Affiliation:
1Cardiovascular Department, “San Carlo” Hospital, 85100 Potenza, Italy
Email: imagingcardio@gmail.com
ORCID: https://orcid.org/0000-0002-6017-020X
Explor Cardiol. 2025;3:101280 DOI: https://doi.org/10.37349/ec.2025.101280
Received: September 03, 2025 Accepted: October 17, 2025 Published: November 11, 2025
Academic Editor: Alexandre Abizaid, Institute Dante Pazzanese de Cardiologia, Brazil
Pulmonary congestion and coronary microvascular dysfunction are central to hemodynamic adaptation before and after transcatheter aortic valve replacement (TAVR). This perspective proposes an exploratory, physiology-anchored framework that integrates lung ultrasound (LUS) and transthoracic Doppler of the left anterior descending artery (LAD) to track these domains on the same bedside platform. A concise anterior four-site LUS sampling captures early, posture- and stress-dependent interstitial congestion, while LAD Doppler provides a non-invasive window on resting diastolic coronary flow velocity (CFV) and CFV reserve (CFVR). In severe aortic stenosis, valve unloading with TAVR typically reduces filling pressures and restores diastolic coronary flow, though CFV and CFVR responses vary with the balance between myocardial oxygen demand and improved hyperemia. Interpreting these signals alongside standard echocardiographic indices (E/e’, LAVI, SPAP, and Doppler velocity index) ensures physiological coherence and avoids misattribution. Rather than focusing on numeric thresholds, this approach emphasizes trajectories—how B-lines, CFV, and CFVR evolve from baseline to post-TAVR follow-up. Concordant improvement supports decongestion and microvascular recovery, whereas discordance prompts investigation of right-sided or extra-cardiac mechanisms. Readily implemented with existing echocardiographic equipment, the combined LUS-LAD strategy offers a scalable, hypothesis-generating tool to explore pulmonary-coronary interactions, guide decongestive and anti-ischemic therapy, and inform future feasibility and reproducibility studies in the TAVR population.
Pulmonary congestion follows a physiological cascade in which rises in left-ventricular end-diastolic pressure and pulmonary capillary wedge pressure (PCWP) disrupt the Starling balance at the alveolar-capillary barrier, leading to interstitial edema that increases the local water/air ratio within subpleural interlobular septa and generates B-lines on lung ultrasound (LUS) [1–3]. Clinical and radiographic signs are late, insensitive, and nonspecific; LUS captures the interstitial stage when therapeutic leverage is greatest [1–3]. Notably, about one in three patients who are “dry” at rest may develop B-lines only at peak stress—exercise or pharmacologic—anticipating clinical decompensation and refining risk [4–6].
B-lines have a dual nature. “Wet” B-lines—water-driven—change within minutes: They increase with exercise, volume loading, or supine posture, and decrease with decongestion or upright position. “Dry” B-lines—connective-tissue-driven—are brighter, less mobile, and comparatively fixed; their recognition benefits from pattern analysis and, increasingly, machine learning [2, 7]. Right-sided venous mechanisms (elevated systemic venous pressure with impaired lymphatic drainage), as observed in pre- or post-capillary pulmonary hypertension or right-ventricular dysfunction, can also produce B-lines despite normal left-sided filling pressures [1–3].
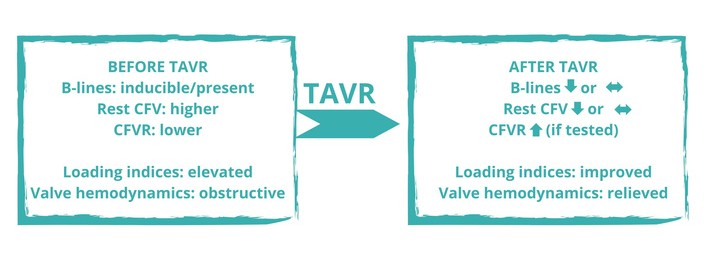
In severe aortic stenosis, coronary flow often shows systolic flow reversal and attenuated diastolic augmentation, both of which improve after valve replacement. Transcatheter aortic valve replacement (TAVR) rapidly enhances forward flow and reduces filling pressures, with early changes in diastolic indices (for example, E/e’) and pulmonary pressures [8–10]. Coronary flow velocity reserve (CFVR) is frequently blunted before valve correction and tends to improve post-TAVR, while resting CFV may decrease, remain stable, or rise depending on the balance between reduced myocardial oxygen demand and restored hyperemia [11, 12]. Independent of valve disease, high resting CFV has been linked to adverse outcomes in chronic coronary syndromes [13]. These observations support an integrated LUS-left anterior descending artery (LAD) Doppler approach to follow pulmonary decongestion and coronary microvascular adaptation around TAVR.
This perspective is hypothesis-generating; proposed applications are exploratory and intended to inform feasibility studies rather than prescribe clinical practice.
Examples of anterior sampling are illustrative, not prescriptive; layouts may vary by laboratory experience and patient habitus. Image acquisition is driven by the clinical question, not a rigid workflow.
Typical LUS settings include a 3.5–5 MHz phased-array or convex probe, depth 10–15 cm, low mechanical index, and presets focused on the pleura. LAD Doppler is obtained from apical or modified parasternal windows with angle correction ≤ 30°, sample volume at mid-to-distal LAD, sweep speed ≥ 100 mm/s, and averaging of at least 3 cycles (≥ 5 in AF).
We seek two complementary views of the same hemodynamic story. The first is the lung’s interstitial water signal: a compact anterior sampling (e.g., four sites) with LUS of the anterior chest—replicable and quick—captures whether water accumulates where it should not and whether that signal is posture- and stress-dependent. The second is the coronary microvascular signal, obtained with transthoracic LAD Doppler to follow resting diastolic CFV and, when feasible, CFVR. Conventional TTE anchors interpretation with loading indices [E/e’, left atrial volume index (LAVI), systolic pulmonary artery pressure (SPAP)] and valve unloading [mean gradient, Doppler velocity index (DVI)] so that lung-coronary changes make physiological sense [4–11] (Figure 1).
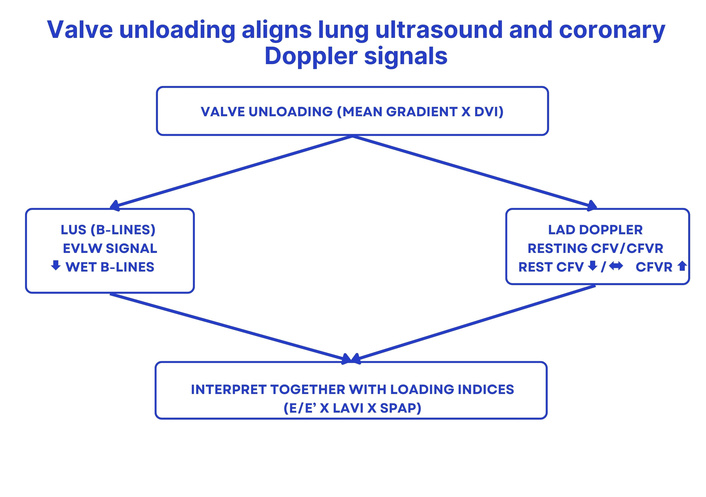
Concept map. Valve unloading (mean gradient and DVI) aligns lung and coronary signals: LUS B-lines—an EVLW signal—decrease; resting CFV decreases or remains stable; CFVR increases. Interpretation is integrated with loading indices (E/e’, LAVI, SPAP). Arrows indicate hypothesized qualitative changes; schematic, not to scale. DVI: Doppler velocity index; LUS: lung ultrasound; B-lines: vertical artifacts indicating interstitial lung water; LAD: left anterior descending artery; CFV: coronary flow velocity (resting diastolic Doppler); CFVR: coronary flow velocity reserve; E/e’: ratio of early mitral inflow velocity to early diastolic mitral annular velocity; LAVI: left atrial volume index; SPAP: systolic pulmonary artery pressure; EVLW: extravascular lung water. ↓: decrease; ↔: no relevant change; ↑: increase.
Two practical lenses maintain consistency: serial comparability (same posture, similar timing, consistent presets) matters more than technological minutiae; and context—right-sided venous hypertension, respiratory disease, heart-rate, or pressure shifts—must accompany image interpretation to avoid false attribution [1–3, 5, 14, 15].
Reading LUS reflects how interstitial signals change with load. B-lines are vertical, laser-like artifacts rising from the pleura to the far field, erasing A-lines. When they wax and wane with posture, stress, or therapy, they behave as a wet signal of interstitial water. When persistent, bright, and immobile despite such maneuvers, they likely reflect a dry substrate (fibrosis or interstitial disease). Stress can reveal a third pattern: patients “dry” at rest who generate B-lines only at peak and clear them quickly in recovery—an early warning that the cardiopulmonary system is approaching decompensation [1–7, 15].
Coronary Doppler is interpreted with similar discipline. Resting diastolic CFV reflects myocardial demand; CFVR captures the capacity to augment flow, which often recovers after valve intervention. These signals must be read alongside E/e’, LAVI, SPAP, and valve unloading (mean gradient/DVI), to arbitrate whether a lung change is left-sided, right venous, or extra-cardiac, and whether a coronary change plausibly relates to unloading [8–14].
Patterns matter more than points. Concordant improvement (fewer B-lines; lower E/e’; stable or falling resting CFV; higher CFVR) indicates decongestion and microvascular rebalancing. Discordance (persistent B-lines with normal E/e’ or unchanged CFVR despite good valve hemodynamics) is not noise but information—prompting evaluation for right-sided congestion, pulmonary disease, anemia, or unrelated microvascular dysfunction [1–3, 8–14].
Rather than defining numeric targets, we illustrate trajectories that clinicians can follow over time.
Lung side: observe the 4-site B-line score from baseline through early post-implant and follow-up; note stress-induced congestion and its recovery kinetics [4–6, 15].
Coronary side: track whether resting CFV stabilizes at a lower demand set-point and whether CFVR regains headroom after unloading [11, 12].
Anchor all readings to contemporaneous loading indices (E/e’, LAVI, SPAP) and valve hemodynamics (mean gradient, DVI) so interpretation remains physiological [8–12].
Over time, these signals form recognizable qualitative maps:
Decongestor: B-lines ↓; resting CFV ↓/↔; CFVR ↑; loading indices improve.
Venous-right: B-lines persist while loading indices exclude left-sided elevation; consider right-venous or extra-cardiac contributors.
Demand-driven: resting CFV remains high despite adequate valve hemodynamics; reassess heart rate, blood pressure (BP), anemia, or microvascular factors [1–3, 8–14] (Figure 2).
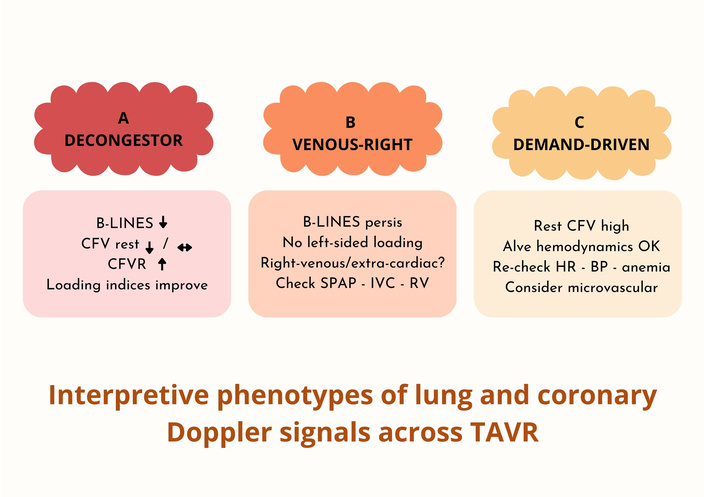
Three qualitative phenotypes integrating lung ultrasound and coronary Doppler signals. (A) Decongestor: B-lines ↓; resting CFV ↓/↔; CFVR ↑; loading indices improve. (B) Venous-right: B-lines persist while loading indices do not indicate left-sided elevation; consider right-venous or extra-cardiac drivers (check SPAP/IVC/RV). (C) Demand-driven: resting CFV remains high despite adequate valve hemodynamics; re-check HR/BP/anemia; consider microvascular factors. Schematic, qualitative map; not to scale. CFV: coronary flow velocity; CFVR: coronary flow velocity reserve; SPAP: systolic pulmonary artery pressure; IVC: inferior vena cava; RV: right ventricle; HR: heart rate; BP: blood pressure; TAVR: transcatheter aortic valve replacement. ↓: decrease; ↔: no relevant change; ↑: increase.
This perspective deliberately avoids operational specifics of any ongoing or planned protocol. Instead, it sketches a roadmap of questions that can be addressed with routine imaging, emphasizing feasibility, reproducibility, and clinical translatability.
Dynamics of interstitial congestion: characterize within-patient LUS B-line evolution from rest to stress and recovery across the peri-procedural trajectory [1–6, 14, 15].
Microvascular adaptation: describe how resting diastolic CFV and CFVR adjust after valve unloading, integrating them with valve hemodynamics and left ventricle (LV) loading [8–12, 14].
Stress phenotyping: when stress echocardiography is indicated, define inducible B-line patterns and test concordance with wall motion and pulmonary surrogates [4–6, 14, 15].
Left vs. right attribution: develop practical attribution rules combining LUS with inferior vena cava (IVC), SPAP, and right ventricle (RV) indices to avoid mislabeling right-venous phenotypes [1–3, 14].
Quality and harmonization: promote shared acquisition standards (4-site LUS; LAD Doppler angles and averaging), report reproducibility, and use artificial intelligence (AI) counting as an adjunct [5, 7, 15].
Equity and generalizability: test feasibility across body habitus, COPD/obesity, rhythm disturbances, and care settings [1–6, 14].
Each domain is exploratory, adaptable to local governance, and aimed at defining feasibility, reproducibility, and patient-relevant outcomes.
Label B-lines as left-sided only when aligned with elevated E/e’, LAVI, or SPAP; otherwise, consider right-venous or extra-cardiac sources. When attribution remains uncertain, report discordance explicitly rather than imposing a left-sided label.
Three principles keep interpretation balanced:
Posture and timing shape meaning—wet B-lines grow supine and at peak stress, recede in recovery; consistent posture preserves interpretability [1–6].
Attribution needs proof: interpret LUS together with loading indices and CFV/CFVR with heart rate, BP, and valve data. Context prevents confident interpretations from becoming convenient but incorrect explanations [1–3, 8–12, 14].
Training and quality: reproducibility depends on operator training, simple quality checks, and structured metadata; AI tools are adjunctive, not replacements for clinical judgment [5, 7, 14, 15].
In symptomatic severe AS, pharmacologic or exercise stress testing may be inappropriate; CFVR assessment can be deferred until after TAVR or performed with low-risk, guideline-concordant protocols. When stress testing is appropriate, vasodilator stress is preferred to inotropic stress for safety and interpretability.
This framework offers bedside immediacy and physiological specificity. LUS detects early interstitial congestion, responding within hours to valve unloading or diuretic therapy and outperforming late clinical or radiographic signs [1–6]. Transthoracic LAD Doppler opens a non-invasive window on coronary microvascular behavior, complementing valve hemodynamics and loading indices [8–12, 14].
Compared with invasive hemodynamics or biomarkers (e.g., NT-proBNP), the paired LUS-LAD approach provides a physiologically coherent, repeatable bedside assessment; unlike CT/CMR, it is radiation-free and readily serializable. It complements but does not replace established imaging or biomarker strategies.
This perspective is exploratory and non-prescriptive.
Attribution is fragile: B-lines may arise from right-venous or extra-cardiac causes, and LAD Doppler depends on heart rate, BP, medications, or insonation angle. Misclassification risk is mitigated—but not eliminated—by combining LUS with IVC, SPAP, and RV indices and maintaining technical rigor [1–3, 11, 12, 14].
Both LUS and LAD Doppler are operator-dependent; reproducibility hinges on training, posture control, standardized presets, and AI-assisted secondary reads with expert adjudication [5, 7, 14, 15]. Feasibility across diverse patient profiles (obesity, COPD, AF) warrants further reporting.
Future work should standardize definitions (wet vs. dry), reporting templates, and quality metrics. Mechanistic studies may test whether changes in B-lines and CFV/CFVR mediate the link between valve unloading and functional recovery. Data-driven phenotyping may uncover distinct pulmonary-coronary coupling profiles requiring tailored follow-up.
This perspective proposes a pragmatic, physiology-anchored way to monitor patients across the TAVR pathway. Pairing concise anterior LUS (e.g., four sites) with transthoracic LAD Doppler allows tracking two complementary signals—extravascular lung water and coronary microvascular behavior—on the shared bedside platform.
Interpretation relies on trajectories, not static values: How B-lines, CFV, and CFVR evolve from baseline to post-implant and follow-up, together with loading and valve indices. Interpreted this way, imaging moves beyond description to provide actionable confirmation of decongestion and microvascular rebalancing or, when discordant, to prompt targeted investigation.
Translation into practice is straightforward: brief posture-controlled LUS clips, focused LAD Doppler aligned to diastolic flow; structured metadata, and disciplined reading. The next step is harmonization—shared templates and quality metrics—to enable cross-center learning.
If adopted and validated, this integrated framework may refine decision-making before and after TAVR, guide therapy titration, and support patient-centered follow-up, providing a concise, scalable way to visualize lung-heart physiology where it matters most—at the point of care.
AI: artificial intelligence
BP: blood pressure
CFV: coronary flow velocity
CFVR: coronary flow velocity reserve
DVI: Doppler velocity index
IVC: inferior vena cava
LAD: left anterior descending artery
LAVI: left atrial volume index
LUS: lung ultrasound
RV: right ventricle
SPAP: systolic pulmonary artery pressure
TAVR: transcatheter aortic valve replacement
This perspective frames a conceptual pathway and opens questions around integrating lung ultrasound B-lines and transthoracic LAD Doppler across TAVR care. It does not prescribe a protocol nor disclose operational specifics; any examples are illustrative to ground the discussion. Concepts are vendor-agnostic; brand-specific implementations are out of scope.
MFC: Writing—original draft, Conceptualization, Validation, Supervision. GDA: Formal analysis, Investigation, Methodology, Visualization, Writing—review & editing. FC: Formal analysis, Investigation. MMDF: Formal analysis, Investigation. MGV: Formal analysis, Investigation. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1318
Download: 120
Times Cited: 0
