Affiliation:
1Center for Cancer Genomics, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing 211100, Jiangsu, China
2Faculty of Medicine, Saad Dahleb University Blida-1, Blida 09000, Algeria
Email: manel.mimis@hotmail.fr
ORCID: https://orcid.org/0000-0001-9179-8502
Affiliation:
3Division of Life Science and Applied Genomics Center, Hong Kong University of Science and Technology, Hong Kong 999077, China
Affiliation:
1Center for Cancer Genomics, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing 211100, Jiangsu, China
Affiliation:
3Division of Life Science and Applied Genomics Center, Hong Kong University of Science and Technology, Hong Kong 999077, China
Affiliation:
1Center for Cancer Genomics, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing 211100, Jiangsu, China
3Division of Life Science and Applied Genomics Center, Hong Kong University of Science and Technology, Hong Kong 999077, China
Email: hxue@ust.hk
ORCID: https://orcid.org/0000-0002-8133-9828
Explor Neurosci. 2025;4:1006108 DOI: https://doi.org/10.37349/en.2025.1006108
Received: January 06, 2025 Accepted: July 24, 2025 Published: September 09, 2025
Academic Editor: Marcello Iriti, Milan State University, Italy
Aim: We previously observed oxidative stress and neuroinflammation caused behavioral and neurochemical changes in young Gabrb2 (gamma-aminobutyric acid type A receptor β2 subunit) knockout (KO) mice. Aging was moderated in a D-galactose-induced accelerated aging mouse model by an oral Chinese medicinal herbal formula BYPA consisting of Bupleurum chinense, Corydalis yanhusuo, Polygonum multiflorum, and Albizia julibrissin. The present study aimed to examine first whether Gabrb2-KO phenotypes observed in young adult mice would remain in aged mice, and whether BYPA may display a role of anti-aging in naturally aged mice.
Methods: A range of behavioral tests were performed on naturally aged Gabrb2-KO and wild-type (WT) mice treated with BYPA. Oxidation stress level was evaluated by MDA (malondialdehyde) test, and the expressions of antioxidant enzymes (superoxide dismutase and catalase) were measured using RT-qPCR (reverse transcription-quantitative polymerase chain reaction).
Results: Behavioral tests on aged Gabrb2-KO mice showed hyper-locomotor activity, social function deficit, decreased levels of anxiety and depression, consistent with a previous study on young Gabrb2-KO mice. Oral administration of BYPA ameliorated anxiety, activity, and depression. Remarkably, BYPA protected facial tissues with regrowth of significantly lost hairs and whiskers due to aging. It also reduced oxidative stress levels and enhanced the expression of antioxidant enzymes.
Conclusions: The present study showed that schizophrenia-like behavioral changes were exhibited by aged Gabrb2-KO mice, similar to what was reported earlier, suggesting that the observed behavioral changes did not result from any developmental delay, but a direct result of Gabrb2-KO, reconfirming the critical role of Gabrb2 in schizophrenia etiology. Since the BYPA herbal formula moderated the oxidative status and enhanced the expressions of antioxidant enzymes in D-galactose-accelerated aging as well as naturally aged mice, it might furnish a useful health supplement to both the schizophrenic and the aged populations, due to its significant antioxidation and anti-inflammation effects exerted in the brain.
Schizophrenia is a severe mental disease that causes social, emotional, and cognitive problems [1], characterized by a strong genetic component with over 100 associated genetic loci [2]. Gabrb2 (gamma-aminobutyric acid type A receptor β2 subunit), which encodes the GABAA (gamma-aminobutyric acid type A receptor) β2 subunit, which combines with the α1 and γ2 subunits to form the major GABAA receptor subtype in the mammalian brain [3], has been associated with schizophrenia in different populations [4–9]. Mechanistically, its altered splicing and epigenetic regulation [10–12], as well as its genotype-dependent expression and the N-glycosylation of its gene product [13, 14], are evident in schizophrenia, accompanied by positive selection, recombination, and genetic imprinting [12, 15]. Furthermore, young adult Gabrb2-KO mice 3 to 10 weeks of age displayed schizophrenia-like behavior and epilepsy that were responsive to treatment with the anti-psychotic drug risperidone [16], along with reduced depression and anxiety plausibly due to alterations of extra-synaptic inhibition through GABAA receptors. These mice exhibited decreased number of neurons, increased number of microglia, and elevated levels of inflammatory markers and oxidative stress [16].
Aging is defined as the progressive loss of body functions accompanied by decreased fertility and increased mortality [17]. It is a risk factor for neurodegenerative diseases exemplified by Alzheimer’s disease and Parkinson’s disease, and it has become a foremost health issue of the world on account of the rapid expansion of the aged populations [18]. Neuroinflammation and oxidative stress in the brain are important manifestations of aging and neurodegenerative diseases [19]. Antioxidant and anti-inflammatory supplements that may reduce neurodegeneration are widely sought after as aids to longevity [20, 21]. The decline in cognitive functions among the aged can also be exacerbated by schizophrenia, where cognitive impairment constitutes a common symptom [22, 23]. Accordingly, effective interventions to reduce neural oxidative stress and inflammation represent an outstanding aim of health care for both the schizophrenics and the aged.
In this regard, our investigations of the anti-aging effect of traditional Chinese medicinal herbs have yielded an oral preparation that ameliorated loss of spatial working memory, reduced oxidative stress, and the pro-inflammatory cytokines TNF-α (tumor necrosis factor-alpha) and IL-6 (interleukin-6) in galactose-induced aging mice with no significant side effects [24]. This BYPA formula consists of the medicinal herbs Radix Bupleurum chinense DC (“B”), Rhizoma Corydalis yanhusuo WT Wang (“Y”), Caulis Polygonum multiflorum Thunb (“P”), and Flos Albizia julibrissin Durazz (“A”) [25, 26]. Among its constituent herbs, polyacetylenes of herb B are known to exhibit antidepressant effects by inhibiting monoamine reuptake in vitro [27]; l-tetrahydropalmatine (l-THP) of herb Y has fast-acting anxiolytic and anti-manic effects mediated by dopamine D2 and GABAA receptors with little significant side effects [28], whereas herbs P and A can contribute to sedative effects [29, 30].
Accordingly, the objective of the present study was to examine whether any of the schizophrenic-like symptoms of young adult Gabrb2-KO mice would persist in aged ones, and to determine the utility of BYPA in regulating aging-related symptoms.
Animal procedures were approved by the AEC (animal ethics committee) of HKUST (Hong Kong University of Science and Technology), identified by the approval number AEP-2022-0038 titled “Study on Gabrb2 associated functional and behavioral effects using mouse model”. All experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Gabrb2-KO mice (hybrid of C57BL/6-129/SvEv) and WT control mice of both sexes [16] were bred and grown at the in-house animal facility until they were more than 7 months old, with the majority more than 12 months old and several of them more than 24 months old.
Ear punching was used to collect mouse tissue for genotyping to confirm the genotypes of the KO and WT mice. Alkaline lysis (75 µL) buffer was added to the tissue, heated for 1 h at 95°C, and shaken for 30 min. The mixture was cooled to 4°C and neutralized; a 5 µL aliquot was employed for PCR amplification using specific primers for the Neo (neomycin resistance gene) and exon 7 of the Gabrb2 gene (Table 1). Amplified products were centrifuged for 10 s, then subjected to 2% agarose gel electrophoresis to determine the genotypes.
Primers sequence for RT-qPCR in this study.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Sod2 | GTGTCTGTGGGAGTCCAAGG | AGCGGAATAAGGCCTGTTGT |
| Cat | AGGATGTGGTTTTCACTGACG | TTTGCCTTGGAGTATCTGGTG |
| ACTB | CTAGGCACCAGGGTGTGATG | GGGGTACTTCAGGGTCAGGA |
The herbal combination was prepared from Radix Bupleuri chinense DC, Rhizoma Corydalis yanhusuo, Caulis Polygonum multiflorum, and Flos Albizia julibrissin. In brief, the herbs were finely powdered and boiled for 2 h in 2 L of 5% acetic acid twice. The supernatants were collected, filtered, reboiled, and dried at 92°C. Ethanol (100%) was added to the powder and reboiled and redried at 92°C. WT-aged mice were separated into a vehicle (Veh) group receiving a daily oral administration of 0.9% NaCl for 4 weeks, and a herbal group receiving, additionally, a daily oral administration of BYPA in 0.9% NaCl (60 mg/kg).
Behavioral tests were performed on aged WT and Gabrb2-KO mice to examine whether Gabrb2-KO phenotypes observed in young adult mice would remain in aged mice, and also performed on aged WT mice for both Veh and BYPA groups to search for the anti-aging effects in naturally aged mice.
The hole-board consisted of a 60 × 60 cm square-shaped floor with 20 cm high walls on each side. Four holes with 3 cm diameter were evenly distributed on the floor. Each mouse was placed at the center of the floor at the start of the test, and allowed to roam for 5 min, during which the number of head-dips into the holes and the number of climbs onto the walls were counted. This test assessed the anxiety-like behavior of the mouse and the sedative effect of any drug administered to it. A decrease in the frequency of climbs or head-dips signified a sedative effect by the drug [31].
The three-chamber test was used to assess social affiliation (or sociability) and preference for social novelty [32]. The apparatus consisted of a rectangular-shaped transparent box with three chambers (a central chamber and two side chambers) and openings between the chambers. The chambers were 19 cm × 45 cm in dimension, and the openings between the chambers could be closed off using a removable plexiglas partition. An upside-down pencil cup was placed inside each side chamber to hold a stranger mouse. Before the test, the test mouse was positioned in the central chamber and allowed to roam for 5 min to familiarize itself with the apparatus. A stranger-1 mouse with no previous contact with the test mouse and similar in age was placed inside the cup in one of the side chambers. The plexiglass partitions between the three chambers were removed for 10 min. We measured the social affiliation in terms of the number and total duration of the visits exhibited by the test mouse for the cup containing the stranger-1 mouse relative to the empty cup. Thereupon, a novel stranger-2 mouse was placed in the cup in the other side chamber, and the number and total duration of visits by the test mouse to the novel stranger-2 mouse relative to the now familiar stranger-1 mouse were recorded in order to determine the preference of the test mice for social novelty.
A plastic, transparent circular container that was exposed at the top was employed in this test. Three strips of tape were fixed to the bottom of the transparent container to create six equal segments of the circle. The locomotor movement of a mouse across different segments was measured by the frequency of its tail crossing between any two segments during a 5-minute test period. If its tail crossed a segment while the mouse was rotating on a spot within the same segment, it would not be counted.
The elevated plus maze consisted of a “+”-shaped elevated platform with four arms and a central square platform. Two opposite arms were closed arms with walls, while the remaining two were open arms without walls. The maze was elevated to 40 cm above ground. At the start, the mouse was positioned on the central platform and allowed to move around the maze for 5 min. During this period, its entries into the closed and open arms were counted and timed separately, while entries into the central platform were not counted. Increased frequency or duration of visits to the open arms over the closed arms signified an anxiolytic effect [33].
The tail suspension test was used to detect depression-like behavior. The apparatus consisted of a box with a horizontal bar set 15 cm above the floor. The mouse was taped by its tail to the horizontal bar and subjected to 6 min of hanging. The immobility time within the 6 min was recorded. This test assessed the effects of a drug on the level of the mouse’s depression, and a decrease in immobility time caused by the drug testified to the antidepressant effect of the drug [34].
Mice were euthanized using cervical dislocation, which was approved by the AEC. The brain of WT-aged mice (Veh and BYPA groups) was removed and homogenized in phosphate buffer saline, pH 7.2. Oxidative stress was estimated by the reaction between TBA (thiobarbituric acid) and MDA using the spectrophotometric assay at 532 nm [35].
The analysis of the expression of antioxidant enzyme genes in the brain of WT-aged mice (Veh and BYPA groups), including Sod2 (superoxide dismutase) and Cat (catalase), was performed by RT-qPCR. Total RNA (ribonucleic acid) was extracted using TRIzol reagent (Invitrogen Corp, CA, 15596026) according to the manufacturer’s instructions. Total RNA was reverse transcribed using QuantiTect® reverse transcription kit (Qiagen, 4368814). RT-qPCR was duplicated for each sample using FastStart Universal SYBR Green Master (Roche, 45-4913850001). ACTB (Beta-Actin, 4333762F) was used as a reference. The primers used in these experiments are described in Table 1.
Results were expressed as mean ± SD. All statistical analyses were performed using Prism 8.0 (Graphpad Software), and statistical significance was established by p < 0.05. Data were analyzed by means of a one-way ANOVA test followed by Tukey’s post hoc test or student’s unpaired t-test.
A spectrum of schizophrenia-like behavioral changes was observed in young adult Gabrb2-KO mice relative to WT mice [16]. In the present study, the aged Gabrb2-KO mice also displayed an increased number of stereotypic climbs in the head-dip test, although not in the number of stereotypic head-dips (Figure 1A).
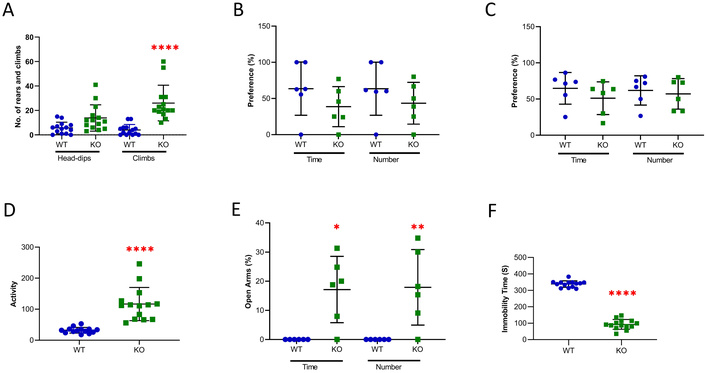
Behavioral tests on aged WT and Gabrb2-KO mice. A) Hole board test: The number of head-dips into the circles and the number of climbs on the walls were counted during a 5-minute interval (WT, n = 14; KO, n = 14). B) Social affiliation: The assessment of both time and number of visitations to the stranger-1 mouse for a 10-minute interval (WT, n = 6; KO, n = 6). C) Social novelty: The assessment of both time and number of visitations to the stranger-2 mouse for a 10-minute interval (WT, n = 6; KO, n = 6). D) Locomotor activity test: The movement of the mouse across each segment was measured by counting the number of times its tail crossed a segment for a total of 5 min (WT, n = 14; KO, n = 14). E) Elevated-plus maze test showing percentile time spent in or entries into open arms (WT, n = 6; KO, n = 6). F) Tail suspension test where the mouse was taped by the tail to the horizontal bar and subject to 6 min hanging. The immobility time was recorded within 6 min (WT, n = 14; KO, n = 14). WT mice are shown in blue and KO mice in green. Statistical analysis was performed using student’s t-test for locomotor activity and tail suspension tests and using one-way ANOVA with Tukey’s post hoc test for other behavioral tests. Data are expressed in mean ± SD. * p < 0.05, ** p < 0.01, **** p < 0.0001. Average age (WT = 15.5 months, KO = 15.1 months).
In the three-chamber test, the KO mice did not differ significantly from the WT mice in the number or duration of visits to the cup containing the stranger-1 mouse relative to the empty cup without any stranger mouse (Figure 1B). There was no significant difference in the number or duration of visits to the cup containing the novel stranger-2 mouse relative to the cup containing the stranger-1 mouse, which had become the familiar mouse (Figure 1C). Therefore, Gabrb2-KO did not affect significantly either the social affiliation of the test mice or the extent of their preference for social novelty behavior.
In the locomotor activity test, the Gabrb2-KO mice showed a much higher level of activity than the WT mice (Figure 1D), plausibly as a manifestation of their psychotic agitation.
In the elevated plus maze test, the Gabrb2-KO mice showed an elevated number and total duration of entries into the open arms compared to the WT mice (Figure 1E), indicative of diminished anxiety in the KO mice. In the tail suspension test, the Gabrb2-KO mice showed reduced immobility time compared to the WT mice (Figure 1F), suggesting that the KO mice were less susceptible to depression than the WT mice.
The BYPA-treated group received a daily oral administration of BYPA for 4 weeks, and the Veh group of animals received daily oral administration of vehicle (0.9% NaCl). Daily oral treatment with BYPA significantly decreased locomotor activity relative to the control mice receiving only administration of vehicle with p < 0.01 (Figure 2A). In the elevated-plus maze test for anxiety, a significant increase in the number of entries into open arms in the elevated plus maze test with p < 0.05 (Figure 2B), which indicates a decreased level of anxiety. In the tail suspension test to assess depression level, a significant decrease in immobility time was observed with p < 0.05 (Figure 2C), indicating a reduced level of depression relative to WT.
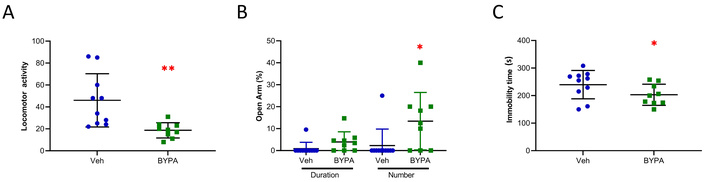
Behavioral tests for BYPA herbal formula treatment on aged WT mice. A) Locomotor activity test: The movement of the mouse across each segment was measured by counting the number of times its tail crossed a segment for a total of 5 min (Veh, n = 10; BYPA, n = 9). B) Elevated-plus maze test showing percentile time spent in or entries into open arms (Veh, n = 11; BYPA, n = 9). C) Tail suspension test where the mouse was taped by the tail to the horizontal bar and subject to 6 min hanging. The immobility time was recorded within 6 min (Veh, n = 10; BYPA, n = 9). Veh group mice are shown in blue and BYPA group mice in green. Statistical analysis was performed using student’s t-test for locomotor activity and tail suspension tests and using one-way ANOVA with Tukey’s post hoc test for other behavioral tests. Data are expressed in mean ± SD. * p < 0.05, ** p < 0.01. Average age (Veh = 16.7 months, KO = 15.8 months).
In the young adult Gabrb2-KO mice, the brain level of oxidative stress measured by MDA was enhanced, and the levels of the inflammatory cytokines TNF-α and IL-6 were also elevated, suggesting that the schizophrenic-like phenotypes of these mice were associated with an oxidant/antioxidant imbalance skewed toward an excessively oxidative status [16].
Since aging is characterized by inflammation with ROS (reactive oxygen species), resulting in an inflammaging process plausibly arising from immuno-decay [36, 37], the phenotypic overlap of inflammation and excessive oxidation between the disorders of schizophrenia and aging is striking. Accordingly, in view of the anti-aging activities of BYPA on mice under accelerated aging through galactose treatment [24], the effects of BYPA on naturally aged mice were analyzed in Figure 3. The results showed that BYPA reduced the brain level of MDA (Figure 3A) and enhanced the expression of Cat (Figure 3B), both to p < 0.05.
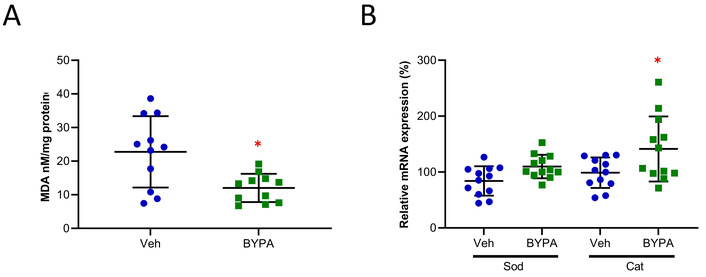
MDA analysis and mRNA expression of antioxidant enzymes in aged WT mice. A) MDA analysis: measured by the reaction between TBA and MDA and expressed per mg protein of sample analyzed (Veh, n = 11; BYPA, n = 11). B) mRNA expression for Sod2 and Cat in the brain was measured using RT-qPCR (Veh, n = 12; BYPA, n = 12). Veh group mice are shown in blue and BYPA group mice in green. Statistical analysis was performed using student’s unpaired t-test for the MDA test and one-way ANOVA with Tukey’s post hoc test for RT-qPCR. Data are expressed in mean ± SD. * p < 0.05. Average age (Veh = 16.7 months, KO = 15.8 months).
Some of the aged WT-mice showed facial hair loss before the start of a test for facial hair regeneration. Hair regrowth was checked constantly to confirm our previous results on galactose-induced aging mice [24]. Importantly, the BYPA group displayed facial hair regeneration after four weeks of daily treatment in contrast to the Veh, which showed no significant facial hair regeneration (Figure 4).

Facial hair regeneration in the BYPA group. A) Photographs of a Veh group mouse (A1) before treatment and (A2) after 4 weeks of treatment with vehicle. B) Photographs of a BYPA group mouse (B1) before treatment and (B2) after 4 weeks of treatment with BYPA.
The present study examined behavioral symptoms of aged Gabrb2-KO mice in comparison to WT mice using behavioral tests, including social inclinations, and also affective tests for anxiety and depression. The findings of several differences between the aged KO mice and normal aged mice have confirmed the problem posed by old-age schizophrenia, including neuroinflammation with increased pro-inflammatory cytokines, as well as increased oxidative stress, and microglial activation, together with hyperactivity, decreases in cell proliferation in the hippocampus, and epilepsy [16]. Moreover, we previously confirmed that Gabrb2 was associated with schizophrenia [38–40] and was subjected to altered methylation, confirming epigenetic regulation of its expression [11]. Epigenetic changes promote accelerated aging and persisting schizophrenia symptoms in Gabrb2-KO mice [38]; all these findings pointed to the plausible utility of aged KO mice as animal models in this regard.
Gabrb2-KO mice exhibited behavioral and cognitive changes similar to those observed in schizophrenia, including: Neuroinflammation with increased oxidative stress, increased pro-inflammatory cytokines, and microglial activation, as well as the comorbidities of hyperactivity, decreases in cell proliferation in the hippocampus, and epilepsy [16].
Although schizophrenic patients often experience a moderation of some of their disturbing symptoms, old-age schizophrenia has emerged as a highly distressing disease. While the aged manifest fewer positive symptoms, their negative and cognitive symptoms tend to persist. It is estimated that old-age schizophrenia in New Hampshire is twice as expensive to manage as depression and three times as difficult to manage as a physical disease. There are also two apparent sources of old-age schizophrenia, with 80–85% early-onset patients developing the disease before age 60, and 15–20% late-onset patients developing the disease after age 60 [22]. It is unclear whether these two groups differ only in the timing of the occurrence of their symptoms or in the mechanisms of their etiology as well.
The encounter of oxidative stress in aging [40] and in Gabrb2-KO mice [16] represents a striking co-occurrence, suggesting that there could be a mechanistic link between aging and schizophrenia in accord with the view that aging might be a disorder, such as an oxidative or immunological disease [36, 37]. BYPA, which is a combination of four potential medicinal herbs based on traditional Chinese medicine (TCM), showed anti-aging and anxiolytic effects as well as reduced oxidative stress and neuroinflammation in an induced aging model with no significant side effects [24]. The present study brought about anti-aging changes also among the naturally WT-aged mice. BYPA lowered the brain level of MDA and increased the expression of anti-inflammatory enzymes (Cat). Increased oxidative stress is common in many neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease [39, 40]. BYPA may be a useful therapy to prevent or delay the occurrence of aging-related diseases. BYPA extracts showed significant anxiolytic and sedative effects, with repair of facial skin and regrowth of facial hair. In this regard, BYPA is suggested to be a potential treatment for insomnia and anxiety [28]. All these findings are supportive of the usage of BYPA as an anti-aging health supplement for the aged population, including aged schizophrenics.
The present study carried out behavioral experiments with two genotypes of the same strain of mice to provide insights into aging and anti-aging from different perspectives. On one hand, behavioral experiments with naturally aged Gabrb2-KO mice demonstrated that the behavioral effects of genetic defects persisted, providing a stable mouse model for relevant neuropsychiatric pharmacology studies, including anti-neuroinflammation, since Gabrb2-KO has been shown by us previously to induce extensive neuroinflammation in mice. On the other hand, BYPA, an anti-neuroinflammatory herbal formula previously reported by us [24], has been surprisingly demonstrated herein with anti-aging effects on hair protection and recovery. BYPA has been strongly recommended to be administered to naturally aged Gabrb2-KO mice. However, compromised fertility in naive KO mice and the long period of mice breeding and needing to orally administer BYPA for 4 weeks after behavioral tests, together with their susceptibility to audiogenic epilepsy, make them fragile and incapable of accomplishing the experiment.
Knowledge generated from this study paves the way to the development of useful anti-neuroinflammation and anti-aging therapeutics and nutraceuticals to address the needs of rapidly aging societies.
AEC: animal ethics committee
Cat: catalase
GABAA: gamma-aminobutyric acid type A receptor
Gabrb2: gamma-aminobutyric acid type A receptor β2 subunit
IL-6: interleukin-6
RNA: ribonucleic acid
TNF-α: tumor necrosis factor-alpha
Veh: vehicle
The authors are thankful to the Laboratory Animal Facility at Hong Kong University of Science and Technology for professional services in maintaining the Gabrb2-KO mice colony used in this study. Special appreciations are paid to PharmacoGenetics Limited, Hong Kong, China, for providing the valuable preparation of BYPA for the present study.
MB: Conceptualization, Investigation, Formal analysis, Visualization, Writing—original draft, Writing—review & editing. AU: Writing—original draft. GS: Investigation. WKM: Writing—original draft. HX: Conceptualization, Visualization, Writing—original draft, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The article has been approved by the AEC (animal ethics committee) of HKUST (Hong Kong University of Science and Technology), identified by the approval number AEP-2022-0038 titled “Study on Gabrb2 associated functional and behavioral effects using mouse model”.
Not applicable.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
The authors are grateful for the support of the Innovation and Technology Fund of China Pharmaceutical University, the Ministry of Education of China, and the Hong Kong University of Science and Technology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1681
Download: 22
Times Cited: 0
