This article belongs to the special issue Novel Therapeutic Approaches for the Treatment of Depression

Aim:
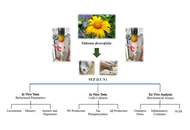
Alzheimer’s disease (AD) is a chronic neurodegenerative brain dysfunction and the most common form of dementia, especially in the elderly, and is considered a serious problem for health systems worldwide. It is a multifactorial and progressive condition, characterized by memory loss, personality changes and decline in cognitive function, in addition to neuropsychiatric complications such as depression, anxiety, sleep disorders, and others, further reducing the quality of life of patients with AD. Since the introduction of galantamine in AD therapy, medicinal plants and herbal remedies are gaining increasing interest as complementary and alternative interventions and are a valuable source for the development of drug candidates for AD. This work aims to explore Tithonia diversifolia ethanol extract (EETD), which showed an acetylcholinesterase (AChE) inhibitory activity like rivastigmine, as a new candidate for molecular targets of AD.
Methods:
Mice were submitted to intracerebroventricular (I.C.V.) streptozotocin (STZ)-induced AD (2.5 mg/mL) and separated into different groups: sham, vehicle, rivastigmine (0.6 mg/kg), and EETD (0.1, 1.0, and 3.0 mg/kg). After AD induction, the animals were treated for 24 days and submitted to behavioral tests of memory, anxiety and depression. After the tests, the animals were sacrificed and the hippocampus was removed for assays of oxidative stress, AChE activity and markers of neuroinflammation. In vitro studies evaluated the effect of the extract on tau hyperphosphorylation, beta-amyloid (Aβ), and nitric oxide (NO) production.
Results:
EETD promoted a reduction in STZ-induced behavioral parameters of depression and anxiety, as well as reversed memory deficits. Biochemical assays revealed that EETD increased antioxidant defenses, as well as decreased levels of neuroinflammation markers. In addition, EETD partially inhibited Aβ production.
Conclusions:
The results together suggest that the plant exhibits therapeutic relevance in AD. However, studies are needed to identify the phytoconstituents responsible for such effects.
Aim:
Alzheimer’s disease (AD) is a chronic neurodegenerative brain dysfunction and the most common form of dementia, especially in the elderly, and is considered a serious problem for health systems worldwide. It is a multifactorial and progressive condition, characterized by memory loss, personality changes and decline in cognitive function, in addition to neuropsychiatric complications such as depression, anxiety, sleep disorders, and others, further reducing the quality of life of patients with AD. Since the introduction of galantamine in AD therapy, medicinal plants and herbal remedies are gaining increasing interest as complementary and alternative interventions and are a valuable source for the development of drug candidates for AD. This work aims to explore Tithonia diversifolia ethanol extract (EETD), which showed an acetylcholinesterase (AChE) inhibitory activity like rivastigmine, as a new candidate for molecular targets of AD.
Methods:
Mice were submitted to intracerebroventricular (I.C.V.) streptozotocin (STZ)-induced AD (2.5 mg/mL) and separated into different groups: sham, vehicle, rivastigmine (0.6 mg/kg), and EETD (0.1, 1.0, and 3.0 mg/kg). After AD induction, the animals were treated for 24 days and submitted to behavioral tests of memory, anxiety and depression. After the tests, the animals were sacrificed and the hippocampus was removed for assays of oxidative stress, AChE activity and markers of neuroinflammation. In vitro studies evaluated the effect of the extract on tau hyperphosphorylation, beta-amyloid (Aβ), and nitric oxide (NO) production.
Results:
EETD promoted a reduction in STZ-induced behavioral parameters of depression and anxiety, as well as reversed memory deficits. Biochemical assays revealed that EETD increased antioxidant defenses, as well as decreased levels of neuroinflammation markers. In addition, EETD partially inhibited Aβ production.
Conclusions:
The results together suggest that the plant exhibits therapeutic relevance in AD. However, studies are needed to identify the phytoconstituents responsible for such effects.
DOI: https://doi.org/10.37349/en.2025.1006109
This article belongs to the special issue Progress in Alzheimer's disease research: etiology, molecular mechanisms involved in disease progression, and advances in therapies aimed at slowing or reversing neurodegeneration

Aim:
We previously observed oxidative stress and neuroinflammation caused behavioral and neurochemical changes in young Gabrb2 (gamma-aminobutyric acid type A receptor β2 subunit) knockout (KO) mice. Aging was moderated in a D-galactose-induced accelerated aging mouse model by an oral Chinese medicinal herbal formula BYPA consisting of Bupleurum chinense, Corydalis yanhusuo, Polygonum multiflorum, and Albizia julibrissin. The present study aimed to examine first whether Gabrb2-KO phenotypes observed in young adult mice would remain in aged mice, and whether BYPA may display a role of anti-aging in naturally aged mice.
Methods:
A range of behavioral tests were performed on naturally aged Gabrb2-KO and wild-type (WT) mice treated with BYPA. Oxidation stress level was evaluated by MDA (malondialdehyde) test, and the expressions of antioxidant enzymes (superoxide dismutase and catalase) were measured using RT-qPCR (reverse transcription-quantitative polymerase chain reaction).
Results:
Behavioral tests on aged Gabrb2-KO mice showed hyper-locomotor activity, social function deficit, decreased levels of anxiety and depression, consistent with a previous study on young Gabrb2-KO mice. Oral administration of BYPA ameliorated anxiety, activity, and depression. Remarkably, BYPA protected facial tissues with regrowth of significantly lost hairs and whiskers due to aging. It also reduced oxidative stress levels and enhanced the expression of antioxidant enzymes.
Conclusions:
The present study showed that schizophrenia-like behavioral changes were exhibited by aged Gabrb2-KO mice, similar to what was reported earlier, suggesting that the observed behavioral changes did not result from any developmental delay, but a direct result of Gabrb2-KO, reconfirming the critical role of Gabrb2 in schizophrenia etiology. Since the BYPA herbal formula moderated the oxidative status and enhanced the expressions of antioxidant enzymes in D-galactose-accelerated aging as well as naturally aged mice, it might furnish a useful health supplement to both the schizophrenic and the aged populations, due to its significant antioxidation and anti-inflammation effects exerted in the brain.
Aim:
We previously observed oxidative stress and neuroinflammation caused behavioral and neurochemical changes in young Gabrb2 (gamma-aminobutyric acid type A receptor β2 subunit) knockout (KO) mice. Aging was moderated in a D-galactose-induced accelerated aging mouse model by an oral Chinese medicinal herbal formula BYPA consisting of Bupleurum chinense, Corydalis yanhusuo, Polygonum multiflorum, and Albizia julibrissin. The present study aimed to examine first whether Gabrb2-KO phenotypes observed in young adult mice would remain in aged mice, and whether BYPA may display a role of anti-aging in naturally aged mice.
Methods:
A range of behavioral tests were performed on naturally aged Gabrb2-KO and wild-type (WT) mice treated with BYPA. Oxidation stress level was evaluated by MDA (malondialdehyde) test, and the expressions of antioxidant enzymes (superoxide dismutase and catalase) were measured using RT-qPCR (reverse transcription-quantitative polymerase chain reaction).
Results:
Behavioral tests on aged Gabrb2-KO mice showed hyper-locomotor activity, social function deficit, decreased levels of anxiety and depression, consistent with a previous study on young Gabrb2-KO mice. Oral administration of BYPA ameliorated anxiety, activity, and depression. Remarkably, BYPA protected facial tissues with regrowth of significantly lost hairs and whiskers due to aging. It also reduced oxidative stress levels and enhanced the expression of antioxidant enzymes.
Conclusions:
The present study showed that schizophrenia-like behavioral changes were exhibited by aged Gabrb2-KO mice, similar to what was reported earlier, suggesting that the observed behavioral changes did not result from any developmental delay, but a direct result of Gabrb2-KO, reconfirming the critical role of Gabrb2 in schizophrenia etiology. Since the BYPA herbal formula moderated the oxidative status and enhanced the expressions of antioxidant enzymes in D-galactose-accelerated aging as well as naturally aged mice, it might furnish a useful health supplement to both the schizophrenic and the aged populations, due to its significant antioxidation and anti-inflammation effects exerted in the brain.
DOI: https://doi.org/10.37349/en.2025.1006108

Aim:
This study aimed to assess the relationship between clinician adherence to International League Against Epilepsy (ILAE) management guidelines and seizure freedom in adult patients with epilepsy at a Mexican tertiary care center.
Methods:
This retrospective cross-sectional study analyzed 404 adult outpatients with epilepsy from an institutional database (January–October 2013). Data were collected on demographic characteristics, seizure types, diagnostic workup completeness, treatment regimens, weight-adjusted dosing, self-reported adherence, and seizure freedom (defined as being seizure-free for at least 3 months). Statistical analysis included chi-squared tests (χ2) for categorical variables and multivariate logistic regression to identify independent predictors of seizure freedom.
Results:
Of 404 patients analyzed (58.7% female, mean age 33 ± 13 years), 49.3% achieved seizure freedom. Generalized seizures (including primary and secondarily generalized seizures) were most common (66%), followed by focal seizures (30%). Diagnostic studies included an electroencephalogram in 80% and a magnetic resonance imaging scan in 75% of patients. Monotherapy was used in 50.7%, polytherapy in 44.6%, with weight-adjusted dosing achieved in 92%. Self-reported treatment adherence was 81%. Factors significantly associated with seizure freedom included treatment adherence (51.4% vs. 27.3% in non-adherent patients, χ2 = 13.56, p < 0.001), monotherapy vs. polytherapy (71.7% vs. 62.9%, χ2 = 46.07, p < 0.001), and adequate weight-adjusted dosing (44.9% vs. 32.3%, χ2 = 5.97, p = 0.01).
Conclusions:
Adherence to ILAE management guidelines, particularly regarding monotherapy selection, weight-adjusted dosing, and treatment adherence, was significantly associated with improved seizure freedom rates. These findings underscore the importance of implementing evidence-based epilepsy management protocols systematically in clinical practice.
Aim:
This study aimed to assess the relationship between clinician adherence to International League Against Epilepsy (ILAE) management guidelines and seizure freedom in adult patients with epilepsy at a Mexican tertiary care center.
Methods:
This retrospective cross-sectional study analyzed 404 adult outpatients with epilepsy from an institutional database (January–October 2013). Data were collected on demographic characteristics, seizure types, diagnostic workup completeness, treatment regimens, weight-adjusted dosing, self-reported adherence, and seizure freedom (defined as being seizure-free for at least 3 months). Statistical analysis included chi-squared tests (χ2) for categorical variables and multivariate logistic regression to identify independent predictors of seizure freedom.
Results:
Of 404 patients analyzed (58.7% female, mean age 33 ± 13 years), 49.3% achieved seizure freedom. Generalized seizures (including primary and secondarily generalized seizures) were most common (66%), followed by focal seizures (30%). Diagnostic studies included an electroencephalogram in 80% and a magnetic resonance imaging scan in 75% of patients. Monotherapy was used in 50.7%, polytherapy in 44.6%, with weight-adjusted dosing achieved in 92%. Self-reported treatment adherence was 81%. Factors significantly associated with seizure freedom included treatment adherence (51.4% vs. 27.3% in non-adherent patients, χ2 = 13.56, p < 0.001), monotherapy vs. polytherapy (71.7% vs. 62.9%, χ2 = 46.07, p < 0.001), and adequate weight-adjusted dosing (44.9% vs. 32.3%, χ2 = 5.97, p = 0.01).
Conclusions:
Adherence to ILAE management guidelines, particularly regarding monotherapy selection, weight-adjusted dosing, and treatment adherence, was significantly associated with improved seizure freedom rates. These findings underscore the importance of implementing evidence-based epilepsy management protocols systematically in clinical practice.
DOI: https://doi.org/10.37349/en.2025.1006107
This article belongs to the special issue Advances in Epilepsy Research

Recent progress in translational neuroscience has significantly advanced our understanding of neurological diseases. Research progress closely went in line with innovations in research methods, which have expanded our insights considerably beyond previous limits. However, despite the development of disease-modifying treatments, therapeutic options in brain diseases still lag behind fundamental discoveries in basic neuroscience. This perspective examines the factors that hinder clinical progress in translational neuroscience and provides solutions on how to overcome them. Editorial board members of Exploration of Neuroscience were interrogated about the most prominent challenges they see in translational neuroscience and about possible ways to overcome these issues. Key challenges were seen at the interface between experimental research and clinical studies by several members, both from the basic and applied neuroscience fields, which include the selection of appropriate study readouts and endpoints. The establishment of refined study endpoints, combined with biomarkers capable of predicting treatment responses in human patients, will be crucial for the successful clinical implementation of new therapies. Further obstacles were found in the standardization of experimental models, interventions, and assessments both in animals and humans, as well as in the development of personalized treatment strategies. These challenges can be addressed through more clearly defined experimental procedures that closely match clinical conditions and precision-based approaches that ensure efficient therapeutic responses. As a great opportunity, treatment options targeting pathophysiological processes in multiple brain diseases and disease processes in different organ systems were noted. Significant barriers remain in the funding of investigator-driven clinical trials through public research programs, as well as the education of translational and clinician scientists dedicated to clinical translation. Enhanced communication between experimental neuroscientists and clinicians, with a shared understanding and common language, will be essential for the success of future research endeavors.
Recent progress in translational neuroscience has significantly advanced our understanding of neurological diseases. Research progress closely went in line with innovations in research methods, which have expanded our insights considerably beyond previous limits. However, despite the development of disease-modifying treatments, therapeutic options in brain diseases still lag behind fundamental discoveries in basic neuroscience. This perspective examines the factors that hinder clinical progress in translational neuroscience and provides solutions on how to overcome them. Editorial board members of Exploration of Neuroscience were interrogated about the most prominent challenges they see in translational neuroscience and about possible ways to overcome these issues. Key challenges were seen at the interface between experimental research and clinical studies by several members, both from the basic and applied neuroscience fields, which include the selection of appropriate study readouts and endpoints. The establishment of refined study endpoints, combined with biomarkers capable of predicting treatment responses in human patients, will be crucial for the successful clinical implementation of new therapies. Further obstacles were found in the standardization of experimental models, interventions, and assessments both in animals and humans, as well as in the development of personalized treatment strategies. These challenges can be addressed through more clearly defined experimental procedures that closely match clinical conditions and precision-based approaches that ensure efficient therapeutic responses. As a great opportunity, treatment options targeting pathophysiological processes in multiple brain diseases and disease processes in different organ systems were noted. Significant barriers remain in the funding of investigator-driven clinical trials through public research programs, as well as the education of translational and clinician scientists dedicated to clinical translation. Enhanced communication between experimental neuroscientists and clinicians, with a shared understanding and common language, will be essential for the success of future research endeavors.
DOI: https://doi.org/10.37349/en.2025.1006106
 Previous
Previous