Affiliation:
1Postgraduate Program in Pharmaceutical Science, Universidade do Vale do Itajaí (UNIVALI), Itajaí 88302-901, Brazil
ORCID: https://orcid.org/0009-0001-4474-9529
Affiliation:
1Postgraduate Program in Pharmaceutical Science, Universidade do Vale do Itajaí (UNIVALI), Itajaí 88302-901, Brazil
ORCID: https://orcid.org/0000-0003-3528-646X
Affiliation:
2Chemistry and Pharmaceutical Research Center (NIQFAR), Universidade do Vale do Itajaí (UNIVALI), Itajaí 88302-901, Brazil
Affiliation:
1Postgraduate Program in Pharmaceutical Science, Universidade do Vale do Itajaí (UNIVALI), Itajaí 88302-901, Brazil
ORCID: https://orcid.org/0009-0009-6446-0384
Affiliation:
1Postgraduate Program in Pharmaceutical Science, Universidade do Vale do Itajaí (UNIVALI), Itajaí 88302-901, Brazil
ORCID: https://orcid.org/0009-0007-3841-4590
Affiliation:
1Postgraduate Program in Pharmaceutical Science, Universidade do Vale do Itajaí (UNIVALI), Itajaí 88302-901, Brazil
3Department of Pharmaceutical Sciences, Universidade Regional de Blumenau (FURB), Blumenau 89030-903, Brazil
ORCID: https://orcid.org/0000-0001-6969-7473
Affiliation:
2Chemistry and Pharmaceutical Research Center (NIQFAR), Universidade do Vale do Itajaí (UNIVALI), Itajaí 88302-901, Brazil
ORCID: https://orcid.org/0009-0003-7192-6345
Affiliation:
2Chemistry and Pharmaceutical Research Center (NIQFAR), Universidade do Vale do Itajaí (UNIVALI), Itajaí 88302-901, Brazil
ORCID: https://orcid.org/0009-0005-9778-5282
Affiliation:
1Postgraduate Program in Pharmaceutical Science, Universidade do Vale do Itajaí (UNIVALI), Itajaí 88302-901, Brazil
2Chemistry and Pharmaceutical Research Center (NIQFAR), Universidade do Vale do Itajaí (UNIVALI), Itajaí 88302-901, Brazil
ORCID: https://orcid.org/0000-0002-9102-6980
Affiliation:
1Postgraduate Program in Pharmaceutical Science, Universidade do Vale do Itajaí (UNIVALI), Itajaí 88302-901, Brazil
2Chemistry and Pharmaceutical Research Center (NIQFAR), Universidade do Vale do Itajaí (UNIVALI), Itajaí 88302-901, Brazil
ORCID: https://orcid.org/0000-0002-8947-2965
Affiliation:
4College of Pharmacy, Dankook University, Cheonan 31116, Korea
ORCID: https://orcid.org/0009-0003-4484-255X
Affiliation:
4College of Pharmacy, Dankook University, Cheonan 31116, Korea
ORCID: https://orcid.org/0009-0002-9125-1798
Affiliation:
4College of Pharmacy, Dankook University, Cheonan 31116, Korea
ORCID: https://orcid.org/0000-0002-1065-6746
Affiliation:
1Postgraduate Program in Pharmaceutical Science, Universidade do Vale do Itajaí (UNIVALI), Itajaí 88302-901, Brazil
2Chemistry and Pharmaceutical Research Center (NIQFAR), Universidade do Vale do Itajaí (UNIVALI), Itajaí 88302-901, Brazil
Email: msouza@univali.br
ORCID: https://orcid.org/0000-0002-6297-9635
Explor Neurosci. 2025;4:1006109 DOI: https://doi.org/10.37349/en.2025.1006109
Received: March 21, 2025 Accepted: July 02, 2025 Published: September 10, 2025
Academic Editor: Ryszard Pluta, Medical University of Lublin, Poland
The article belongs to the special issue Progress in Alzheimer's disease research: etiology, molecular mechanisms involved in disease progression, and advances in therapies aimed at slowing or reversing neurodegeneration
Aim: Alzheimer’s disease (AD) is a chronic neurodegenerative brain dysfunction and the most common form of dementia, especially in the elderly, and is considered a serious problem for health systems worldwide. It is a multifactorial and progressive condition, characterized by memory loss, personality changes and decline in cognitive function, in addition to neuropsychiatric complications such as depression, anxiety, sleep disorders, and others, further reducing the quality of life of patients with AD. Since the introduction of galantamine in AD therapy, medicinal plants and herbal remedies are gaining increasing interest as complementary and alternative interventions and are a valuable source for the development of drug candidates for AD. This work aims to explore Tithonia diversifolia ethanol extract (EETD), which showed an acetylcholinesterase (AChE) inhibitory activity like rivastigmine, as a new candidate for molecular targets of AD.
Methods: Mice were submitted to intracerebroventricular (I.C.V.) streptozotocin (STZ)-induced AD (2.5 mg/mL) and separated into different groups: sham, vehicle, rivastigmine (0.6 mg/kg), and EETD (0.1, 1.0, and 3.0 mg/kg). After AD induction, the animals were treated for 24 days and submitted to behavioral tests of memory, anxiety and depression. After the tests, the animals were sacrificed and the hippocampus was removed for assays of oxidative stress, AChE activity and markers of neuroinflammation. In vitro studies evaluated the effect of the extract on tau hyperphosphorylation, beta-amyloid (Aβ), and nitric oxide (NO) production.
Results: EETD promoted a reduction in STZ-induced behavioral parameters of depression and anxiety, as well as reversed memory deficits. Biochemical assays revealed that EETD increased antioxidant defenses, as well as decreased levels of neuroinflammation markers. In addition, EETD partially inhibited Aβ production.
Conclusions: The results together suggest that the plant exhibits therapeutic relevance in AD. However, studies are needed to identify the phytoconstituents responsible for such effects.
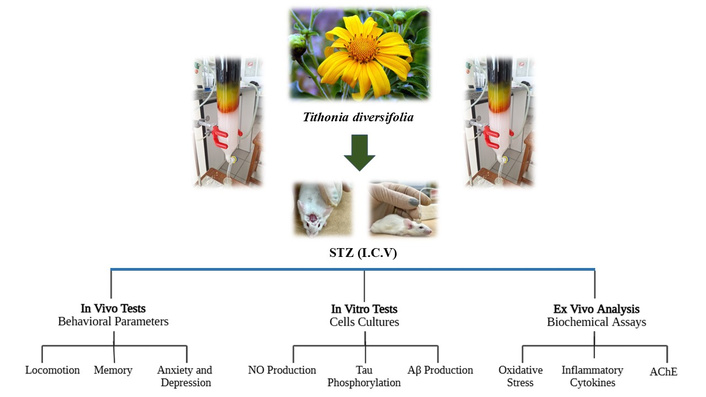
Alzheimer’s disease (AD) is a neurodegenerative disorder associated with aging, characterized by the progressive loss of neurons in different regions of the central nervous system (CNS). This neuronal decline impairs cognitive and memory functions, directly affecting daily activities and social interactions [1]. Furthermore, the disease accounts for 60% to 80% of all dementia cases, affects over 40 million people worldwide, and its prevalence is expected to rise significantly in the coming years [1–3].
Although its etiology remains unclear, and is currently considered to have a multifactorial background, the disease exhibits as histopathological characteristics the formation of senile plaques resulting from the accumulation of beta-amyloid (Aβ) peptides, as well as the presence of neurofibrillary tangles resulting from the hyperphosphorylation of tau protein [1, 4]. Studies have shown changes typical of AD in the course of post-ischemia brain neurodegeneration, i.e., progressive brain and hippocampal atrophy, increased amyloid production, and modification of tau protein [5], which somewhat changes the paradigm related to the possible causes of the disease and the possible development of new experimental models for the discovery of possible therapeutic targets. Substantial evidence also indicates that oxidative stress plays a central role in disease progression by promoting neuroinflammation and triggering intense microglial activation, which results in neuronal apoptosis, particularly affecting cholinergic neurons [6–8].
In this context, oxidative stress arises from an imbalance between the production of reactive oxygen species (ROS) and the brain’s endogenous antioxidant defense mechanisms, ultimately leading to tissue damage [6, 9, 10]. Brain regions such as the cerebral cortex and hippocampus, both essential for cognitive processing, are especially vulnerable to this type of injury [11]. In parallel, the cholinergic system, critical for learning and memory, is disrupted in AD, with a reduction in choline acetyltransferase activity impairing acetylcholine (ACh) synthesis, and an increase in acetylcholinesterase (AChE) activity accelerating its degradation at synapses [12].
Current treatment options include AChE inhibitors and NMDA receptor antagonists, which aim to increase ACh levels in the brain and reduce excitotoxicity. However, these therapies provide only symptomatic relief and are often associated with adverse effects that hinder treatment adherence [13]. Consequently, the search for new therapeutic alternatives remains a priority. In this context, in the search for potential targets of pharmacological interest for AD, many compounds obtained from natural sources with therapeutic potential for AD are being studied, boosted mainly by galantamine, isolated from Galanthus nivalis, in the management of the disease [14]. In this aspect, Tithonia diversifolia (Hemsl.) can be a good option for study.
As previously reported in our group’s work with this species, T. diversifolia is a medicinal plant with a wide geographic distribution, popularly known as “arnica”, “margaridão”, or “mão de Deus”. This plant is widely used in folk medicine, mainly in Mexico, Venezuela, and Brazil, to treat bruising and muscle pain, infectious and inflammatory processes [15]. The antioxidant and anti-inflammatory effects of the plant have already been reported in the literature and, recently, data obtained in our laboratories showed that the extract of T. diversifolia presented anti-inflammatory activity by inhibiting the production of cytokines and nitric oxide (NO) peripherally, as well as the migration of leukocytes [16]. In addition, it has been reported that the extract inhibited AChE and butyrylcholinesterase (BuChE) activities in vitro tests [17]. Therefore, the objective of the present study was to pharmacologically validate the plant as a potential target for the treatment of AD.
The extract was obtained according to procedures previously described [16]. The aerial parts were dried in a circulating air oven for 7 days. Leaves and stems were ground by hand, obtaining 80.715 g of dry weight. After this, the extract was submitted to two extraction steps with 95% ethanol. The first was a 6-h dynamic maceration at 700 rpm, yielding 3.38 g of extract. The second, using the plant residue, was a 6-day static maceration, yielding 2.49 g. Both extracts were filtered, evaporated at 60°C, dried, and then combined to ensure exhaustive extraction of plant compounds.
Male Swiss mice (25–30 g) obtained from the Central Animal Facility of the Universidade do Vale do Itajaí (UNIVALI) were kept in groups with access to food and water ad libitum and under a 12-h light-dark cycle (lights on at 7:00 AM) and controlled temperature (23 ± 1°C). These procedures followed the standards of the UNIVALI Animal Use Ethics Committee (CEUA/UNIVALI), protocol number 022/17, obtained in December 2017 with an addendum in 2023, and are in accordance with the Guide for the Care and Use of Laboratory Animals. Behavioral experiments were conducted from 1:00 PM to 6:00 PM. The animals were divided into six experimental groups: (G1) sham (surgery and vehicle infusion only); (G2) vehicle [streptozotocin (STZ)-induced and treated with phosphate buffered saline (PBS)]; (G3–G5) induced by STZ and treated orally (PO) with EETD at 0.1, 1.0 and 3.0 mg/kg; (G6) positive control [induced by STZ and treated with rivastigmine 0.6 mg/kg, treated intraperitoneally (IP)]. After the behavioral procedures, the animals were euthanized with a deep anesthetic overdose through the IP application of a solution of Xylazine 2% (Xilazin®) 30 mg/kg and Ketamine (Cetamin®) 300 mg/kg. Their brains were carefully removed and prepared for biochemical assays. The euthanasia method adopted is based on the Euthanasia Practice guideline document of the National Council for the Control of Animal Experimentation (CONCEA), a federal agency to which all CEUAs are linked in Brazil. This procedure was performed in accordance with the ethics committee for animal use at Univali (CEUA/UNIVALI, which is linked to CONCEA).
The following drugs and reagents were used in this study. STZ and rivastigmine were purchased from Sigma-Aldrich (St. Louis, MO, USA), Ketamine was obtained (Vetbrands, FL, USA), xylazine (Agener União, SP, BR), and lidocaine with epinephrine 2% (Cristália, SP, BR). The reagents used to obtain EETD were obtained from VETEC (RJ, BR) and LABSYNTH (SP, BR). All drugs were dissolved in saline (except STZ, which was dissolved in artificial cerebrospinal fluid prepared as described [18] and infused at room temperature).
This procedure was performed as described by Pinton et al. (2010) [19] with adaptations [18, 20–22]. Mice were anesthetized with a combination of xylazine and ketamine (10 and 100 mg/kg, respectively, via IP route) and received two intracerebroventricular STZ (I.C.V. STZ) injections of (2.5 μL, 2.5 mg/mL), 48 h apart, targeting the cerebral ventricle. Injections were made using a Hamilton syringe and were guided by the bregma. Treatments began on the third day after the first injection and continued until day 24. Behavioral assessments (ambulation, anxiety, and memory) were conducted between days 17 and 24. From the 17th to 24th, mice had the assessment of ambulation, anxiety, and memory according to the experimental design presented in Figure 1.
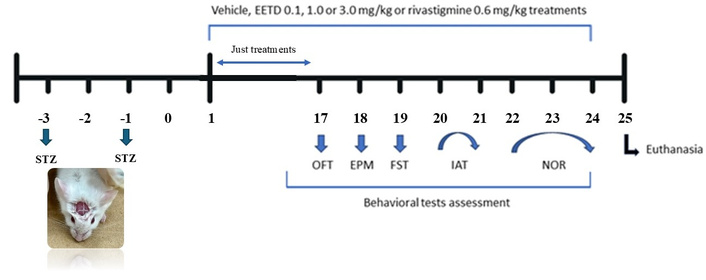
Timeline of experimental protocol. Male Swiss mice were administered by intracerebroventricular (I.C.V.) injection with 2.5 mg/mL of STZ. Experimental treatments started 48 h after the last infusion (day 1). A total of 60 animals were used in the study. The animals were divided into groups (n = 10) according to treatment with vehicle, EETD [0.1, 1.0, or 3.0 mg/kg, administered orally (PO)] or rivastigmine [0.6 mg/kg, intraperitoneally (IP)], starting from day 1 to day 17 without interruption and continuing from day 17 until day 24 (period in which the behavioral tests took place). A group of animals (sham operated/n = 10) underwent the same I.C.V. procedure with artificial liquor, instead of STZ, used to control the procedure. Behavioral parameters were assessed with the same animals starting on day 1 until the 24th. Finally, the animals were euthanized 24 h after the last behavior test and had their hippocampus dissected for subsequent biochemical analysis. EETD: Tithonia diversifolia ethanol extract; OFT: open field test; EPM: elevated plus maze; FST: forced swimming test; IAT: inhibitory avoidance test; NOR: novel object recognition; STZ: streptozotocin.
To assess possible treatment effects on locomotion, mice were placed in a 50 cm × 50 cm × 39 cm open field divided into nine squares. The test was conducted 60 min after EETD (0.1, 1.0, or 3.0 mg/kg, PO) or vehicle administration and 30 min after rivastigmine (0.6 mg/kg, IP). The number of line crossings was recorded during 6 min [20–22].
To assess anxiety-related behavior, mice were subjected to the elevated plus maze (EPM) test 60 min after administration of EETD (0.1, 1.0, or 3.0 mg/kg) or vehicle administered PO, and 30 min after rivastigmine (0.6 mg/kg, IP). The apparatus had two open and two closed arms, elevated 70 cm from the floor. Each animal was placed in the center, and total arm entries and time spent in each arm were recorded over 5 min [18].
The forced swimming test (FST) was chosen to evaluate behavioral parameters of depression. It is a model developed by Porsolt and collaborators first in rats and later adapted for mice and adapted to our laboratory by Tolardo et al. (2010) [23]. In the test, the animals received the treatments and were subsequently transferred individually to a glass tank (46 cm high × 20 cm diameter) containing 20 cm of water at 24 ± 1°C at a controlled temperature (24 ± 1°C) and forced to swim for a period of 6 min. The behavioral parameter observed was the immobility time. Each animal was considered to be in a state of either floating or immobility when it produced minimal movements necessary to keep its head above water, preventing it from sinking. The test was conducted 60 min after EETD (0.1, 1.0, or 3.0 mg/kg, PO) or vehicle administration, and 30 min after rivastigmine (0.6 mg/kg, IP).
The novel object recognition (NOR) test has been widely used to evaluate the effect of substances on the special memory of animals, especially mice [24, 25]. The experiment was conducted in 3 stages, each lasting 10 min. In the first stage (day 1), the animals were placed in an open field where they remained freely exploring the apparatus. In the second stage (24 h after the first), two objects (A and B) that were completely identical (color and size) were introduced into the apparatus, positioned equilaterally to each other, and the interaction time with each object was timed. In the third stage (24 h after the second), object B was removed and replaced by a new object (different in color and shape to object B, but of the same size), and the interaction time between the previously known object and the new object was timed [20, 24]. RI (%) = [(time spent on the new object/time spent on the already known object + time spent on the new object) × 100%]. It was considered a positive effect on memory results above 50%.
This test evaluates the aversive memory. The apparatus used was an automated box (Insight®) measuring 50 cm × 25 cm × 25 cm. The base included copper grid bars (1 mm in diameter, spaced 1 cm apart) and a wooden platform. The procedure consisted of two phases: training and testing. During training, each mouse was placed on the platform, and the time taken to step down was recorded. Upon stepping down, the animal received a 2-second foot shock (0.4 mA). After 24 h, the test session was conducted using the same setup, but without shocks. Treatments were administered immediately after the training session, and memory performance was evaluated based on the difference in step-down latency between the two sessions [20, 22, 24].
Once the behavioral assays were completed, the animals were euthanized with an overdose of deep anesthetic (as previously described), and the brains were collected and dissected to obtain the hippocampus. As is routinely done in our laboratories, the samples were homogenized in phosphate buffer (pH 7.4) in a dilution ratio of 1:3 (w/v) for immediate quantification of reduced glutathione (GSH) and malondialdehyde (MDA) levels.
For GSH evaluation [26], aliquots of tissue homogenate were deproteinized with 12.5% trichloroacetic acid and centrifuged at 900 × g for 15 min at 4°C. A 10 μL portion of the resulting supernatant was mixed with 280 μL of 0.4 M Tris-HCl buffer (pH 8.9) and 10 μL of 10 mM 5,5ʼ-dithiobis(2-nitrobenzoic acid) (DTNB). After 20 min, absorbance was measured at 405 nm, and GSH concentrations were determined by interpolation against a standard curve (1–10 μg/mL), with results expressed as μg/g [24]. Lipid peroxidation was measured by the MDA quantification. A 100 µL aliquot of the homogenate was mixed with 200 µL of 0.5% thiobarbituric acid in 20% trichloroacetic acid and incubated at 95°C for 60 min. After cooling to room temperature, samples were centrifuged at 3,000 × g for 10 min, and the absorbance of the supernatant was measured at 532 nm. MDA concentrations were calculated using a standard curve generated with known MDA concentrations and expressed as nmol/mg [27].
Moreover, the homogenates were centrifuged at 9,000 × g for 15 min, and the supernatants were used for biochemical analyses of superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST), glutathione reductase (GR) and glutathione peroxidase (GPx). For SOD activity measurement, analysis was conducted as described previously [28] with some modifications [24] where the supernatant obtained from homogenized samples was incubated with pyrogallol (1 mM) in a buffer system containing Tris-HCl (1 mM) and EDTA (5 mM), adjusted to pH 8.5. After 20 min of incubation, the reaction was stopped by the addition of hydrochloric acid (1 M), and absorbance was recorded at 405 nm. Enzymatic activity was defined as the amount of SOD required to suppress 50% of pyrogallol auto-oxidation compared to control samples, and results were expressed as mmol·min–1·mg–1.
For the CAT assay, performance was conducted in accordance with Aebi [29] with adaptations [30]. For the assay, aliquots of the supernatant were added to a reaction medium consisting of Tris-HCl–EDTA buffer (200 mM, pH 8.5) supplemented with 20 mM hydrogen peroxide, once this activity is determined as the enzymatic capacity to degrade 1 μmol of hydrogen peroxide per minute under standard conditions (25°C, pH 7.0) [29]. Enzymatic activity was quantified by spectrophotometric analysis at 240 nm, and values were expressed as μmol·min–1·mg–1.
The GST assay was performed according to Habig et al. (1974) [31] with adaptations [30] using supernatant aliquots in a reaction mixture containing 1 mM 1-chloro-2,4-dinitrobenzene (CDNB), 1 mM GSH, and 100 mM potassium phosphate buffer (pH 6.5). The conjugation of CDNB with GSH was monitored spectrophotometrically at 340 nm over a 90-second interval. Enzymatic activity was calculated based on the molar extinction coefficient of GSH (9.6 mM–1·cm–1) and expressed as mmol·min–1·mg–1.
GR activity was measured by monitoring the NADPH-dependent reduction of oxidized glutathione (GSSG), as described by Carlberg and Mannervik [32]. Briefly, assays were performed in a phosphate buffer (0.1–0.2 M, pH 7.0) containing 2 mM EDTA, with final reaction concentrations of 0.1 mM NADPH and 1.0 mM GSSG at 25°C. Sample aliquots were added to pre-equilibrated reaction buffer, and the enzymatic reaction was initiated by GSSG addition. The decrease in absorbance at 340 nm, corresponding to NADPH oxidation, was recorded continuously for 1–3 min. Enzyme activity was calculated using the molar extinction coefficient of NADPH (6.22 mM–1·cm–1) and normalized to protein content. GR activity was determined by measuring the rate of NADPH oxidation and expressed as μmol·min–1·mg–1.
GPx activity was assessed in a 96-well plate by monitoring NADPH oxidation at 340 nm for 1 min (10-second intervals) in a reaction mixture containing sodium azide, β-NADPH, yeast GR, and GSH in sodium phosphate buffer (pH 7.0). The reaction was initiated by the addition of hydrogen peroxide, and results were expressed as nmol·min–1·mg–1. The analysis was performed as described previously [33] with adaptations [34]. For all the measurements, protein concentrations were determined using the Bradford method [35].
Finally, nitrite production was determined by the Griess reaction [36]. After centrifugation of hippocampal homogenates, 50 µL of the supernatant was incubated with 100 µL of Griess reagent at room temperature for 10 min. Absorbance was measured at 525 nm using a microplate reader, and nitrite concentrations were determined from a standard curve (0–100 µM) and were expressed as µmol/g.
Tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) levels were measured from hippocampal tissue homogenates previously prepared as previously described. For this purpose, mouse ELISA kits purchased from RayBiotech Inc. (Norcross, GA, USA) and R&D Systems Inc. (Minneapolis, USA), respectively, were used. The procedures were performed according to the manufacturer’s instructions and the results were presented as pg/mg protein for TNF-α and IL-6.
AChE activity was measured according to the method of Ellman [37] and standardized in our laboratories by Cazarin et al. (2021) [24]. Approximately 900 µL of 24 mM K-phosphate buffer (pH 7.2), 50 µL of 1 mM 5,5’-dithio-bis-(2-nitrobenzoic acid) (DTNB), 50 µL of supernatant solution S1 (0.2–0.4 mg protein) and different concentrations of TXR (0.45, 0.89, and 1.77 µM) were contained in the assay medium. To start the reaction, 25 µL of 0.8 mM ACh was added and monitored at 412 nm for 4 min at 25°C in a spectrophotometer. AChE activity was expressed in µmol of ACh hydrolyzed/h/mg protein [24].
The procedures for cell culture were adapted from the previously reported protocols by Lee et al. (2023) [38] and Kim et al. (2022) [39]. The BV2 (murine microglial), HT22 (murine hippocampal neuronal), and [Chinese hamster ovary (CHO) cells stably expressing amyloid precursor protein (APP)] APP-CHO cell lines were utilized as described in the original studies [38, 39]. BV2 and HT22 cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. In contrast, APP-CHO cells were cultured in RPMI-1640 (cell culture medium commonly used to grow mammalian cells) containing 10% heat-inactivated FBS and 1 μg/mL geneticin. All cell lines were incubated at 37°C in a humidified incubator with 5% CO2.
To evaluate cell viability, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was conducted. BV2 cells were plated at a density of 2.0 × 104 cells per well in 96-well plates and allowed to adhere for 3 h. HT22 cells were seeded at 1.0 × 104 cells/well and incubated for 24 h, while APP-CHO cells were plated at 2.0 × 104 cells/well and cultured overnight. After the initial incubation, the cells were serum-deprived for 1 h and subsequently treated with test compounds dissolved in dimethyl sulfoxide (DMSO). Following 24 h of treatment, 10 μL of MTT solution (5 mg/mL) was added to each well, and the plates were incubated at 37°C for an additional 3 h. The supernatant was then discarded, and the resulting formazan crystals were dissolved in 100 μL of DMSO. Absorbance was measured at 540 nm using a BioTek Epoch microplate reader (BioTek Instruments). Each experiment was carried out in triplicate as per the reference protocol [39].
For the quantification of NO production, BV2 cells were seeded into 24-well plates at a concentration of 2.0 × 105 cells/well and cultured for 24 h. Following incubation, the culture medium was replaced with serum-free DMEM containing test samples (prepared in DMSO). After 1 h of pre-treatment, lipopolysaccharide (LPS) was added at a final concentration of 1 μg/mL, and the cells were incubated for an additional 24 h at 37°C. Subsequently, 100 μL of the culture supernatant from each well was transferred to a 96-well plate and mixed with an equal volume of Griess reagent. The mixture was incubated in the dark at room temperature for 30 min, and the absorbance was measured. All assays were performed in triplicate, following the previously established method [39].
APP-CHO cells were seeded in 6-well plates at a density of 6.0 × 105 cells/well and incubated overnight. After serum starvation for 1 h, cells were treated with DMSO-diluted test samples and incubated for 24 h at 37°C. Cells were then rinsed with PBS, lysed in Laemmli buffer, and boiled at 100°C for 10 min. Proteins were separated by 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF) membranes, and blocked with 5% skim milk in PBS for 1 h. Membranes were incubated overnight at 4°C with primary antibodies: APP/β-amyloid (1:1,000, Cell Signaling Technology), BACE1 (1:1,000, EMD Millipore), and α-tubulin (1:25,000, Sigma-Aldrich). After PBS-T washes (3 × 15 min), membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:2,500, Bio-Rad). Protein bands were visualized using ECL (Western Blotting Substrate) reagent (Menlo Park, CA, USA) and detected using the ChemiDoc™ XRS+ (Bio-Rad) [38].
HT22 cells were seeded in 6-well plates at a density of 6.0 × 105 cells/well in 1,000 μL of medium and incubated for 24 h. The medium was then replaced with serum-free DMEM, and DMSO-diluted test samples were added. After 1 h, okadaic acid (80 nM) was introduced, followed by 16 h incubation at 37°C. Cells were then rinsed with PBS and lysed in Laemmli sample buffer. Cell lysates were boiled for 15 min at 100°C before analysis by western blotting. For the western blot analysis, proteins were separated via 7.5% SDS-PAGE, transferred to PVDF membranes (Bio-Rad, Hercules, CA, USA), and blocked with 5% bovine serum albumin in TBS for 2 h. Membranes were incubated overnight at 4°C with primary antibodies including AT8 (1:1,000, Thermo Fisher Scientific, Middlesex, MA, USA) and α-tubulin (1:1,000, Santa Cruz Biotechnology, Dallas, TX, USA). After TBST washes (3× for 15 min each), membranes were incubated with HRP-conjugated secondary antibodies (1:1,000, Thermo Fisher Scientific) for 1 h at room temperature. ECL detection reagent (ATTA corporation, Tokyo, Japan) was used for visualization, and protein bands were detected using a ChemiDoc™ XRS+ (Bio-Rad). The procedure was followed as previously described [40].
The parametric results were expressed as mean ± standard deviation (SD) and statistical significance was obtained by one or two-way analysis of variance (ANOVA), followed by Tukey’s test, when applicable. Kruskal-Wallis test followed by Dunn’s test was used to evaluate non-parametric results, which were expressed as median ± interquartile ranges. The Kolmogorov-Smirnov normality test was applied to verify the data’s normality. Moreover, power analysis was performed to determine all sample sizes. Differences were significant when P < 0.05, by using the GraphPad Prism version 5.00 for Windows (GraphPad Software, California, USA) program. To complement the inferential statistical tests, analyses of effect sizes and statistical power were conducted using Microsoft Excel 2016 (Microsoft Corp., Redmond, WA, USA). Specifically, Cohen’s d was calculated to quantify the magnitude of differences between two means in pairwise comparisons, while Cohen’s f was employed to assess the effect size for analyses involving more than two groups, such as in ANOVA models.
The effects of treatments on locomotor performance, behavioral parameters of anxiety and depression in animals with STZ-induced AD are shown in Figure 2. I.C.V. STZ promoted a significant decrease at open arms entries (P < 0.001, Cohen’s d = –2.59) (Figure 2A) and exploration time (P < 0.01, Cohen’s d = –2.82) (Figure 2B) compared to sham group and mice treated with EETD at all doses used, had a significant increase in the frequency (P < 0.001, Cohen’s d = 2.48; P < 0.001, Cohen’s d = 5.99 and P < 0.001, Cohen’s d = 6.71 respectively for 0.1, 1.0 and 3.0 mg/kg of EETD) and time of permanence (P < 0.001, Cohen’s d = 9.83; P < 0.001, Cohen’s d = 5.19 and P < 0.001, Cohen’s d = 6.89 respectively for 0.1, 1.0 and 3.0 mg/kg) in the open arms when subjected to EPM suggesting anxiolytic-like effect (Figure 2A and B) when compared to the vehicle group. Furthermore, the treatments did not change the locomotor performance (Figure 2C) of the mice, according to the results obtained in the open field test experiment. Besides, the results showed that the EETD has an antidepressant-like effect, which was evidenced by the decrease in the immobility time of the animals when submitted to the FST (Figure 2D) compared to the vehicle group (P < 0.001, Cohen’s d = –1.94; P < 0.001, Cohen’s d = –2.54 and P < 0.0001, Cohen’s d = –4.57 respectively for 0.1, 1.0 and 3.0 mg/kg). Rivastigmine at the dose used also significantly promoted anxiolytic and antidepressant-like effects when compared to the vehicle group (P < 0.001 and P < 0.0001, Cohen’s d = 13.81 and Cohen’s d = –3.90, respectively).
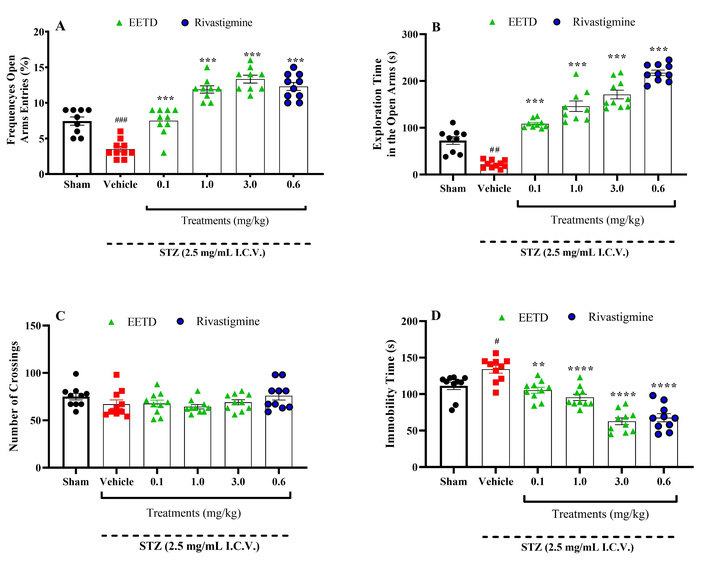
Behavioral effects of EETD 0.1, 1.0, or 3.0 mg/kg treatments on anxiety- and depression-like parameters and locomotory activity of I.C.V. STZ-induced AD in mice. (A) Frequency of entries into open arms; (B) time to explore in open arms; (C) number of crossings; (D) immobility time. Effect of EETD [0.1, 1.0, or 3.0 mg/kg, administered orally (PO)] or rivastigmine [0.6 mg/kg, intraperitoneally (IP)] in mice submitted to STZ 2.5 mg/mL intracerebroventricular (I.C.V.) injection. Behavior parameters assessed on the Elevated Plus Maze (EPM) are shown in Figure 2A and B (open arms frequency entries and open arms time exploration, respectively); open field test (OFT) in Figure 2C (number of crossings) and forced swimming test (FST) in Figure 2D (immobility time). Results are presented by mean ± SD, n = 10 per group. Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test. **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared to the vehicle group. #P < 0.05, ##P < 0.01, and ###P < 0.001 compared to the sham group (animals that received no I.C.V. STZ administration). EETD: Tithonia diversifolia ethanol extracts; STZ: streptozotocin.
The findings indicate that administering STZ (2.5 mg/mL, I.C.V.) caused a decline in cognitive function in the animals, evidenced by the vehicle group’s consistent descent latency during both training and testing sessions (Figure 3A). However, groups with STZ-induced AD that received EETD treatment at doses of 1.0 and 3.0 mg/kg (the highest doses used) exhibited a statistically significant improvement in cognitive deficits (P < 0.001 for both doses, Cohen’s d = 7.24 and 6.13, respectively) (Figure 3A and B). This indicates that EETD increased the time it took for animals to descend from the platform in the inhibitory avoidance test (IAT), suggesting a beneficial effect on memory compared to the vehicle group. Additionally, rivastigmine (0.6 mg/kg, IP) significantly enhanced memory in animals with AD (P < 0.001, Cohen’s d = 13.03) when compared to the vehicle group, as expected.
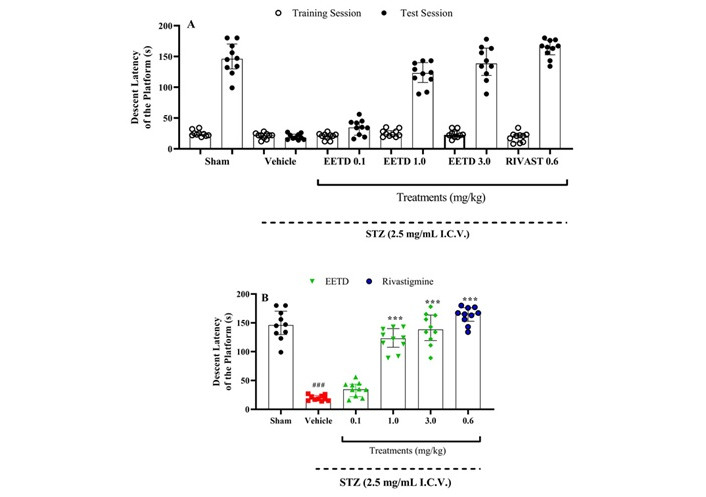
Behavioral effects of EETD 0.1, 1.0, or 3.0 mg/kg treatments on aversive memory of I.C.V. STZ-induced AD mice submitted to inhibitory avoidance test (IAT). (A) Results from training and testing sessions, respectively; (B) results refer to the test session only. Behavior evaluation on IAT. In panel A, the first column of each treatment represents the training session and the second column represents the testing session. Panel B shows differences among groups for step-down latency in the test session. In each column, data are expressed as medians followed by interquartile ranges (25–75). Data were submitted to analysis of variance (ANOVA) with Dunn’s test and showed ***P < 0.001 compared to the vehicle group. ###P < 0.001 compared to sham group (animals that received no I.C.V. STZ administration) for the average descent index (Mann-Whitney test). n = 10 per group. Cohen’s f = 3.09. EETD: Tithonia diversifolia ethanol extract; RIVAST: rivastigmine; STZ: streptozotocin.
Figure 4 shows the results related to NOR. In panel A, it can be observed that the group that received STZ and was treated with vehicle had a significant reduction (P < 0.05) in the time spent exploring the new object (B) when compared to the sham group. However, animals treated with EETD (1.0 and 3.0 mg/kg) as well as with rivastigmine had a longer exploration time on the new object (P < 0.05 and P < 0.001, P < 0.01, respectively, compared to the vehicle (Figure 4A). The beneficial effects of treatments with EETD and rivastigmine are best observed in panel B, which represents the recognition index of the new object. The results demonstrate that the administration of STZ resulted in a decline in the cognitive function of the animals, as demonstrated in the vehicle group (P < 0.001, Cohen’s d = –4.85 compared to the sham group), where the old object that was already familiar to them was not recognized. However, in the groups with AD induced by STZ and treated with EETD at doses of 1.0 and 3.0 mg/kg, it was possible to observe a reduction in the cognitive deficit, through an increase in the recognition index of the new object in a statistically significant way when compared to the vehicle group (P < 0.001, Cohen’s d = 5.55 and 5.77, respectively).
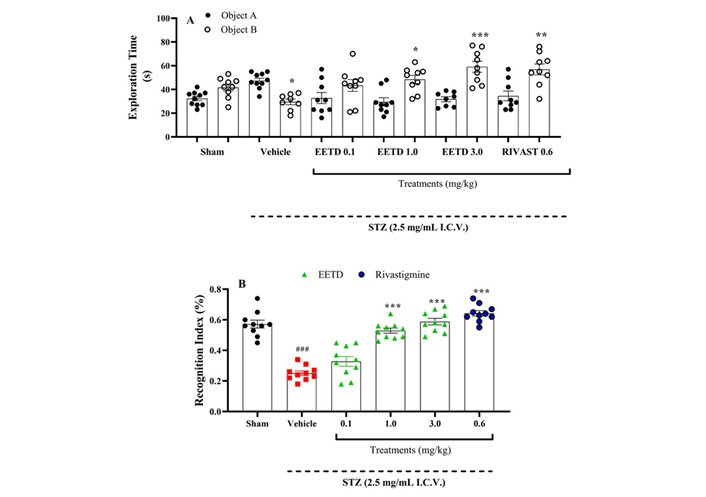
Behavior evaluation of EETD 0.1, 1.0, or 3.0 mg/kg treatments on recognition index of I.C.V. STZ-induced AD in mice submitted to novel object recognition (NOR). (A) Exploration time of objects A and B; (B) recognition index. Effect of EETD [0.1, 1.0, or 3.0 mg/kg, administered orally (PO)] or rivastigmine [0.6 mg/kg, intraperitoneally (IP)] in mice submitted to STZ 2.5 mg/mL intracerebroventricular (I.C.V.) injection. Behavior evaluation on NOR demonstrates the differences in exploration time among familiar and new objects in Figure 4A. Figure 4B shows the recognition index for groups. Results are represented by mean ± SD, n = 10 per group. Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to vehicle group. ###P < 0.001 compared to sham group (animals that received no I.C.V. STZ administration). Cohen’s f = 2.17, indicating a substantial effect size. EETD: Tithonia diversifolia ethanol extract; STZ: streptozotocin.
The results are shown in Figure 5, where it is demonstrated that I.C.V. STZ injection indicates a decrease in CAT (Figure 5A), SOD (Figure 5B), GST (Figure 5C), GR (Figure 5D), GPx (Figure 5E) and GSH (Figure 5F) compared to sham group (P < 0.0001). The 3.0 mg/kg EETD treatment highly significantly elevated levels of GSH (P < 0.0001, Cohen’s d = 2.87), increased CAT (P < 0.05, Cohen’s d = 2.50), GPx (P < 0.01, Cohen’s d = 1.91) and SOD activity (P < 0.001, Cohen’s d = 4.54) compared to the vehicle-treated group. It was also observed in the experiments that the treatment of animals with rivastigmine promoted a significant increase (P < 0.01 or P < 0.0001) in these markers (except for GSH) compared to the vehicle group. Additionally, the results also showed that STZ was able to significantly increase the levels of MDA (P < 0.0001, Cohen’s d = 40.45) (Figure 6A) and nitrite (P < 0.01, Cohen’s d = 2.02) (Figure 6B) in the brain of the animals. Interestingly, the treatment with EETD and rivastigmine were able to decrease MDA and nitrite levels induced by STZ (P < 0.0001, Cohen’s d = –11.30 and Cohen’s d = –19.78 for MDA; P < 0.05, Cohen’s d = –1.94 and P < 0.01, Cohen’s d = –2.90 for nitrite; EETD and rivastigmine, respectively).
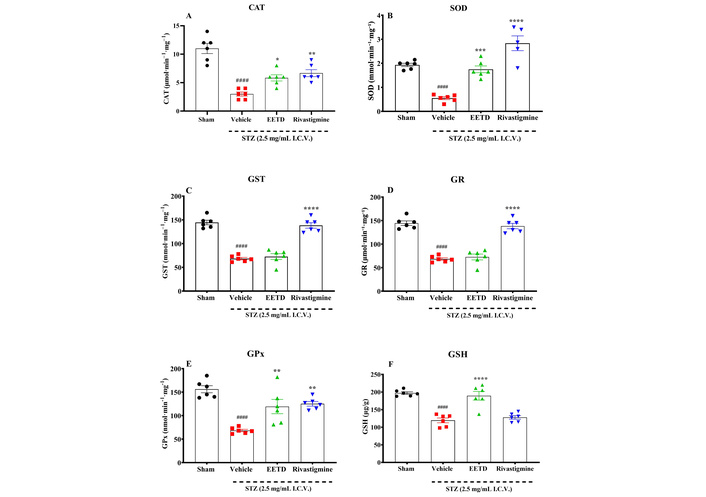
Antioxidant effects of EETD 3.0 mg/kg treatment in the hippocampus of mice submitted to I.C.V. STZ-induced AD. (A) CAT activity evaluation; (B) SOD activity; (C) GST evaluation; (D) GR levels; (E) GPx evaluation; (F) GSH levels. Effect of 3.0 mg/kg EETD treatment administered orally (PO) or 0.6 mg/kg rivastigmine intraperitoneally (IP) in the hippocampus of mice submitted to intracerebroventricular (I.C.V.) STZ 2.5 mg/mL injection. Results are represented by mean ± SD, n = 6 per group. Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 compared to vehicle group. ####P < 0.0001 compared to sham group (animals that received no I.C.V. STZ administration). CAT: catalase; SOD: superoxide dismutase; GST: glutathione S-transferase; GR: glutathione reductase; GPx: glutathione peroxidase; GSH: reduced glutathione; EETD: Tithonia diversifolia ethanol extract; STZ: streptozotocin.
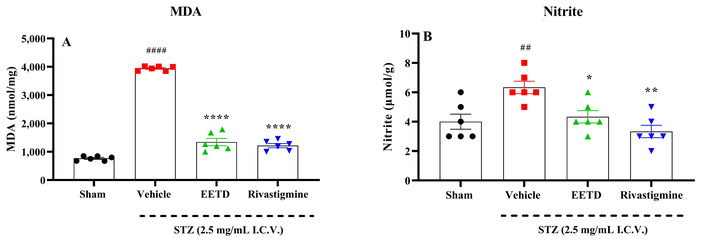
Lipidic peroxidation and nitrite levels in the hippocampus of mice submitted to I.C.V. STZ-induced AD and administered with 3.0 mg/kg EETD. (A) Lipid peroxidation; (B) nitrite levels. Effect of 3.0 mg/kg EETD treatment administered orally (PO) or 0.6 mg/kg rivastigmine intraperitoneally (IP) in the hippocampus of mice submitted to intracerebroventricular (I.C.V.) STZ 2.5 mg/mL injection. Panels A and B show, respectively, the modulation of lipid peroxidation and nitrite levels. Results are represented by mean ± SD, n = 6 per group. Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05, **P < 0.01, and ****P < 0.0001 compared to vehicle group. ##P < 0.01, ###P < 0.001, and ####P < 0.0001 compared to sham group (animals that received no I.C.V. STZ administration). MDA: malondialdehyde; EETD: Tithonia diversifolia ethanol extract; STZ: streptozotocin; AD: Alzheimer’s disease.
The results presented in Table 1 show that AChE activity was significantly reduced in animals treated with EETD at doses of 1.0 mg/kg (P < 0.05) and 3.0 mg/kg (P < 0.001), as well as in those treated with rivastigmine 0.6 mg/kg (P < 0.0001), compared to the group that received STZ and vehicle control.
Effect of EETD on AChE activity.
| Treatments (mg/kg) | AChE activity(µmol hydrolyzed ACh/h/mg protein) |
|---|---|
| Vehicle | 620.5 ± 2.45 |
| EETD 1.0 | 483.8 ± 1.85* |
| EETD 3.0 | 374.3 ± 2.17*** |
| Rivastigmine 0.6 | 320.0 ± 1.89**** |
Results are represented by mean ± SD, n = 3 per group. Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05, ***P < 0.001, and ****P < 0.0001 compared to vehicle group. EETD: Tithonia diversifolia ethanol extract; ACh: acetylcholine; AChE: acetylcholinesterase.
The results presented in Table 2 demonstrate that the increased hippocampal levels of cytokines such as TNF-α and IL-6 by STZ administration were significantly reduced by treatments with EETD and rivastigmine (P < 0.001, P < 0.0001).
Effect of treatments on TNF-α and IL-6 in the hippocampus of animals induced by STZ.
| Treatments(mg/kg) | TNF-α(pg/mg protein) | IL-6(pg/mg protein) |
|---|---|---|
| Sham | 6.78 ± 1.40 | 5.82 ± 0.97 |
| Vehicle | 32.00 ± 2.18### | 22.00 ± 1.38### |
| EETD 1.0 | 28.00 ± 1.85 | 23.00 ± 2.18 |
| EETD 3.0 | 15.30 ± 2.17**** | 12.32 ± 2.70*** |
| Rivastigmine 0.6 | 12.00 ± 2.19**** | 8.00 ± 2.19*** |
Effects of EETD 1.0 or 3.0 mg/kg administered orally (PO) and rivastigmine treatment (0.6 mg/kg, IP) on TNF-α and IL-6 in the hippocampus of mice submitted to STZ 2.5 mg/mL I.C.V. injection. Results are represented by mean ± SD, n = 10 per group. Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test. ***P < 0.001, and ****P < 0.0001 compared to vehicle group. ###P < 0.001 compared to sham group (animals that received no I.C.V. STZ administration). TNF-α: tumor necrosis factor alpha; IL-6: interleukin-6; EETD: Tithonia diversifolia ethanol extract; STZ: streptozotocin.
The effect of EETD on neuroinflammation was assessed by measuring LPS-induced NO production in BV2 cells. Treatment with the extract at concentrations exceeding 12.5 μg/mL reduced BV2 cell viability to below 80% compared to the DMSO-treated control group, indicating cytotoxicity, as shown in Figure 7A. Therefore, non-cytotoxic concentrations below 6.25 μg/mL were used for further analysis. The effect of the extract on NO production was evaluated using the Griess reagent. As shown in Figure 7B, EETD significantly reduced LPS-induced NO production in a dose-dependent manner compared to the LPS-treated group.
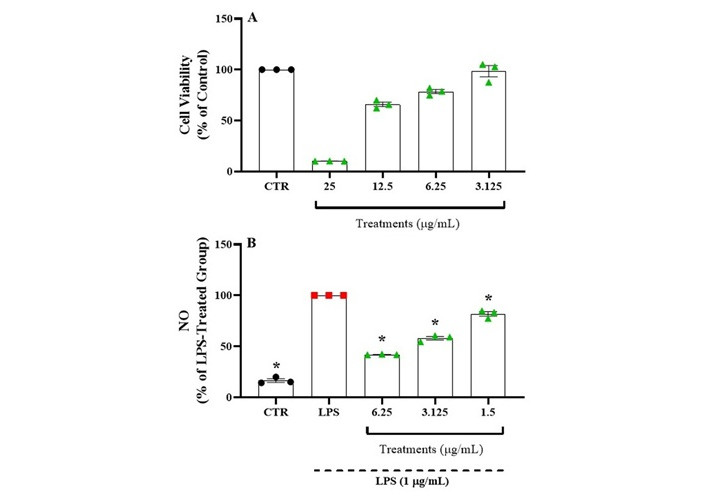
Effect of EETD on neuroinflammation in BV2 cells. (A) Cell viability; (B) NO production. In panel A, the previous results for cell viability and choice of treatment doses with the extract are shown. In panel B, the results related to NO production are shown. Results are expressed as the mean ± SD. Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05 compared to the LPS group. CTR: control; LPS: lipopolysaccharide; NO: nitric oxide; EETD: Tithonia diversifolia ethanol extract.
The impact of EETD on tau hyperphosphorylation induced by okadaic acid was assessed via western blot analysis using the AT8 antibody, which detects phosphorylated Tau at Ser202 and Thr205. The viability of HT22 cells remained unaffected by extract treatment at concentrations up to 12.5 μg/mL.
Treatment with okadaic acid significantly increased tau hyperphosphorylation, detected by the AT8 antibody. However, treatment with EETD did not alter tau hyperphosphorylation levels (Figure 8).
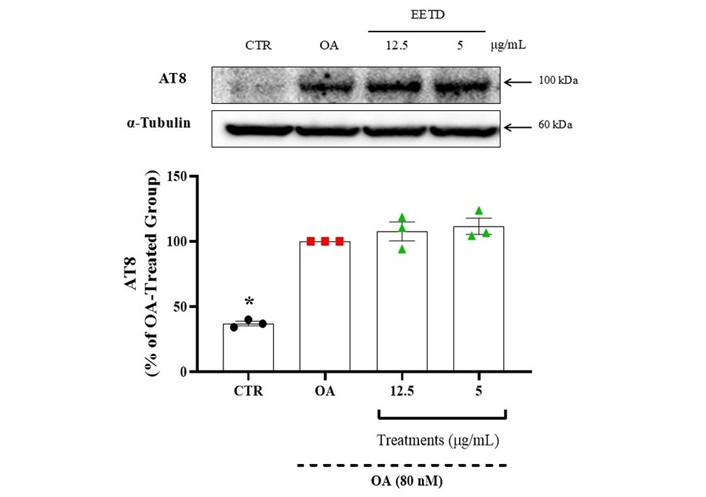
Effect of EETD on tau hyperphosphorylation in HT22 cells. Results are expressed as the mean ± SD. Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05 compared to the okadaic acid group. CTR: control; OA: okadaic acid; EETD: Tithonia diversifolia ethanol extract.
The effect of EETD on Aβ production was evaluated by measuring soluble amyloid precursor protein beta (sAPPβ), a fragment generated by β-secretase cleavage of APP in APP-CHO cells. Extract concentrations up to 10 μg/mL did not affect cell viability, indicating an absence of cytotoxicity.
To assess Aβ production, western blot analysis was performed. As shown in Figure 9, EETD had minor effects on Aβ production, though the changes were not statistically significant.
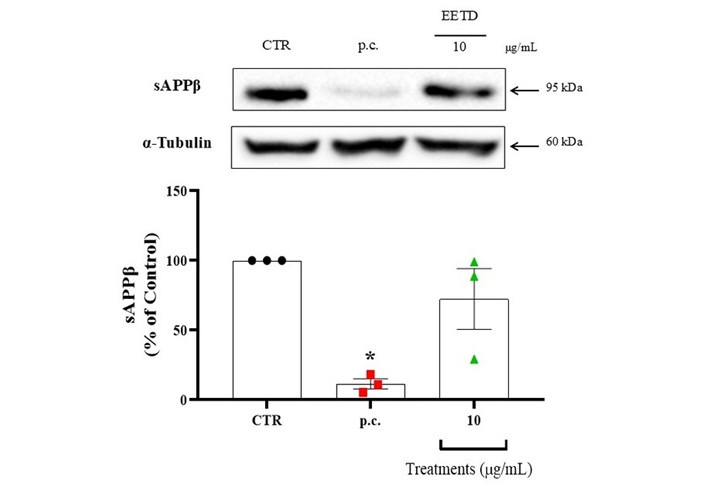
Effect of EETD on Aβ production. Effect of EETD on Aβ production in APP-CHO cells. Results are expressed as the mean ± SD. Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05 compared to the control group. CTR: control; p.c.: positive control; EETD: Tithonia diversifolia ethanol extract; sAPPβ: soluble amyloid precursor protein beta.
This study evaluated the pharmacological effects of EETD at doses of 0.1, 1.0, and 3.0 mg/kg in I.C.V. STZ injections as an AD model. The I.C.V. STZ has been associated with decreased glucose utilization and, as a consequence, produces several brain metabolic and behavioral disturbances [41], leading to neuron death. In this way, it is proposed that the I.C.V. STZ model can be recognized as a preclinical model of AD [18–22, 41] and for developing therapeutic strategies for its treatment [42]. One of the major research questions in the field of AD is the identification and development of novel therapeutic agents. A review article by Zheng and Wang (2025) [43] provides an overview of recent advances in drug development for AD. Despite significant progress in understanding the underlying pathophysiology of AD, only aducanumab has been approved by the Food and Drug Administration (FDA) for the treatment of AD since 2003 [44]. Several neurotransmission systems are involved in cognitive processes, but when it comes to AD, the central focus is the cholinergic system, on which current AD treatment is based. ACh plays an essential role in cognition, and impaired cortical cholinergic neurotransmission causes cognitive impairment [45]. Although current therapeutic options are based on pharmacodynamic mechanisms that cause ACh levels to be established by AChE inhibition, the effects of these drugs are palliative and the adverse effects catastrophic, leading to non-adherence to therapy [46]. This highlights the need for new therapeutic strategies to combat this centuries-old disease, not only evaluating effects on key targets in the pathogenesis of the disease but also reducing its comorbidities. To increase therapeutic strategies for complex diseases such as AD, plants with therapeutic potential have come onto the scene. T. diversifolia has attracted significant attention in pharmacological research due to its potential medicinal properties, especially anti-inflammatory and antioxidant response [16, 17]. Despite only one work in literature presenting the potential of T. diversifolia as a promising tool for AD, the brilliant article brings the potential inhibitory activity against AChE and BuChE in a concentration-dependent manner [47]. Our results showed that 1.0 and 3.0 mg/kg had the potential inhibitory activity against AChE activity, corroborating results previously cited, probably attributed such activity to due presence of abundant phenolic compounds already demonstrated in analysis of HPLC-DAD [17, 47].
Memory loss is among the first symptoms reported by patients suffering from AD and their caregivers. Working memory, spatial memory, and short- and long-term declarative memories are the first types affected early during the disease, but other types of memories suffer the effects of the neurodegeneration process [48]. Our studies demonstrated that treatment of animals with EETD significantly reduced the deleterious effects of STZ when evaluated in two classic memory models, the IAT and the object recognition test, which evaluate aversive memory and spatial memory and are widely used in studies with substances with anti-Alzheimer potential [49].
In AD, cognitive impairment and behavioral changes are not the only symptoms described in patients. Anxiety and depression are common symptoms that are associated with the progression of AD [2], and new pharmacological therapies that can alleviate the comorbidities associated with AD would be welcome in terms of improving the quality of life of patients and their families. Thus, in the current research for pharmacological targets, behavioral tests such as EPM (to evaluate anxiety parameters) and FST (to evaluate depression parameters) are commonly used, respectively, in preclinical trials in research with anxiolytics [50] and antidepressants [51]. The results presented in this work showed an interesting effect of EETD 0.1, 1.0, and 3.0 mg/kg on the anxiogenic and depressive effects promoted by the injection of STZ, suggesting that in addition to the anti-Alzheimer’s potential, the plant exhibits anxiolytic and antidepressant-type effects.
The aim of this study was also to investigate possible mechanisms of action of the anti-Alzheimer’s effect of EETD. With this objective, the potential neuroprotective effects of EETD were also investigated via in vitro assays by examining their impact on neuroinflammation, tau hyperphosphorylation, and Aβ production, three key pathological processes implicated in neurodegenerative diseases, particularly AD [52–54]. These three pathological processes are generally present in experimental Alzheimer’s models, such as the STZ model and other more modern ones, such as the Alzheimer’s ischemic model, largely based on the consequences of ischemia observed in the brain and hippocampus of patients.
Our results demonstrated that EETD significantly reduced LPS-induced NO production in BV2 microglial cells at non-cytotoxic concentrations (< 6.25 μg/mL). Since excessive NO production is a hallmark of neuroinflammation and is associated with microglial activation in neurodegenerative conditions [52], this finding suggests that EETD may possess anti-inflammatory properties that could mitigate neuroinflammation-driven neuronal damage. The dose-dependent suppression of NO production further supports the potential of EETD as a modulator of microglial activity. Several natural compounds, such as flavonoids and polyphenols, have been reported to exhibit similar anti-neuroinflammatory effects by inhibiting inflammatory pathways like NF-κB and MAPK [54, 55]. Future studies should explore whether EETD influences these pathways to suppress NO production.
In contrast, EETD did not affect tau hyperphosphorylation in HT22 cells treated with okadaic acid, a well-established phosphatase inhibitor that induces tau hyperphosphorylation [56, 57]. The AT8 antibody, which detects phosphorylation at Ser202 and Thr205, confirmed that tau hyperphosphorylation was significantly increased by okadaic acid treatment. However, EETD failed to prevent or reduce this pathological modification. This result suggests that, while EETD may have anti-inflammatory potential, it does not directly modulate tau phosphorylation. Given the complex regulatory mechanisms governing tau pathology, EETD may lack activity against kinases such as glycogen synthase kinase-3 beta (GSK-3β) or cyclin-dependent kinase 5 (CDK5), which are known to drive tau hyperphosphorylation [57, 58]. Alternatively, longer exposure durations or different concentrations might be necessary to observe an effect. Some natural compounds, such as resveratrol and curcumin, have been reported to reduce tau hyperphosphorylation by modulating GSK-3β activity [59]; however, no such effect was observed with EETD in this study.
Regarding Aβ production, our data showed that EETD had only minor, statistically insignificant effects on sAPPβ levels in APP-CHO cells, suggesting that it does not strongly influence β-secretase activity or Aβ generation. Since Aβ accumulation is a central event in AD pathology, therapeutic strategies often target β- or γ-secretase to reduce Aβ production [60, 61]. The lack of a significant effect of EETD suggests that its neuroprotective properties, if any, are likely not mediated through direct modulation of Aβ processing. However, it remains to be determined whether EETD might influence Aβ clearance or aggregation, which are also critical aspects of AD pathology. Some plant-derived compounds, such as epigallocatechin gallate (EGCG), rutin, and quercetin, have been shown to promote Aβ clearance via autophagy pathways [62], and further research is needed to explore whether EETD might have similar effects.
In addition to drug development, the role of oxidative stress in synaptic dysfunction in AD is discussed in a review by Zhang et al. (2024) [63]. The review explores innovative therapeutic strategies based on understanding the complexity of molecular mechanisms involved in AD. It emphasizes the potential of targeting oxidative stress to improve synaptic function in AD. The present study showed the decrease of the main antioxidant brain defenses promoted by I.C.V. STZ and that the EETD 3.0 mg/kg reversed this effect, suggesting that extract compounds may affect the ROS generation and depuration, improving the antioxidant defenses. Our results complement the findings on the antioxidant potential of the plant and corroborate several studies that investigate the antioxidant activities of T. diversifolia extracts [16, 17, 47].
Furthermore, as already reported, Ojo et al. (2022) [17] characterized the phenolic constituents of T. diversifolia leaves and assessed their antioxidant properties. The study revealed that T. diversifolia extracts are rich in phenolic compounds with antioxidant activity. These findings suggest that T. diversifolia extracts possess significant antioxidant potential, which is attributed to their phenolic constituents and can be considered a promising strategy for AD [64]. Besides, the authors also demonstrated that the free radical scavenging ability is better when compared to the vitamin C standard. In this context, these findings show that the strategy of the use of this plant could be driven by memory loss, as a big symptom of AD.
This study also evaluated the effects of EETD on two markers of neuroinflammation in the hippocampus of mice subjected to STZ infusion, TNF-α, and IL-6. In AD animal models, neuroinflammation is reported to occur alongside and be associated with pathological Aβ deposition [65]. Cytokines, produced under both normal and pathological conditions, are major contributors to neuroinflammation [66], modulating neural functions such as neurogenesis, synaptic plasticity, synaptic scaling, and long-term potentiation—all of which influence cognition [67]. Nearly all neuroinflammatory mechanisms involved in AD are influenced by cytokines [67, 68]. IL-6, while considered a pro-inflammatory cytokine, plays a crucial role in brain function regulation; at low levels, it supports processes like neurogenesis, hippocampal long-term potentiation, and neural plasticity [69, 70]. Likewise, TNF-α has dual roles depending on the physiological context: under normal conditions, it regulates neuronal activity, but in pathological states, it exerts cytotoxic effects and may trigger cell death, including apoptosis [71]. In the present study, treatment with EETD significantly reduced hippocampal levels of TNF-α and IL-6 in STZ-treated animals, indicating a cytoprotective role of the plant extract in modulating neuroinflammatory responses.
In the study of Alzheimer’s, it is true that animal models are extremely different from patients, both in terms of the mechanisms that trigger neuroinflammation, the amyloid cascade, the formation of tau-related neurofibrillary tangles, and the duration of the disease. Without dismissing the role of preclinical models, Granzotto and collaborators (2024) [72] critically discuss their limitations, emphasizing the need for careful consideration of how experiments are designed and results interpreted. Indeed, this need applies not only to Alzheimer’s but also to other diseases whose neuropharmacological mechanisms, as well as the search for possible therapeutic targets, use animal models.
In summary, within the limitations of the model used, the results together demonstrate the neuroprotective capacity of T. diversifolia against STZ-induced oxidative stress, as well as against cognitive loss in animals when evaluated in memory tests. The results also demonstrate that the plant exhibits antidepressant, anticholinesterase, and anxiolytic effects. Associated with its ability to reduce pro-inflammatory cytokines and partially inhibit tau hyperphosphorylation and Aβ deposition, the results together suggest that the plant exhibits therapeutic relevance in AD. However, studies are needed to identify the phytoconstituents responsible for such effects.
ACh: acetylcholine
AChE: acetylcholinesterase
AD: Alzheimer’s disease
ANOVA: analysis of variance
APP: amyloid precursor protein
Aβ: beta-amyloid
BuChE: butyrylcholinesterase
CAT: catalase
CEUA: Animal Use Ethics Committee
CHO: Chinese hamster ovary
CONCEA: Control of Animal Experimentation
DMEM: Dulbecco’s Modified Eagle’s Medium
DMSO: dimethyl sulfoxide
EETD: Tithonia diversifolia ethanol extract
EPM: elevated plus maze
FBS: fetal bovine serum
FST: forced swimming test
GPx: glutathione peroxidase
GSH: reduced glutathione
GSK-3β: glycogen synthase kinase-3 beta
GR: glutathione reductase
GST: glutathione S-transferase
HRP: horseradish peroxidase
I.C.V.: intracerebroventricular
IAT: inhibitory avoidance test
IL-6: interleukin-6
IP: intraperitoneally
LPS: lipopolysaccharide
MDA: malondialdehyde
MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
NO: nitric oxide
NOR: novel object recognition
PBS: phosphate buffered saline
PO: orally
PVDF: polyvinylidene difluoride
ROS: reactive oxygen species
sAPPβ: soluble amyloid precursor protein beta
SDS-PAGE: sodium dodecyl sulfate-polyacrylamide gel electrophoresis
SOD: superoxide dismutase
STZ: streptozotocin
TNF-α: tumor necrosis factor alpha
The authors would like to thank UNIVALI (Universidade do Vale do Itajaí) and Dankook University, Chungnam-Do for the support to the execution of this work. We would also like to thank Prof. Dr. Vivian de Mello Cionek for her statistical support.
GMG: Conceptualization, Methodology. CAC and VCF: Writing—original draft. JGB, HIE, ACdS, MEV, AM, MSK, CHL, and SYP: Methodology. APD and MH: Writing—review & editing. MMdS: Conceptualization, Supervision, Methodology, Writing—original draft, Data curation, Software, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The present study was approved by the CEUA/UNIVALI (Animal Use Ethics Committee/Universidade do Vale do Itajaí) with the experimental protocols approved with opinion number 022/17.
Not applicable.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
This study was financially supported by funding agencies such as CNPq (National Council for Scientific and Technological Development), CAPES (Coordination for the Improvement of Higher Education Personnel), and FAPESC (Santa Catarina Research Support Foundation) through the granting of master’s research scholarships to Graziella Martins Guimaraes [FAPESC-00000439/2024], Heloisa Immianovsky Eisendecker [CNPq-130633/2024], doctoral scholarships to Ana Caroline dos Santos [CAPES-206/2022], Ana Paula Dalmagro [CAPES/PROSUC-88887.147590/2017], Camila André Cazarin [CAPES/PROSUC-88887.647998/2021-00] and scientific productivity scholarship with bench fee to Angela Malheiros [CNPq-311059/2023-6], and Márcia Maria de Souza [CNPq-306355/2022-1]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1950
Download: 35
Times Cited: 0
Atif Salim Khatib ... Daniya Tasnim
Octavio García ... Jesús Antonio Villegas-Piña
