









Polycystic ovary syndrome (PCOS) is a common endocrine–metabolic condition that carries a higher cardiovascular risk than currently reflected by traditional screening tools. Emerging evidence suggests that resting tachycardia and autonomic dysfunction may serve as early, non-invasive indicators of cardiovascular dysregulation in this population. This review synthesizes current data on resting heart rate (RHR), heart rate variability (HRV), and direct autonomic markers in women with PCOS, drawing from human studies published between 2000 and 2025. Across 32 eligible studies, most reported increased sympathetic activity, reduced parasympathetic tone, elevated RHR, and impaired HRV patterns observed even in normal-weight or metabolically mild PCOS phenotypes. These alterations correlate with endothelial dysfunction, arterial stiffness, and subclinical atherosclerosis, underscoring their cardiovascular relevance. Mechanistic insights highlight the contributions of insulin resistance, hyperandrogenism, inflammation, adipokine imbalance, chemoreflex sensitization, and altered cortisol metabolism to autonomic disruption. Despite consistent findings, methodological variability in HRV protocols and inadequate adjustment for major confounders limit definitive interpretation. RHR, due to its simplicity and accessibility, including through wearable devices, holds promise as a supportive early risk signal; however, it should not be used in isolation. Future studies must adopt standardized autonomic measurements, including diverse cohorts, and evaluate whether modifying autonomic markers translates into improved cardiometabolic outcomes. Integrating RHR and HRV with metabolic and endocrine markers may enhance early cardiovascular risk stratification in women with PCOS.
Polycystic ovary syndrome (PCOS) is a common endocrine–metabolic condition that carries a higher cardiovascular risk than currently reflected by traditional screening tools. Emerging evidence suggests that resting tachycardia and autonomic dysfunction may serve as early, non-invasive indicators of cardiovascular dysregulation in this population. This review synthesizes current data on resting heart rate (RHR), heart rate variability (HRV), and direct autonomic markers in women with PCOS, drawing from human studies published between 2000 and 2025. Across 32 eligible studies, most reported increased sympathetic activity, reduced parasympathetic tone, elevated RHR, and impaired HRV patterns observed even in normal-weight or metabolically mild PCOS phenotypes. These alterations correlate with endothelial dysfunction, arterial stiffness, and subclinical atherosclerosis, underscoring their cardiovascular relevance. Mechanistic insights highlight the contributions of insulin resistance, hyperandrogenism, inflammation, adipokine imbalance, chemoreflex sensitization, and altered cortisol metabolism to autonomic disruption. Despite consistent findings, methodological variability in HRV protocols and inadequate adjustment for major confounders limit definitive interpretation. RHR, due to its simplicity and accessibility, including through wearable devices, holds promise as a supportive early risk signal; however, it should not be used in isolation. Future studies must adopt standardized autonomic measurements, including diverse cohorts, and evaluate whether modifying autonomic markers translates into improved cardiometabolic outcomes. Integrating RHR and HRV with metabolic and endocrine markers may enhance early cardiovascular risk stratification in women with PCOS.
DOI: https://doi.org/10.37349/ec.2026.101295

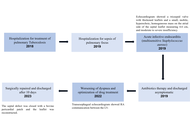
The Gerbode defect is characterized by a high ventricular septal defect associated with a defect in the septal leaflet of the tricuspid valve, allowing blood to enter the right atrium from the left ventricle. It accounts for approximately 0.08% of intracardiac shunts and may be congenital or acquired. We describe a rare case of Gerbode defect secondary to tricuspid valve endocarditis. A 58-year-old male patient presented with acute infective endocarditis due to Staphylococcus aureus, related to central venous access. Echocardiography showed a tricuspid valve with thickened leaflets and a small mobile image on the atrial side of the septal leaflet, as well as moderate to severe regurgitation. After completion of the antibiotic regimen with resolution of the infectious condition, the patient was discharged asymptomatic, and a new echocardiogram showed no vegetation on the tricuspid valve. During outpatient follow-up, he presented dyspnea on mild exertion, and consecutive echocardiograms showed moderate tricuspid insufficiency and significant pulmonary hypertension with a pulmonary artery systolic pressure of 83 mmHg (reference: 30 mmHg). He underwent right and left cardiac catheterization, which showed a Gerbode defect, and a transesophageal echocardiogram showed a shunt in the subaortic region measuring 6 to 8 mm, with a maximum gradient of 56 mmHg. He underwent elective surgery to correct the Gerbode defect and tricuspid valve repair, with a good clinical result. The Gerbode defect is rare, and the diagnosis can be challenging because it simulates other conditions. Treatment consists of closing the defect when it generates refractory symptoms or complications. The reported case was surgically corrected, with a good result and favorable evolution.
The Gerbode defect is characterized by a high ventricular septal defect associated with a defect in the septal leaflet of the tricuspid valve, allowing blood to enter the right atrium from the left ventricle. It accounts for approximately 0.08% of intracardiac shunts and may be congenital or acquired. We describe a rare case of Gerbode defect secondary to tricuspid valve endocarditis. A 58-year-old male patient presented with acute infective endocarditis due to Staphylococcus aureus, related to central venous access. Echocardiography showed a tricuspid valve with thickened leaflets and a small mobile image on the atrial side of the septal leaflet, as well as moderate to severe regurgitation. After completion of the antibiotic regimen with resolution of the infectious condition, the patient was discharged asymptomatic, and a new echocardiogram showed no vegetation on the tricuspid valve. During outpatient follow-up, he presented dyspnea on mild exertion, and consecutive echocardiograms showed moderate tricuspid insufficiency and significant pulmonary hypertension with a pulmonary artery systolic pressure of 83 mmHg (reference: 30 mmHg). He underwent right and left cardiac catheterization, which showed a Gerbode defect, and a transesophageal echocardiogram showed a shunt in the subaortic region measuring 6 to 8 mm, with a maximum gradient of 56 mmHg. He underwent elective surgery to correct the Gerbode defect and tricuspid valve repair, with a good clinical result. The Gerbode defect is rare, and the diagnosis can be challenging because it simulates other conditions. Treatment consists of closing the defect when it generates refractory symptoms or complications. The reported case was surgically corrected, with a good result and favorable evolution.
DOI: https://doi.org/10.37349/ec.2026.101296

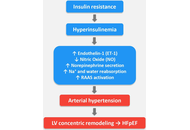
Heart failure (HF) is still one of the most common causes of death today. The vast majority of heart diseases end up leading to HF, which therefore has a high prevalence in the adult population (on average 1–2%), and which increases enormously (over 10%) after the age of 65, becoming the most frequent cause of hospitalization for these subjects. It is therefore necessary to increase efforts to deepen our understanding of the pathophysiological mechanisms that lead to HF and its worsening, particularly with regard to hormonal-metabolic derangements as contributors to HF development and progression. This, in the hope of being able, in the near future, to intervene on them, reducing the prevalence of this pathology and its economic impact on countries’ healthcare spending. To this aim, we performed a narrative review of the scientific literature on the interactions between both insulin and the growth hormone/insulin-like growth factor-1 axis and the cardiovascular system, and in particular, to verify the role that these hormones may play in the development and negative progression of HF.
Heart failure (HF) is still one of the most common causes of death today. The vast majority of heart diseases end up leading to HF, which therefore has a high prevalence in the adult population (on average 1–2%), and which increases enormously (over 10%) after the age of 65, becoming the most frequent cause of hospitalization for these subjects. It is therefore necessary to increase efforts to deepen our understanding of the pathophysiological mechanisms that lead to HF and its worsening, particularly with regard to hormonal-metabolic derangements as contributors to HF development and progression. This, in the hope of being able, in the near future, to intervene on them, reducing the prevalence of this pathology and its economic impact on countries’ healthcare spending. To this aim, we performed a narrative review of the scientific literature on the interactions between both insulin and the growth hormone/insulin-like growth factor-1 axis and the cardiovascular system, and in particular, to verify the role that these hormones may play in the development and negative progression of HF.
DOI: https://doi.org/10.37349/ec.2026.101294

Background:
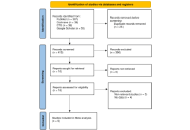
Heart failure (HF) remains a growing global health problem, with nearly half of all cases attributed to HF with preserved ejection fraction (HFpEF) and its precursor, left ventricular diastolic dysfunction (LVDD). Although echocardiography is the diagnostic gold standard, its high cost and limited availability restrict its use for large-scale screening. In contrast, the electrocardiogram (ECG) is inexpensive and widely accessible. Recent advances in artificial intelligence (AI) have created opportunities to leverage ECG data for the early detection of cardiac dysfunction. The objective of this study was to systematically review and meta-analyze the diagnostic performance of AI-based ECG models for detecting cardiac dysfunction.
Methods:
The QUADAS-2 tool was used to assess the risk of bias. Pooled sensitivity and specificity were estimated using a bivariate random-effects model, with heterogeneity quantified using the I2 statistic. Pre-specified subgroup analyses were conducted according to clinical endpoint and AI model type.
Results:
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, nine eligible studies evaluating AI algorithms applied to ECG data for the detection of HFpEF were identified. Considerable methodological and population heterogeneity was observed across studies. Risk of bias was generally low for reference standards, although concerns were noted in patient selection. The pooled specificity of AI-ECG models was high at 0.83 [95% confidence interval (CI): 0.74–0.89], while pooled sensitivity was 0.82 (95% CI: 0.70–0.90). Both estimates demonstrated extremely high heterogeneity (I2 > 96%). Subgroup analyses by endpoint and model type did not explain this variability.
Discussion:
AI-enhanced ECG models show good diagnostic accuracy, specifically in ruling out cardiac dysfunction due to their high specificity. However, the high and unexplained heterogeneity across these studies limits the immediate generalizability of the results. Large, prospective validation studies across diverse populations are essential before these models can be confidently adopted into routine clinical practice.
Background:
Heart failure (HF) remains a growing global health problem, with nearly half of all cases attributed to HF with preserved ejection fraction (HFpEF) and its precursor, left ventricular diastolic dysfunction (LVDD). Although echocardiography is the diagnostic gold standard, its high cost and limited availability restrict its use for large-scale screening. In contrast, the electrocardiogram (ECG) is inexpensive and widely accessible. Recent advances in artificial intelligence (AI) have created opportunities to leverage ECG data for the early detection of cardiac dysfunction. The objective of this study was to systematically review and meta-analyze the diagnostic performance of AI-based ECG models for detecting cardiac dysfunction.
Methods:
The QUADAS-2 tool was used to assess the risk of bias. Pooled sensitivity and specificity were estimated using a bivariate random-effects model, with heterogeneity quantified using the I2 statistic. Pre-specified subgroup analyses were conducted according to clinical endpoint and AI model type.
Results:
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, nine eligible studies evaluating AI algorithms applied to ECG data for the detection of HFpEF were identified. Considerable methodological and population heterogeneity was observed across studies. Risk of bias was generally low for reference standards, although concerns were noted in patient selection. The pooled specificity of AI-ECG models was high at 0.83 [95% confidence interval (CI): 0.74–0.89], while pooled sensitivity was 0.82 (95% CI: 0.70–0.90). Both estimates demonstrated extremely high heterogeneity (I2 > 96%). Subgroup analyses by endpoint and model type did not explain this variability.
Discussion:
AI-enhanced ECG models show good diagnostic accuracy, specifically in ruling out cardiac dysfunction due to their high specificity. However, the high and unexplained heterogeneity across these studies limits the immediate generalizability of the results. Large, prospective validation studies across diverse populations are essential before these models can be confidently adopted into routine clinical practice.
DOI: https://doi.org/10.37349/ec.2026.101293

Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare but potentially life-threatening inherited arrhythmia disorder, often presenting in childhood or adolescence. Early and accurate diagnosis is critical, as untreated CPVT carries a high risk of sudden cardiac death, particularly in young individuals. This case underscores the importance of maintaining a high index of clinical suspicion and employing a systematic diagnostic approach. We highlight the value of integrating clinical history, family background, and targeted investigations in evaluating young adults presenting with sudden cardiac arrest. Prompt recognition and diagnosis of CPVT may be lifesaving and have significant implications for both patients and their families.
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare but potentially life-threatening inherited arrhythmia disorder, often presenting in childhood or adolescence. Early and accurate diagnosis is critical, as untreated CPVT carries a high risk of sudden cardiac death, particularly in young individuals. This case underscores the importance of maintaining a high index of clinical suspicion and employing a systematic diagnostic approach. We highlight the value of integrating clinical history, family background, and targeted investigations in evaluating young adults presenting with sudden cardiac arrest. Prompt recognition and diagnosis of CPVT may be lifesaving and have significant implications for both patients and their families.
DOI: https://doi.org/10.37349/ec.2026.101292

Left ventricular pseudoaneurysm is a rare acquired cardiac abnormality that frequently occurs after myocardial infarction or a previous cardiac procedure. Blunt chest trauma accounts for this uncommon entity in sporadic cases. However, this disease does not have any specific clinical findings, so it is necessary to monitor the suspected patient closely. The standard noninvasive techniques for diagnosing left ventricular pseudoaneurysms are transthoracic echocardiography and thoracic computed tomography. Untreated ventricular pseudoaneurysms carry a considerable risk of rupture, ranging from 30% to 45%. So, an urgent surgical treatment is often required. Herein, we aimed to present a 34-year-old male who underwent emergency surgery as a result of cardiac perforation three hours after a traffic accident and developed a giant left ventricular pseudoaneurysm 19 months after discharge. The giant pseudoaneurysm was successfully repaired. This case highlights the need for long‑term surveillance after blunt cardiac trauma to detect late pseudoaneurysm formation.
Left ventricular pseudoaneurysm is a rare acquired cardiac abnormality that frequently occurs after myocardial infarction or a previous cardiac procedure. Blunt chest trauma accounts for this uncommon entity in sporadic cases. However, this disease does not have any specific clinical findings, so it is necessary to monitor the suspected patient closely. The standard noninvasive techniques for diagnosing left ventricular pseudoaneurysms are transthoracic echocardiography and thoracic computed tomography. Untreated ventricular pseudoaneurysms carry a considerable risk of rupture, ranging from 30% to 45%. So, an urgent surgical treatment is often required. Herein, we aimed to present a 34-year-old male who underwent emergency surgery as a result of cardiac perforation three hours after a traffic accident and developed a giant left ventricular pseudoaneurysm 19 months after discharge. The giant pseudoaneurysm was successfully repaired. This case highlights the need for long‑term surveillance after blunt cardiac trauma to detect late pseudoaneurysm formation.
DOI: https://doi.org/10.37349/ec.2026.101291

Redesigning cardiovascular services at the local level is a pressing task for decentralized health systems facing the rising burden of chronic cardiovascular disease. In northern Modena (Emilia-Romagna, Italy), a post-restructuring reorganization exposed the limits of hospital-centric models and the need for integrated, patient-centered care. In 2021, Santa Maria Bianca Hospital, Mirandola—a first-level, non-interventional facility serving a largely rural population—launched a program to build a digitally integrated, prevention-oriented cardiology network. This review distills that field experience into a scalable framework for organizing peripheral cardiovascular services. The Mirandola Cardiology Network evolved along six operational domains: (1) reactivation of the cardiology unit with community outreach; (2) expansion of outpatient services and telecardiology; (3) a day hospital platform for chronic heart failure management; (4) digital transformation of the echocardiography service; (5) development of an advanced imaging center integrating coronary computed tomography (CT) angiography and planned cardiac magnetic resonance imaging (MRI); and (6) consolidation of professional education, research, and network-wide governance. By combining digital tools, non-invasive imaging, and multidisciplinary collaboration, the model established continuity of care across inpatient, outpatient, and community settings while improving access to diagnostics and appropriateness of care. Although prospective or comparative outcomes are not presented, process indicators and implementation milestones suggest scalability and sustainability, with potential to reduce avoidable admissions and streamline clinical pathways. The Mirandola experience shows that innovation in cardiology is feasible in peripheral settings when investment in technology, governance, and training is aligned with a coherent, value-based vision. It offers actionable guidance for decentralized systems seeking to implement digitally enabled, community-focused cardiology consistent with contemporary recommendations on territorial care and chronic disease management.
Redesigning cardiovascular services at the local level is a pressing task for decentralized health systems facing the rising burden of chronic cardiovascular disease. In northern Modena (Emilia-Romagna, Italy), a post-restructuring reorganization exposed the limits of hospital-centric models and the need for integrated, patient-centered care. In 2021, Santa Maria Bianca Hospital, Mirandola—a first-level, non-interventional facility serving a largely rural population—launched a program to build a digitally integrated, prevention-oriented cardiology network. This review distills that field experience into a scalable framework for organizing peripheral cardiovascular services. The Mirandola Cardiology Network evolved along six operational domains: (1) reactivation of the cardiology unit with community outreach; (2) expansion of outpatient services and telecardiology; (3) a day hospital platform for chronic heart failure management; (4) digital transformation of the echocardiography service; (5) development of an advanced imaging center integrating coronary computed tomography (CT) angiography and planned cardiac magnetic resonance imaging (MRI); and (6) consolidation of professional education, research, and network-wide governance. By combining digital tools, non-invasive imaging, and multidisciplinary collaboration, the model established continuity of care across inpatient, outpatient, and community settings while improving access to diagnostics and appropriateness of care. Although prospective or comparative outcomes are not presented, process indicators and implementation milestones suggest scalability and sustainability, with potential to reduce avoidable admissions and streamline clinical pathways. The Mirandola experience shows that innovation in cardiology is feasible in peripheral settings when investment in technology, governance, and training is aligned with a coherent, value-based vision. It offers actionable guidance for decentralized systems seeking to implement digitally enabled, community-focused cardiology consistent with contemporary recommendations on territorial care and chronic disease management.
DOI: https://doi.org/10.37349/ec.2026.101290

Background:
Myosin inhibitors have been shown to improve exercise capacity and symptoms, as well as reduce the left ventricular outflow tract (LVOT) gradient. This study explores the efficacy of mavacamten versus aficamten in hypertrophic cardiomyopathy (HCM) patients.
Methods:
From the inception to October 2024, the electronic databases [Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, and ClinicalTrials.gov] were searched. Using a random-effects model and a frequentist framework, specific effect sizes [mean difference (MD) and risk ratio (RR)] were pooled.
Results:
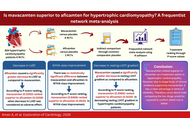
This network meta-analysis included six randomized controlled trials (RCTs). A total of 826 individuals with HCM were included; 443 of them received a cardiac myosin inhibitor, while 383 received placebo. Comparison of aficamten versus mavacamten through a common comparator, placebo, showed that aficamten caused a lesser decrease in resting LVOT gradient than that of mavacamten [MD = 14.74, 95% CI (3.02; 26.47)]. Therefore, mavacamten ranked higher (P-score = 0.9966) than aficamten (P-score = 0.5034) in decreasing resting LVOT gradient. Aficamten significantly reduced left ventricular ejection fraction (LVEF) in contrast to mavacamten [MD = –5.59, 95% CI (–10.43; –0.75)]. According to P-score ranking, mavacamten (0.5053) ranked higher than aficamten (0.0059). For New York Heart Association (NYHA) class improvement, there was no statistically significant difference between the two groups [MD = –0.37, 95% CI (–1.79; 1.06)]. But P-score ranked mavacamten (0.8466) higher than aficamten (0.6533).
Discussion:
Mavacamten ranked superior to aficamten in HCM management. However, this ranking is based not on the absolute clinical benefit but on the network point estimates. Additionally, due to a larger body of clinical evidence supporting mavacamten, it has a clear advantage in terms of reliability. Therefore, more direct trials comparing the two drugs would be required to confirm which one is better and provide conclusive evidence.
Background:
Myosin inhibitors have been shown to improve exercise capacity and symptoms, as well as reduce the left ventricular outflow tract (LVOT) gradient. This study explores the efficacy of mavacamten versus aficamten in hypertrophic cardiomyopathy (HCM) patients.
Methods:
From the inception to October 2024, the electronic databases [Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, and ClinicalTrials.gov] were searched. Using a random-effects model and a frequentist framework, specific effect sizes [mean difference (MD) and risk ratio (RR)] were pooled.
Results:
This network meta-analysis included six randomized controlled trials (RCTs). A total of 826 individuals with HCM were included; 443 of them received a cardiac myosin inhibitor, while 383 received placebo. Comparison of aficamten versus mavacamten through a common comparator, placebo, showed that aficamten caused a lesser decrease in resting LVOT gradient than that of mavacamten [MD = 14.74, 95% CI (3.02; 26.47)]. Therefore, mavacamten ranked higher (P-score = 0.9966) than aficamten (P-score = 0.5034) in decreasing resting LVOT gradient. Aficamten significantly reduced left ventricular ejection fraction (LVEF) in contrast to mavacamten [MD = –5.59, 95% CI (–10.43; –0.75)]. According to P-score ranking, mavacamten (0.5053) ranked higher than aficamten (0.0059). For New York Heart Association (NYHA) class improvement, there was no statistically significant difference between the two groups [MD = –0.37, 95% CI (–1.79; 1.06)]. But P-score ranked mavacamten (0.8466) higher than aficamten (0.6533).
Discussion:
Mavacamten ranked superior to aficamten in HCM management. However, this ranking is based not on the absolute clinical benefit but on the network point estimates. Additionally, due to a larger body of clinical evidence supporting mavacamten, it has a clear advantage in terms of reliability. Therefore, more direct trials comparing the two drugs would be required to confirm which one is better and provide conclusive evidence.
DOI: https://doi.org/10.37349/ec.2026.101289

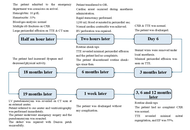
Rheumatoid arthritis (RA) confers an elevated cardiovascular disease (CVD) risk through systemic inflammation, arterial stiffness, and myocardial dysfunction. The ratio of carotid-femoral pulse wave velocity (PWV) to left ventricular global longitudinal strain (LV-GLS) has been proposed as a novel index of ventricular-arterial coupling. This study investigated whether a one-year physical activity intervention improves the PWV/LV-GLS ratio in RA patients without known CVD. Eighteen participants with RA from the prospective PARA 2010 study underwent baseline and one-year assessments. PWV was measured by oscillometry, and LV-GLS by speckle-tracking echocardiography. Physical activity was promoted through supervised circuit training and additional moderate-to-vigorous activity. At one year, very active participants [≥ 1,000 metabolic equivalent of task (MET)-minutes/week, n = 10] showed a relative reduction in PWV/LV-GLS ratio compared with baseline, while no change was observed in less active participants (< 1,000 MET-minutes/week, n = 8). Between-group comparison at follow-up demonstrated significantly lower PWV/LV-GLS ratio in very active versus less active patients (p < 0.05). No significant between-group differences were observed when PWV and LV-GLS were analyzed separately. In conclusion, a high level of physical activity was associated with improved ventricular-arterial coupling, reflected by a lower PWV/LV-GLS ratio, in RA patients. These findings support a potential dose-dependent effect of physical activity on subclinical cardiovascular function in RA. The clinical trial was registered at http://www.isrctn.com, unique identifier: ISRCTN88886304.
Rheumatoid arthritis (RA) confers an elevated cardiovascular disease (CVD) risk through systemic inflammation, arterial stiffness, and myocardial dysfunction. The ratio of carotid-femoral pulse wave velocity (PWV) to left ventricular global longitudinal strain (LV-GLS) has been proposed as a novel index of ventricular-arterial coupling. This study investigated whether a one-year physical activity intervention improves the PWV/LV-GLS ratio in RA patients without known CVD. Eighteen participants with RA from the prospective PARA 2010 study underwent baseline and one-year assessments. PWV was measured by oscillometry, and LV-GLS by speckle-tracking echocardiography. Physical activity was promoted through supervised circuit training and additional moderate-to-vigorous activity. At one year, very active participants [≥ 1,000 metabolic equivalent of task (MET)-minutes/week, n = 10] showed a relative reduction in PWV/LV-GLS ratio compared with baseline, while no change was observed in less active participants (< 1,000 MET-minutes/week, n = 8). Between-group comparison at follow-up demonstrated significantly lower PWV/LV-GLS ratio in very active versus less active patients (p < 0.05). No significant between-group differences were observed when PWV and LV-GLS were analyzed separately. In conclusion, a high level of physical activity was associated with improved ventricular-arterial coupling, reflected by a lower PWV/LV-GLS ratio, in RA patients. These findings support a potential dose-dependent effect of physical activity on subclinical cardiovascular function in RA. The clinical trial was registered at http://www.isrctn.com, unique identifier: ISRCTN88886304.
DOI: https://doi.org/10.37349/ec.2026.101288
This article belongs to the special issue Exploring Exercise Cardiology: from Molecules to Humans

The editor-in-chief plays a vital role in ensuring a journal’s scientific integrity and quality. Their primary responsibilities include managing the peer-review process, selecting qualified reviewers, and making final decisions on manuscript acceptance, revision, or rejection. In cases of scientific misconduct, conflicts of interest, authorship disputes, or ethical concerns, the editor has the ultimate authority.
An editor’s vision for the journal shapes which manuscripts are reviewed and accepted, influencing the journal’s academic direction. While the role offers benefits such as scientific prestige, greater research visibility, and financial compensation, it also entails significant ethical responsibilities. Academic editor malpractice refers to any actions that violate ethical standards or compromises the integrity of the peer-review process.
Editors typically serve five-year terms, often with the possibility of renewal, and are frequently evaluated based on the journal’s impact factor trend. However, their role extends beyond editorial duties—they act as gatekeepers, literary agents, accountants, mediators, and judges, navigating the complex relationships among authors, reviewers, and publishers.
Editors of major journals hold an extraordinary amount of power within the publication process. They act as an umpire to judge the scientific research that is being published. Like an umpire, they must know about the sport and rules of play, but they themselves should never be in the competition. The problem is that this ideal is not always met. Whether the subject is the efficacy of an antihypertensive drug, the value of a new costly biomarker, or the origin of a pandemic, editors often make decisions for multi-parametric—and also extra-scientific—reasons. On this basis, some papers are published while others are declined, and the stream of scientific evidence can be polluted.
In summary, the editor-in-chief is a cornerstone of academic publishing, ensuring that scientific quality and integrity are upheld while balancing multiple responsibilities.
The editor-in-chief plays a vital role in ensuring a journal’s scientific integrity and quality. Their primary responsibilities include managing the peer-review process, selecting qualified reviewers, and making final decisions on manuscript acceptance, revision, or rejection. In cases of scientific misconduct, conflicts of interest, authorship disputes, or ethical concerns, the editor has the ultimate authority.
An editor’s vision for the journal shapes which manuscripts are reviewed and accepted, influencing the journal’s academic direction. While the role offers benefits such as scientific prestige, greater research visibility, and financial compensation, it also entails significant ethical responsibilities. Academic editor malpractice refers to any actions that violate ethical standards or compromises the integrity of the peer-review process.
Editors typically serve five-year terms, often with the possibility of renewal, and are frequently evaluated based on the journal’s impact factor trend. However, their role extends beyond editorial duties—they act as gatekeepers, literary agents, accountants, mediators, and judges, navigating the complex relationships among authors, reviewers, and publishers.
Editors of major journals hold an extraordinary amount of power within the publication process. They act as an umpire to judge the scientific research that is being published. Like an umpire, they must know about the sport and rules of play, but they themselves should never be in the competition. The problem is that this ideal is not always met. Whether the subject is the efficacy of an antihypertensive drug, the value of a new costly biomarker, or the origin of a pandemic, editors often make decisions for multi-parametric—and also extra-scientific—reasons. On this basis, some papers are published while others are declined, and the stream of scientific evidence can be polluted.
In summary, the editor-in-chief is a cornerstone of academic publishing, ensuring that scientific quality and integrity are upheld while balancing multiple responsibilities.
DOI: https://doi.org/10.37349/ec.2026.101286

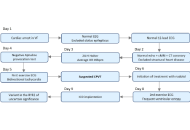
Exercise-related arrhythmia is attracting growing attention, according to the increased popularity of leisure-time sports, which have great benefits and acute risk, although the hemodynamics and therapeutics of exercise-related arrhythmia are poorly understood. We have experienced two cases of different types of exercise-related arrhythmias. In an 80-year-old woman, exercise-induced increase in supraventricular premature contractions (SVPCs) converted to atrial fibrillation (AF) during a control ergometric stress test (EST), but SVPCs were diminished, and AF was not observed in the secondary EST after starting bisoprolol. In a 39-year-old woman, idiopathic premature ventricular contractions (PVCs) appeared immediately after the termination of the control EST but were scarcely induced by the secondary EST under the treatment with bisoprolol. Post-exercise abnormal increase in the double product was suppressed, leading to the possibility of improved exercise tolerance in both cases. A couple of ESTs under the same protocol to compare the arrhythmic behaviors with and without treatment provides a therapeutic strategy in exercise-related arrhythmia, and short-term bisoprolol is concluded to be favorable to the specific types of exercise-related arrhythmia, at least in these two cases.
Exercise-related arrhythmia is attracting growing attention, according to the increased popularity of leisure-time sports, which have great benefits and acute risk, although the hemodynamics and therapeutics of exercise-related arrhythmia are poorly understood. We have experienced two cases of different types of exercise-related arrhythmias. In an 80-year-old woman, exercise-induced increase in supraventricular premature contractions (SVPCs) converted to atrial fibrillation (AF) during a control ergometric stress test (EST), but SVPCs were diminished, and AF was not observed in the secondary EST after starting bisoprolol. In a 39-year-old woman, idiopathic premature ventricular contractions (PVCs) appeared immediately after the termination of the control EST but were scarcely induced by the secondary EST under the treatment with bisoprolol. Post-exercise abnormal increase in the double product was suppressed, leading to the possibility of improved exercise tolerance in both cases. A couple of ESTs under the same protocol to compare the arrhythmic behaviors with and without treatment provides a therapeutic strategy in exercise-related arrhythmia, and short-term bisoprolol is concluded to be favorable to the specific types of exercise-related arrhythmia, at least in these two cases.
DOI: https://doi.org/10.37349/ec.2026.101287
This article belongs to the special issue Exploring Exercise Cardiology: from Molecules to Humans

Pulmonary embolism (PE) is the third most common cause of cardiovascular mortality and presents a significant challenge in acute care settings. EkoSonic Endovascular System (EKOS) (ultrasound assisted catheter directed thrombolysis) and suction thrombectomy have emerged as key treatment options for high and intermediate risk PE. EKOS delivers localized fibrinolytic therapy, whereas thrombectomy provides definitive clot removal using devices such as the FlowTriever System (Inari Medical). However, the optimal treatment strategy, particularly in recurrent PE, remains uncertain. We report a case requiring escalation of therapy from EKOS to suction thrombectomy due to recurrent PE and worsening hemodynamic status despite initial thrombolysis. The patient was initially treated with EKOS for a saddle PE but was rehospitalized with syncope and persistent right ventricular (RV) strain. Given the inadequate response to thrombolysis, suction thrombectomy was performed, leading to marked improvement in RV function and overall clinical status. This case underscores the importance of individualized management and timely escalation when initial therapy is insufficient. Assessment of therapeutic success should include not only symptomatic relief but also resolution of clot burden and RV recovery. A focused literature review comparing EKOS and suction thrombectomy suggests that while both modalities are viable, suction thrombectomy may offer faster hemodynamic improvement in select patients. However, available data remains limited, highlighting the need for further comparative studies. Overall, this case and review support a tailored, multidisciplinary approach to PE management, emphasizing shared decision making and early escalation in patients with clinical deterioration despite initial intervention.
Pulmonary embolism (PE) is the third most common cause of cardiovascular mortality and presents a significant challenge in acute care settings. EkoSonic Endovascular System (EKOS) (ultrasound assisted catheter directed thrombolysis) and suction thrombectomy have emerged as key treatment options for high and intermediate risk PE. EKOS delivers localized fibrinolytic therapy, whereas thrombectomy provides definitive clot removal using devices such as the FlowTriever System (Inari Medical). However, the optimal treatment strategy, particularly in recurrent PE, remains uncertain. We report a case requiring escalation of therapy from EKOS to suction thrombectomy due to recurrent PE and worsening hemodynamic status despite initial thrombolysis. The patient was initially treated with EKOS for a saddle PE but was rehospitalized with syncope and persistent right ventricular (RV) strain. Given the inadequate response to thrombolysis, suction thrombectomy was performed, leading to marked improvement in RV function and overall clinical status. This case underscores the importance of individualized management and timely escalation when initial therapy is insufficient. Assessment of therapeutic success should include not only symptomatic relief but also resolution of clot burden and RV recovery. A focused literature review comparing EKOS and suction thrombectomy suggests that while both modalities are viable, suction thrombectomy may offer faster hemodynamic improvement in select patients. However, available data remains limited, highlighting the need for further comparative studies. Overall, this case and review support a tailored, multidisciplinary approach to PE management, emphasizing shared decision making and early escalation in patients with clinical deterioration despite initial intervention.
DOI: https://doi.org/10.37349/ec.2026.101285

Background:
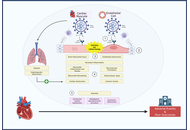
Patients with pre-existing heart failure (HF) are particularly vulnerable to adverse outcomes following coronavirus disease 2019 (COVID-19). Understanding of the long-term cardiovascular sequelae of COVID-19 in this high-risk group is essential to improve post-infection management and outcomes.
Methods:
A systematic review of PubMed, Scopus, Web of Science, and Embase was conducted to identify peer-reviewed studies published between 2020 and 2025. Eligible studies included adults with a confirmed diagnosis of HF prior to COVID-19 infection and reported cardiovascular outcomes assessed at least 12 weeks after the acute phase. Data were extracted on patient demographics, HF subtype, cardiovascular outcomes, quality of life (QoL), and management approaches.
Results:
Forty-five studies met the inclusion criteria, encompassing heterogeneous but predominantly high-income country populations across multiple regions and HF phenotypes. COVID-19 was associated with increased HF symptoms, hospital readmissions 28% [95% confidence interval (CI) 24–32%] at 12 months, and mortality 18% (95% CI 15–22%) at ≥ 12 months. Patients with HF with reduced ejection fraction (HFrEF) had a 1.4-fold greater readmission risk than HF with preserved ejection fraction (HFpEF). Mechanistic data implicated persistent myocardial inflammation, endothelial dysfunction, and autonomic dysregulation. Functional capacity declined, with a mean 68-meter reduction in six-minute walk distance (6MWD). Vaccination was associated with a ~40% reduction in mortality and major adverse cardiovascular events (MACE).
Discussion:
COVID-19 is associated with a sustained cardiovascular burden in individuals with HF, underscoring the importance of long-term surveillance, optimization of guideline-directed medical therapy, and structured rehabilitation. Standardized, prospective studies are needed to elucidate causal mechanisms and refine post-COVID management strategies.
Background:
Patients with pre-existing heart failure (HF) are particularly vulnerable to adverse outcomes following coronavirus disease 2019 (COVID-19). Understanding of the long-term cardiovascular sequelae of COVID-19 in this high-risk group is essential to improve post-infection management and outcomes.
Methods:
A systematic review of PubMed, Scopus, Web of Science, and Embase was conducted to identify peer-reviewed studies published between 2020 and 2025. Eligible studies included adults with a confirmed diagnosis of HF prior to COVID-19 infection and reported cardiovascular outcomes assessed at least 12 weeks after the acute phase. Data were extracted on patient demographics, HF subtype, cardiovascular outcomes, quality of life (QoL), and management approaches.
Results:
Forty-five studies met the inclusion criteria, encompassing heterogeneous but predominantly high-income country populations across multiple regions and HF phenotypes. COVID-19 was associated with increased HF symptoms, hospital readmissions 28% [95% confidence interval (CI) 24–32%] at 12 months, and mortality 18% (95% CI 15–22%) at ≥ 12 months. Patients with HF with reduced ejection fraction (HFrEF) had a 1.4-fold greater readmission risk than HF with preserved ejection fraction (HFpEF). Mechanistic data implicated persistent myocardial inflammation, endothelial dysfunction, and autonomic dysregulation. Functional capacity declined, with a mean 68-meter reduction in six-minute walk distance (6MWD). Vaccination was associated with a ~40% reduction in mortality and major adverse cardiovascular events (MACE).
Discussion:
COVID-19 is associated with a sustained cardiovascular burden in individuals with HF, underscoring the importance of long-term surveillance, optimization of guideline-directed medical therapy, and structured rehabilitation. Standardized, prospective studies are needed to elucidate causal mechanisms and refine post-COVID management strategies.
DOI: https://doi.org/10.37349/ec.2026.101284

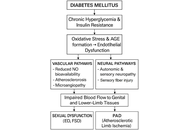
Diabetes mellitus is one of the leading global health concerns, with an increasing burden of cardiovascular morbidity and mortality. Sexual dysfunction and peripheral artery disease are two frequent and interrelated complications in diabetic populations, both serving as potential indicators of systemic vascular and neural damage. The interplay of endothelial dysfunction, atherosclerosis, and diabetic neuropathy provides a mechanistic basis linking these complications with heightened cardiovascular risk. While sexual dysfunction is often underrecognized, it may represent an early marker of vascular impairment. Peripheral artery disease, on the other hand, is a well-established predictor of major cardiovascular events. Patient-centered education and comprehensive management approaches are essential to address these issues and improve outcomes. This narrative review synthesizes current evidence on vascular and neural mechanisms underlying sexual dysfunction and peripheral artery disease in diabetes, highlighting their clinical relevance and implications for cardiovascular risk stratification.
Diabetes mellitus is one of the leading global health concerns, with an increasing burden of cardiovascular morbidity and mortality. Sexual dysfunction and peripheral artery disease are two frequent and interrelated complications in diabetic populations, both serving as potential indicators of systemic vascular and neural damage. The interplay of endothelial dysfunction, atherosclerosis, and diabetic neuropathy provides a mechanistic basis linking these complications with heightened cardiovascular risk. While sexual dysfunction is often underrecognized, it may represent an early marker of vascular impairment. Peripheral artery disease, on the other hand, is a well-established predictor of major cardiovascular events. Patient-centered education and comprehensive management approaches are essential to address these issues and improve outcomes. This narrative review synthesizes current evidence on vascular and neural mechanisms underlying sexual dysfunction and peripheral artery disease in diabetes, highlighting their clinical relevance and implications for cardiovascular risk stratification.
DOI: https://doi.org/10.37349/ec.2025.101283
This article belongs to the special issue The Effect of Sexual Dysfunctions, Peripheral Artery Disease, and Patient Education on the Cardiovascular Risk in Diabetes

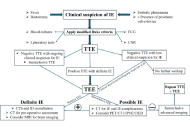
Infective endocarditis (IE) remains a challenging diagnosis, particularly in patients with prosthetic valves, cardiac implantable electronic devices (CIEDs), or nonspecific presentations. With rising rates of healthcare-associated and device-related infections, the need for earlier and more reliable diagnosis has become increasingly important. Multimodality imaging now plays a central role in confirming IE, identifying complications, and guiding management. Echocardiography is the initial test of choice, with transesophageal echocardiography (TEE) offering better sensitivity for vegetations, leaflet perforation, and periannular extension, though its limitations in prosthetic valve endocarditis (PVE) have led to greater reliance on other modalities. Cardiac computed tomography (CT) provides detailed anatomical information that can reveal abscesses, pseudoaneurysms, and prosthetic dehiscence, and is frequently used for surgical planning. Functional imaging with 18F-fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) and white blood cell single-photon emission CT/CT (WBC-SPECT/CT) improves diagnostic accuracy in PVE and CIED infections while also detecting systemic embolic events. Brain magnetic resonance imaging (MRI) has become an important tool to uncover neurologic complications, including silent emboli and mycotic aneurysms. This review summarizes the strengths and limitations of each modality, outlines a stepwise approach to imaging decisions, and considers how findings should be incorporated into overall clinical care. This review also highlights surgical indications, evolving antimicrobial strategies, and the future role of standardized imaging protocols. Taken together, thoughtful use of multimodality imaging is critical to improving outcomes in patients with suspected or confirmed IE.
Infective endocarditis (IE) remains a challenging diagnosis, particularly in patients with prosthetic valves, cardiac implantable electronic devices (CIEDs), or nonspecific presentations. With rising rates of healthcare-associated and device-related infections, the need for earlier and more reliable diagnosis has become increasingly important. Multimodality imaging now plays a central role in confirming IE, identifying complications, and guiding management. Echocardiography is the initial test of choice, with transesophageal echocardiography (TEE) offering better sensitivity for vegetations, leaflet perforation, and periannular extension, though its limitations in prosthetic valve endocarditis (PVE) have led to greater reliance on other modalities. Cardiac computed tomography (CT) provides detailed anatomical information that can reveal abscesses, pseudoaneurysms, and prosthetic dehiscence, and is frequently used for surgical planning. Functional imaging with 18F-fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) and white blood cell single-photon emission CT/CT (WBC-SPECT/CT) improves diagnostic accuracy in PVE and CIED infections while also detecting systemic embolic events. Brain magnetic resonance imaging (MRI) has become an important tool to uncover neurologic complications, including silent emboli and mycotic aneurysms. This review summarizes the strengths and limitations of each modality, outlines a stepwise approach to imaging decisions, and considers how findings should be incorporated into overall clinical care. This review also highlights surgical indications, evolving antimicrobial strategies, and the future role of standardized imaging protocols. Taken together, thoughtful use of multimodality imaging is critical to improving outcomes in patients with suspected or confirmed IE.
DOI: https://doi.org/10.37349/ec.2025.101282

Aim:
Pulmonary vein isolation (PVI) is a widely accepted and effective treatment for atrial fibrillation (AF). Even though success rates have been climbing, some patients experience AF recurrence after ablation. This study aimed to identify predictors of AF recurrence, with a focus on the potential role of premature atrial contractions (PAC).
Methods:
A retrospective single-center analysis was conducted on 185 patients with AF who underwent primo PVI at a single center between 07/2014 and 01/2017. Patients underwent AF ablation using radiofrequency ablation (n = 61), by the CARTO (n = 50) and EnSite (n = 11) mapping systems, and the endoscopic laser balloon (n = 124). Exclusion criteria were combined procedures or the absence of a 24-hour Holter recording three months post-ablation. The primary endpoint was freedom from atrial arrhythmia 12 months after ablation with an application of a 90-day blanking period.
Results:
Survival analysis revealed a significant difference in AF recurrence rates between low and high PAC burden groups (log-rank test, p = 0.004). ROC-analysis identified an optimal PAC burden cut-off of 57 PAC’s over 24 hours (AUC 0.69). This association remained significant in multivariable Cox proportional hazards analysis, with a hazard ratio of 3.38 (p = 0.021).
Conclusions:
PAC burden measured on 24-hour Holter monitoring at three months proved to be an independent predictor of AF recurrence following PVI. Multivariable analysis confirmed a significant hazard ratio of 3.38 for AF recurrence within one year. An optimal predictive threshold of 57 PAC demonstrated high negative predictive value for AF recurrence.
Aim:
Pulmonary vein isolation (PVI) is a widely accepted and effective treatment for atrial fibrillation (AF). Even though success rates have been climbing, some patients experience AF recurrence after ablation. This study aimed to identify predictors of AF recurrence, with a focus on the potential role of premature atrial contractions (PAC).
Methods:
A retrospective single-center analysis was conducted on 185 patients with AF who underwent primo PVI at a single center between 07/2014 and 01/2017. Patients underwent AF ablation using radiofrequency ablation (n = 61), by the CARTO (n = 50) and EnSite (n = 11) mapping systems, and the endoscopic laser balloon (n = 124). Exclusion criteria were combined procedures or the absence of a 24-hour Holter recording three months post-ablation. The primary endpoint was freedom from atrial arrhythmia 12 months after ablation with an application of a 90-day blanking period.
Results:
Survival analysis revealed a significant difference in AF recurrence rates between low and high PAC burden groups (log-rank test, p = 0.004). ROC-analysis identified an optimal PAC burden cut-off of 57 PAC’s over 24 hours (AUC 0.69). This association remained significant in multivariable Cox proportional hazards analysis, with a hazard ratio of 3.38 (p = 0.021).
Conclusions:
PAC burden measured on 24-hour Holter monitoring at three months proved to be an independent predictor of AF recurrence following PVI. Multivariable analysis confirmed a significant hazard ratio of 3.38 for AF recurrence within one year. An optimal predictive threshold of 57 PAC demonstrated high negative predictive value for AF recurrence.
DOI: https://doi.org/10.37349/ec.2025.101281

Pulmonary congestion and coronary microvascular dysfunction are central to hemodynamic adaptation before and after transcatheter aortic valve replacement (TAVR). This perspective proposes an exploratory, physiology-anchored framework that integrates lung ultrasound (LUS) and transthoracic Doppler of the left anterior descending artery (LAD) to track these domains on the same bedside platform. A concise anterior four-site LUS sampling captures early, posture- and stress-dependent interstitial congestion, while LAD Doppler provides a non-invasive window on resting diastolic coronary flow velocity (CFV) and CFV reserve (CFVR). In severe aortic stenosis, valve unloading with TAVR typically reduces filling pressures and restores diastolic coronary flow, though CFV and CFVR responses vary with the balance between myocardial oxygen demand and improved hyperemia. Interpreting these signals alongside standard echocardiographic indices (E/e’, LAVI, SPAP, and Doppler velocity index) ensures physiological coherence and avoids misattribution. Rather than focusing on numeric thresholds, this approach emphasizes trajectories—how B-lines, CFV, and CFVR evolve from baseline to post-TAVR follow-up. Concordant improvement supports decongestion and microvascular recovery, whereas discordance prompts investigation of right-sided or extra-cardiac mechanisms. Readily implemented with existing echocardiographic equipment, the combined LUS-LAD strategy offers a scalable, hypothesis-generating tool to explore pulmonary-coronary interactions, guide decongestive and anti-ischemic therapy, and inform future feasibility and reproducibility studies in the TAVR population.
Pulmonary congestion and coronary microvascular dysfunction are central to hemodynamic adaptation before and after transcatheter aortic valve replacement (TAVR). This perspective proposes an exploratory, physiology-anchored framework that integrates lung ultrasound (LUS) and transthoracic Doppler of the left anterior descending artery (LAD) to track these domains on the same bedside platform. A concise anterior four-site LUS sampling captures early, posture- and stress-dependent interstitial congestion, while LAD Doppler provides a non-invasive window on resting diastolic coronary flow velocity (CFV) and CFV reserve (CFVR). In severe aortic stenosis, valve unloading with TAVR typically reduces filling pressures and restores diastolic coronary flow, though CFV and CFVR responses vary with the balance between myocardial oxygen demand and improved hyperemia. Interpreting these signals alongside standard echocardiographic indices (E/e’, LAVI, SPAP, and Doppler velocity index) ensures physiological coherence and avoids misattribution. Rather than focusing on numeric thresholds, this approach emphasizes trajectories—how B-lines, CFV, and CFVR evolve from baseline to post-TAVR follow-up. Concordant improvement supports decongestion and microvascular recovery, whereas discordance prompts investigation of right-sided or extra-cardiac mechanisms. Readily implemented with existing echocardiographic equipment, the combined LUS-LAD strategy offers a scalable, hypothesis-generating tool to explore pulmonary-coronary interactions, guide decongestive and anti-ischemic therapy, and inform future feasibility and reproducibility studies in the TAVR population.
DOI: https://doi.org/10.37349/ec.2025.101280

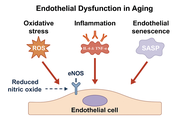
Cardiovascular aging is characterized by progressive endothelial dysfunction and arterial stiffening, two interrelated processes underlying the increased risk of hypertension, coronary artery disease, heart failure, and atrial fibrillation in older individuals. Endothelial dysfunction results from reduced nitric oxide bioavailability, increased oxidative stress, chronic low-grade inflammation, and accumulation of senescent endothelial cells that secrete pro-inflammatory mediators. In parallel, structural alterations of the vascular wall, including elastin fragmentation, collagen deposition, cross-linking by advanced glycation end products, vascular smooth muscle cell phenotypic switching, and calcification, lead to increased stiffness and impaired vascular compliance. These maladaptive changes reinforce one another, creating a vicious cycle in which dysfunctional endothelium accelerates stiffening, while mechanical alterations in turn amplify endothelial injury. Molecular pathways involving NADPH oxidases, mitochondrial dysfunction, NF-κB, JAK/STAT, AMPK, mTOR, sirtuins, and epigenetic regulators integrate oxidative, inflammatory, and metabolic signals that shape vascular aging. Clinically, endothelial dysfunction and vascular stiffness predict cardiovascular events independent of traditional risk factors and serve as emerging biomarkers of biological vascular age. Established therapies such as statins, renin-angiotensin system blockade, structured exercise, and dietary interventions improve vascular function, while novel approaches targeting senescence and redox imbalance are under investigation. Understanding these mechanisms provides opportunities to mitigate vascular aging and extend cardiovascular health span.
Cardiovascular aging is characterized by progressive endothelial dysfunction and arterial stiffening, two interrelated processes underlying the increased risk of hypertension, coronary artery disease, heart failure, and atrial fibrillation in older individuals. Endothelial dysfunction results from reduced nitric oxide bioavailability, increased oxidative stress, chronic low-grade inflammation, and accumulation of senescent endothelial cells that secrete pro-inflammatory mediators. In parallel, structural alterations of the vascular wall, including elastin fragmentation, collagen deposition, cross-linking by advanced glycation end products, vascular smooth muscle cell phenotypic switching, and calcification, lead to increased stiffness and impaired vascular compliance. These maladaptive changes reinforce one another, creating a vicious cycle in which dysfunctional endothelium accelerates stiffening, while mechanical alterations in turn amplify endothelial injury. Molecular pathways involving NADPH oxidases, mitochondrial dysfunction, NF-κB, JAK/STAT, AMPK, mTOR, sirtuins, and epigenetic regulators integrate oxidative, inflammatory, and metabolic signals that shape vascular aging. Clinically, endothelial dysfunction and vascular stiffness predict cardiovascular events independent of traditional risk factors and serve as emerging biomarkers of biological vascular age. Established therapies such as statins, renin-angiotensin system blockade, structured exercise, and dietary interventions improve vascular function, while novel approaches targeting senescence and redox imbalance are under investigation. Understanding these mechanisms provides opportunities to mitigate vascular aging and extend cardiovascular health span.
DOI: https://doi.org/10.37349/ec.2025.101279
This article belongs to the special issue Molecular Mechanisms of Cardiovascular Aging

Background:
Cardiac computed tomography (CT) has evolved from an anatomic test to a platform that quantifies functional, inflammatory, and tissue-characterization biomarkers. We synthesized evidence on the diagnostic and prognostic value of CT-based biomarkers.
Methods:
Systematic review of 29 human studies (2015–2025) appraising low-attenuation plaque (LAP), perivascular fat attenuation index (FAI/PCAT), total/non-calcified plaque burden, epicardial adipose tissue, CT-derived fractional flow reserve (FFR-CT), and CT myocardial perfusion. Study quality was assessed with risk of bias (RoB) 2.0, Newcastle-Ottawa Scale (NOS), and AMSTAR 2.
Results:
CT biomarkers extended risk assessment beyond stenosis severity. LAP burden > 4% predicted myocardial infarction (MI) [hazard ratio (HR) 4.65; 95% CI 2.06–10.5] and per-doubling LAP predicted MI (HR 1.60; 95% CI 1.10–2.34). Perivascular FAI/PCAT showed independent prognostic value: high FAI was associated with ~2-fold higher cardiac mortality (derivation HR 2.15, validation HR 2.06), and RCA PCAT ≥ −70.5 Hounsfield unit (HU) predicted MI (HR 2.45) with additive risk when combined with high-risk plaque (HRP) features (reported up to ~6-fold vs. reference). FFR-CT achieved up to 81% diagnostic accuracy (sensitivity ~86%, specificity ~79%) vs. invasive FFR, improving specificity over CTA alone. Emerging metrics (e.g., total plaque volume, CT perfusion) demonstrated incremental discrimination in selected cohorts, though standardization remains variable.
Discussion:
CT-based biomarkers provide measurable diagnostic and prognostic information on coronary anatomy, function, inflammation, and tissue health. Priorities include standardized acquisition/analysis, multicenter validation, and integration into decision pathways to optimize individualized risk stratification and therapy.
Background:
Cardiac computed tomography (CT) has evolved from an anatomic test to a platform that quantifies functional, inflammatory, and tissue-characterization biomarkers. We synthesized evidence on the diagnostic and prognostic value of CT-based biomarkers.
Methods:
Systematic review of 29 human studies (2015–2025) appraising low-attenuation plaque (LAP), perivascular fat attenuation index (FAI/PCAT), total/non-calcified plaque burden, epicardial adipose tissue, CT-derived fractional flow reserve (FFR-CT), and CT myocardial perfusion. Study quality was assessed with risk of bias (RoB) 2.0, Newcastle-Ottawa Scale (NOS), and AMSTAR 2.
Results:
CT biomarkers extended risk assessment beyond stenosis severity. LAP burden > 4% predicted myocardial infarction (MI) [hazard ratio (HR) 4.65; 95% CI 2.06–10.5] and per-doubling LAP predicted MI (HR 1.60; 95% CI 1.10–2.34). Perivascular FAI/PCAT showed independent prognostic value: high FAI was associated with ~2-fold higher cardiac mortality (derivation HR 2.15, validation HR 2.06), and RCA PCAT ≥ −70.5 Hounsfield unit (HU) predicted MI (HR 2.45) with additive risk when combined with high-risk plaque (HRP) features (reported up to ~6-fold vs. reference). FFR-CT achieved up to 81% diagnostic accuracy (sensitivity ~86%, specificity ~79%) vs. invasive FFR, improving specificity over CTA alone. Emerging metrics (e.g., total plaque volume, CT perfusion) demonstrated incremental discrimination in selected cohorts, though standardization remains variable.
Discussion:
CT-based biomarkers provide measurable diagnostic and prognostic information on coronary anatomy, function, inflammation, and tissue health. Priorities include standardized acquisition/analysis, multicenter validation, and integration into decision pathways to optimize individualized risk stratification and therapy.
DOI: https://doi.org/10.37349/ec.2025.101278

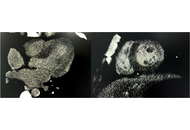
The diagnosis of acute myocarditis requires the exclusion of coronary artery disease (CAD). Coronary CTA (computed tomography angiography) is usually used to evaluate the coronary arteries in young patients. However, the use of coronary CTA for the diagnosis of myocarditis has been rarely reported. Here we present a Han male clinical myocarditis patient who was 18 years old, had a focus of enhancement in the subcardia, and predominantly involving the lateral wall of the left ventricle with iodinated contrast in coronary CTA. The patient was diagnosed with myocarditis. Immunoglobulin, vitamin C antioxidant, and myocardial nutrition were given to the patient for treatment. During follow-up, the patient’s myocardial enzymes gradually decreased to normal, and the original symptoms disappeared. As a non-invasive rapid examination method that can evaluate coronary artery and myocardial lesions at the same time, the utility of myocardial delayed enhancement on CTA may warrant further investigation.
The diagnosis of acute myocarditis requires the exclusion of coronary artery disease (CAD). Coronary CTA (computed tomography angiography) is usually used to evaluate the coronary arteries in young patients. However, the use of coronary CTA for the diagnosis of myocarditis has been rarely reported. Here we present a Han male clinical myocarditis patient who was 18 years old, had a focus of enhancement in the subcardia, and predominantly involving the lateral wall of the left ventricle with iodinated contrast in coronary CTA. The patient was diagnosed with myocarditis. Immunoglobulin, vitamin C antioxidant, and myocardial nutrition were given to the patient for treatment. During follow-up, the patient’s myocardial enzymes gradually decreased to normal, and the original symptoms disappeared. As a non-invasive rapid examination method that can evaluate coronary artery and myocardial lesions at the same time, the utility of myocardial delayed enhancement on CTA may warrant further investigation.
DOI: https://doi.org/10.37349/ec.2025.101277
 Previous
Previous