Affiliation:
2Department of Medical and Surgical Sciences, Magna Graecia University, 88100 Catanzaro, Italy
Affiliation:
1Division of Cardiology, Fatebenefratelli Hospital, 82100 Benevento, Italy
Email: qciampi@gmail.com
ORCID: https://orcid.org/0000-0003-4775-8380
Explor Cardiol. 2025;3:101273 DOI: https://doi.org/10.37349/ec.2025.101273
Received: June 30, 2025 Accepted: September 03, 2025 Published: September 28, 2025
Academic Editor: Dimitrios M Tousoulis, Athens University Medical School, Greece
We report the case of a 58-year-old woman who developed typical chest pain following intravaginal administration of misoprostol, used routinely as a premedication before a hysteroscopy. Misoprostol is a synthetic prostaglandin E1 analogue widely employed for cervical softening and the medical management of an incomplete abortion, and is generally considered safe. The chest pain was accompanied by transient, severe ST-segment elevation (up to 6 mm) in the inferior and inferolateral electrocardiogram (ECG) leads, which resolved promptly after sublingual nitrate administration. A hyperventilation test (5 minutes at 30 breaths per minute) demonstrated a paradoxical reduction in peak diastolic flow velocity in the mid-to-distal left anterior descending coronary artery. Invasive coronary angiography showed smooth, angiographically normal coronary arteries, but provocation with intracoronary acetylcholine induced complete vasospasm of the left circumflex artery. This case underscores the importance of recognizing vasospastic angina even in non-cardiology settings. It highlights the value of targeted noninvasive and invasive diagnostic testing to confirm this often overlooked, underdiagnosed, and undertreated condition.
Vasospastic angina (VSA), characterized by transient coronary artery spasm resulting in myocardial ischemia, is a known but frequently underrecognized cause of chest pain, especially in patients with minimal or no angiographic evidence of coronary stenosis [1]. Misoprostol is a synthetic prostaglandin E1 analogue widely employed for cervical softening and the medical management of incomplete abortion and is generally considered safe. However, it may induce coronary vasospasm, causing angina, myocardial infarction, and cardiac arrest in vulnerable subjects [2–12].
A 58-year-old woman in menopausal status with negative past cardiovascular history and no current drug therapy was admitted to the obstetrics and gynecology department for an elective hysteroscopy. She was pretreated with intravaginal misoprostol, a common practice to soften the cervix and reduce procedural complications such as cervical laceration or perforation.
Approximately three hours after administration, the patient experienced acute, severe substernal chest pain. Electrocardiogram (ECG) revealed significant ST-segment elevation in inferolateral leads (Figure 1). Sublingual nitrates were promptly administered, resulting in the complete resolution of symptoms and ECG changes.
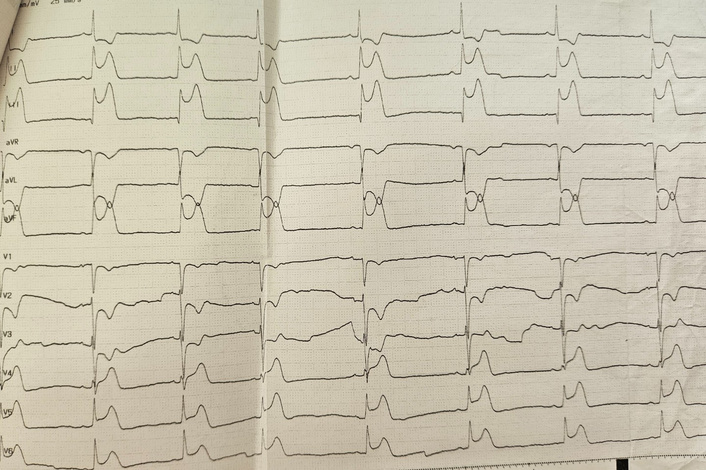
The electrocardiogram showed ST-segment elevation in the inferolateral leads after misoprostol administration.
Following clinical stabilization, the patient underwent a standardized hyperventilation test (5 minutes of deep breathing at 25–50 breaths per minute), with echocardiographic monitoring continued for 5 minutes of post-maneuvering [13, 14]. Hyperventilation induced the expected increase in myocardial oxygen demand, reflected by a rise in heart rate (from 64 to 106 bpm), systolic blood pressure (from 130 to 160 mmHg), and a rate-pressure product (from 8,320 to 16,960 mmHg × bpm). Despite this physiological stress, Doppler echocardiography revealed a paradoxical decrease in coronary flow velocity (CFV) in the mid-distal left anterior descending (LAD) artery from 24.2 cm/s at rest to 13.7 cm/s at peak stress, followed by an increase in the recovery period (CFV 36.1 cm/s), suggesting a vasospastic response (Figure 2, lower panel).
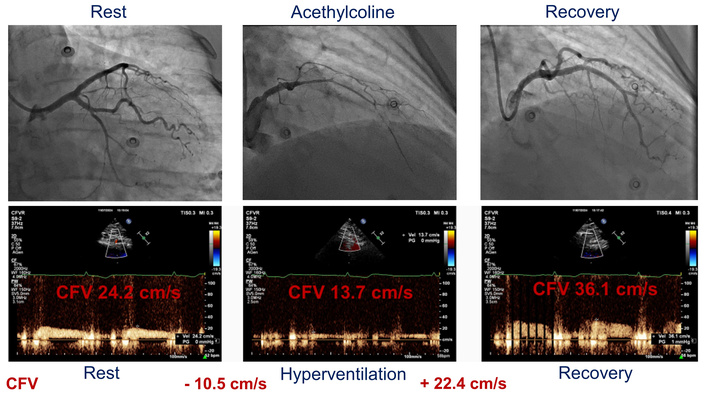
The paradoxical coronary flow pattern during the hyperventilation stress test and its angiographic correlate. lower panel: coronary flow velocity (CFV) in the left anterior descending (LAD) artery evaluated at rest, after hyperventilation, and in the recovery period; upper panel: coronary angiographic findings at rest, during intracoronary acetylcholine administration, and after intracoronary administration of nitrates.
Invasive coronary angiography was subsequently performed, showing angiographically normal coronary arteries (Figure 2, upper panel). Provocative testing with intracoronary acetylcholine induced complete vasospasm of the left circumflex artery (and also of the LAD artery to a lesser degree), at the first step of the infusion (20 µg), as demonstrated by coronary vasoconstriction > 90% plus ischemic ECG changes and characteristic chest pain, confirming the diagnosis of VSA. The patient was discharged on a regimen of calcium channel blockers (verapamil, 240 mg once daily, long-acting formulation orally) and nitrates (isosorbide mononitrate 60 mg once daily, long-acting formulation orally), with instructions to avoid beta-blockers.
At one-year follow-up, she remained asymptomatic, and a hyperventilation echocardiographic stress test was performed on therapy. During the test, the increase in heart rate (from 69 to 85 bpm), systolic blood pressure (from 120 to 150 mmHg), and rate-pressure product (from 8,280 to 12,750 mmHg × bpm) was not associated with ischemic ECG changes, and neither with regional wall motion abnormalities. In contrast to the previous exam, where we observed a drop in the CFV of the LAD artery, a hyperventilation test in this case showed an increase in the CFV of the LAD artery from 26 cm/s at rest to 31.4 cm/s at peak stress, according to the incremental rate-pressure product (Figure 3).
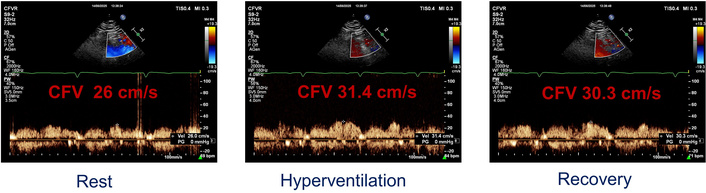
Coronary flow velocity (CFV) in the left anterior descending (LAD) artery evaluated at rest, after hyperventilation, and in the recovery period.
Considering the favorable response, we decided to continue the same drug plan and to reassess the patient every year (Figure 4).
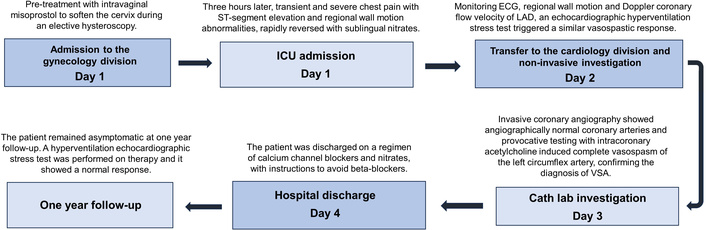
Timeline events and the treatment details in chronological order. ECG: electrocardiogram; LAD: left anterior descending; VSA: vasospastic angina.
This case highlights a rare but clinically significant phenomenon: vasospastic angina triggered by misoprostol in a non-cardiology setting. While the misoprostol is generally safe and routinely used for various gynecological indications, including incomplete abortion and pre-hysteroscopy cervical preparation [15], in susceptible individuals, it can provoke coronary vasospasm.
The diagnosis of vasospastic angina is straightforward and potentially life-saving when appropriately considered, but is often overlooked. The vasospastic effect of misoprostol may be mediated by two primary mechanisms:
Stimulation of prostaglandin E receptor subtype EP3, which promotes vasoconstriction, in contrast to the vasodilatory effects of EP2 and EP4 activation.
A dose-dependent increase in circulating norepinephrine, a potent vasoconstrictor [16].
Vasospasm associated with misoprostol has been reported across various routes of administration, including intravenous, oral, buccal, rectal, and vaginal, and can occur even at low doses. Peak plasma concentrations are highest with intravenous and sublingual administration, while vaginal administration results in a slower but prolonged absorption. Hence, no route or dose can be deemed entirely safe in vulnerable patients [12, 16].
Clinicians, including gynecologists, obstetricians, and cardiologists, should be aware of misoprostol’s potential to induce coronary vasospasm. Although rare, this adverse effect can lead to serious and life-threatening events. Early recognition is critical. Noninvasive testing with hyperventilation echocardiography, followed by confirmatory intracoronary acetylcholine testing, can help establish the diagnosis and initiate appropriate treatment [17]. Therapy should include calcium channel blockers and/or nitrates, while beta-blockers should be avoided.
CFV: coronary flow velocity
ECG: electrocardiogram
LAD: left anterior descending
VSA: vasospastic angina
Ettore C, Emma C, EL, NC, and QC: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. BV: Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The Declaration of Helsinki (2024 version) was adequately complied with, and the study was approved by the institutional ethics committees in its latest versions as part of the more comprehensive Stress Echo 2030 study 291/294/295 Comitato Etico Lazio-1, March 8, 2021; ClinicalTrials.gov Identifier NCT05081115.
Informed consent to participate in the study was obtained from all participants.
Informed consent to publication was obtained from the participant.
Data will not be shared as it involves patient privacy, but may be available from the corresponding author upon reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1633
Download: 153
Times Cited: 0
