Affiliation:
1Pediatric Cardiology Department, Agia Sophia Children’s Hospital, GR11526 Athens, Greece
Email: Andrianouska@gmail.com
ORCID: https://orcid.org/0000-0002-0047-1713
Affiliation:
1Pediatric Cardiology Department, Agia Sophia Children’s Hospital, GR11526 Athens, Greece
Affiliation:
2First Department of Pediatrics, National and Kapodistrian University of Athens, GR11526 Athens, Greece
ORCID: https://orcid.org/0000-0002-1573-7190
Affiliation:
2First Department of Pediatrics, National and Kapodistrian University of Athens, GR11526 Athens, Greece
ORCID: https://orcid.org/0000-0003-4617-0657
Affiliation:
1Pediatric Cardiology Department, Agia Sophia Children’s Hospital, GR11526 Athens, Greece
Explor Cardiol. 2025;3:101271 DOI: https://doi.org/10.37349/ec.2025.101271
Received: May 18, 2025 Accepted: September 03, 2025 Published: September 22, 2025
Academic Editor: Geu-Ru Hong, Yonsei University College of Medicine, Korea
Resistant Kawasaki has been associated with the aggressive development of large coronary aneurysms, despite prompt treatment. In this paper, we present an infant who presented with resistant Kawasaki. Although initially he seemed to defervesce after the initial administration of intravenous immunoglobulin, he developed a new onset of fever, requiring a repeat dose of immunoglobulin. Coronary aneurysms developed rapidly, necessitating a second dose of immunoglobulins and second-line treatments such as anakinra and infliximab. High titers of COVID-19 antibodies have been a confounding factor in the management of that child, as the alternative diagnosis of multisystem inflammatory syndrome in children (MIS-C) was considered. Finally, the clinical and laboratory values were more in keeping with MIS-C.
Kawasaki disease is the most common childhood vasculitis affecting the medium-sized arteries. The estimated incidence is 25 per 100,000 in North America [1]. Intravenous immunoglobulin (IVIG) and aspirin remain the mainstay of treatment, as prompt administration has decreased the incidence of the development of coronary artery aneurysms [1, 2]. However, patients unresponsive to IVIG are at higher risk of developing coronary artery aneurysms.
The second-line treatment for these patients has been studied, yet the optimal treatment has not been determined. Here, we describe a young infant with acute Kawasaki who developed large coronary aneurysms despite initial defervescence after treatment [3].
A two-month-old male infant presented to the local hospital with a two-day history of pyrexia up to 40°C, a concurrent mild diarrheal illness, and slightly decreased feeding. He had received the first dose of the routine childhood immunization of the hexavalent vaccine twenty-four hours prior to the symptoms. There was no history of viral infection.
He was born at 37 + 2 weeks of gestation with an elective cesarean secondary to a previous cesarean with a birth weight of 2,820 g. He is the third child of non-consanguineal healthy parents. Prior to the admission, he had been gaining weight appropriately.
His present weight was 5,200 g, height 54 cm, and head circumference 40 cm.
On examination, he was pyrexial with a fever of 38°C. On inspection, he had a blanching maculopapular rash, with a heart rate of 100 bpm, and blood pressure of 100/57 mmHg. The chest was clear with normal vesicular sounds, and the heart sounds were normal. The femoral arteries were palpable.
A few hours after admission, he had a new onset of pyrexia up to 39.5°C and a pulsating fontanelle. A lumbar puncture was undertaken, showing leucocytes 10/μL, RBC 4,800/μL, glucose 87 mg/dL, protein 50.6 mg/dL, negative culture, and antibiotic treatment was commenced with cefotaxime.
An extended nasopharyngeal viral panel, including SARS-CoV-2, was negative. More, cerebrospinal fluid PCR was negative for extended viral panel as well as bacterial Neisseria meningitides, Streptococcus pneumonia, Hemophilus influenzae type b, and Listeria monocytogenes.
However, two days later, four days after the onset of the fever, the patient developed strawberry tongue, cheilitis, and widespread maculopapular blanching rash over the limbs and torso. An initial echocardiogram was normal, but an abdominal echo showed edema surrounding the gallbladder. A diagnosis of incomplete Kawasaki was made. The patient was treated with IVIG 2 g/kg and high-dose aspirin 80 mg/kg/day. The fever subsided after 72 hours; however, he did develop peripheral and scrotal edema, which resolved.
Despite being afebrile, two days after the initial fever resolution, on the ninth day of illness, a new onset of fever was noted up to 38.2°C, so oral prednisolone was commenced at a dose of 2 mg/kg/day. Subsequently, desquamation of the fingers was noted.
On the 11th day of admission, 13 days after the initial pyrexia, a new echocardiogram showed dilation of the left coronary artery (LCA) with a 2.3 mm diameter (previously 1.7 mm), z score 2.9, and the left anterior descending (LAD) branch had a diameter of 2.2 mm with a z score of 3.87. The right coronary artery (RCA) measured 2.1 mm (previously 1.7 mm) with a z score of 2.9 (Dallaire and Dahdah, JASE 2011 [4]). The next day, as he continued to be pyrexial with a fever up to 37.7°C, he was transferred to our hospital (Table 1).
Timeline.
| Timeline | Intervention |
|---|---|
| Seventy-two hours before admission | 1st dose of the hexavalent routine immunization |
| Day 1: 48 hours before admission | Pyrexia up to 40°C |
| Day 3: admission, pyrexia, bulging fontanelle, diffuse maculopapular rash | Lumbar puncture, cefotaxime 200 mg/kg/day for 7 days total |
| Day 4: rash, diagnosis of “incomplete” Kawasaki | IVIG 2 g/kg |
| Day 5: pyrexial | Aspirin 80 mg/kg/day, also gentamycin 7.5 mg/kg/day and omeprazole 2 mg/kg/day |
| Day 7: peripheral oedema, scrotal swelling | - |
| Day 9: relapse pyrexia 38.2°C | Prednisolone po 2 mg/kg/day |
| Day 10: fever subsided | Change antibiotics to ampicillin-clavulanate |
| Day 11: desquamation of the fingers | - |
| Day 13: cardiac echocardiography | Generalized dilation of the coronary arteries. LCA 2.3 mm, z score 2.9; LAD 2.2 mm, z score 3.87 |
| Day 14: new onset of pyrexia 37.7°C | Transferred to our centre. Aneurysm LCA 2.6 mm, LAD 3.4 mm, RCA 3.1 mm |
| Day 15 | Second dose IVIG 2 g/kg, methylprednisolone 2 mg/kg, anakinra 8 mg/kg, infliximab 25 stat |
| Day 17: apyrexial | Commenced SC tinzaparin 1,000 IU × 1 |
| Day 19 | Decrease methylprednisolone 1.5 mg/kg, SC anakinra 4 mg/kg |
IVIG: intravenous immunoglobulin; LAD: left anterior descending; LCA: left coronary artery; po: per os; RCA: right coronary artery; SC: subcutaneous; -: no data.
At our hospital, a new echocardiogram was performed. It showed good systolic function of the left ventricle, mitral and tricuspid regurgitation, and a small aneurysm of LCA 2.6 mm (z score 3.86), a medium aneurysm of the LAD 3.4 mm (z score 6.41), and RCA 3.1 mm (z score 6.94) (Figure 1A and 1B). Subsequently, a second dose of immunoglobulin was given. The oral prednisolone was changed to intravenous methylprednisolone, followed by a single dose of infliximab. More, anakinra was added 8 mg/kg as it was felt that an intensification of therapy was warranted due to the rapid appearance of medium-sized aneurysms, the persistence of a high white cell count and platelets, and high inflammatory markers. However, the coagulation screening was normal. Regarding the cardiac markers, the troponin was borderline raised (15.9 pg/mL, normal < 14 pg/mL), and the NT proBNP was raised to 220 pg/mL. Finally, the patient’s young age < 6 months placed him in a higher risk category (Table 2). As per American Heart Association (AHA) guidelines, the patient was also given tinzaparin and anakinra, continued at a reduced dose of 4 mg/kg/day.
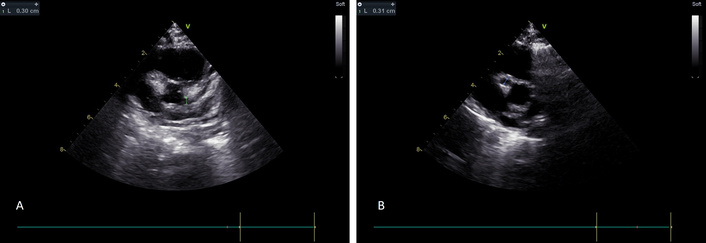
Echocardiogram on day 14. (A) Dilation of the left coronary artery and the left anterior descending branch. (B) Dilation of the right coronary artery.
Laboratory results.
| Laboratory parameters | Day of admission | |||||||
|---|---|---|---|---|---|---|---|---|
| 14/7/2023 | 16/7/2023 | 20/7/2023 | 26/7/2023 | 31/7/2023 | 2/8/2023 | 8/8/2023 | 11/8/2023 | |
| White cell count (× 103/μL) | 8,500 | 24,900 | 21,100 | 24,760 | 29,940 | 19,360 | 17,270 | 24,900 |
| Polymorphs (%) | 33.0 | 50.0 | 51.0 | - | 41.0 | - | 27.5 | 44.0 |
| Lymphocytes (%) | 28.0 | 34.0 | 26.0 | 52.0 | 43.8 | - | 49.8 | 41.0 |
| Monocytes (%) | 9.0 | 6.0 | 10.0 | 13.0 | 8.2 | 14.0 | 13.9 | 12.0 |
| Eosinophils (%) | 0.0 | - | 6.0 | 3.0 | 1.3 | 1.0 | 3.2 | 3.0 |
| RABDO (%) | 30 | 10 | 5 | - | - | - | - | - |
| Hemoglobin (g/dL) | 9.5 | 8.9 | 7.9 | 7.8 | 8.6 | 8.6 | 9.9 | 8.4 |
| Hematocrit (%) | 27.9 | 25.3 | 22.7 | 25.0 | 28.6 | 28.2 | 31.2 | 31.4 |
| Mean corpuscular volume (fL) | 87.7 | 86.0 | 86.5 | 86.1 | 85.9 | 88.6 | 83.5 | 82.6 |
| Mean corpuscular hemoglobin (pg) | 29.7 | 30.2 | 30.0 | 27.0 | 26.2 | 27.1 | 26.5 | 22.1 |
| RDW (%) | 12.9 | 13.6 | 14.6 | - | - | - | - | - |
| Platelets (× 103/μL) | 505,000 | 386,000 | 583,000 | 693,000 | 850,000 | 888,000 | 891,000 | 900,000 |
| ESR (mm/h) | - | - | - | - | 75 | 101 | 55 | 18 |
| CRP (mg/dL) | 32.00 | 31.70 | 24.60 | 70.60 | 10.70 | 7.57 | - | 28.30 |
| Glucose (mg/dL) | 163 | 81 | 85 | 82 | 102 | 101 | 110 | 115 |
| Urea (mg/dL) | 17 | 17 | 7 | 18 | 14 | 39 | 22 | 20 |
| Creatinine (mg/dL) | 0.26 | - | 0.22 | 0.23 | 0.23 | 0.25 | 0.25 | 0.27 |
| Potassium (mmol/L) | 4.4 | 3.8 | 4.7 | 4.9 | 5.6 | 4.8 | 5.3 | 5.0 |
| Sodium (mmol/L) | 134 | 134 | 139 | 136 | 135 | 134 | - | 135 |
| Chloride (mmol/L) | - | 104 | 102 | 100 | 99 | 98 | 97 | 100 |
| Calcium (mmol/L) | - | - | - | 9.4 | 10.6 | 10.6 | 10.8 | 10.1 |
| SGOT (IU/L) | 43 | 22 | 26 | 81 | 52 | 39 | 37 | 34 |
| SGPT (IU/L) | 25 | 14 | 9 | 52 | 51 | 35 | 42 | 30 |
| GGT (IU/L) | - | - | - | 63 | 140 | 115 | 88 | 74 |
| Total bilirubin (mg/dL) | - | - | - | 0.27 | - | - | 0.16 | 0.15 |
| DBILI (mg/dL) | - | - | - | 0.13 | - | - | 0.09 | 0.09 |
| Fibrinogen (mg/dL) | - | - | - | - | - | - | 300 | 332 |
| NT proBNP (pg/mL) | - | - | - | - | - | 220 | - | - |
CRP: C-reactive protein; DBILI: direct bilirubin; ESR: erythrocyte sedimentation rate; GGT: gamma-glutamyl transferase; RABDO: rhabdomyolysis; RDW: red cell distribution width; SGOT: serum glutamate oxaloacetate transaminase; SGPT: serum glutamate pyruvate transaminase; -: no data.
The antibodies for SARS-CoV-2 IgG2 taken on day 17 were positive at 23,847.2 AU/mL (negative < 50 AU/mL).
On day 23, a repeat echocardiogram showed mild mitral and tricuspid regurgitation, LCA 2.6 mm (z score 3.86), LAD ectasia, measuring 3.9 mm (z score 9.66), and RCA with dilation of the proximal part of 4 mm (z score 8.94). The distal part was 2.9 mm (z score 4) (Figure 2).
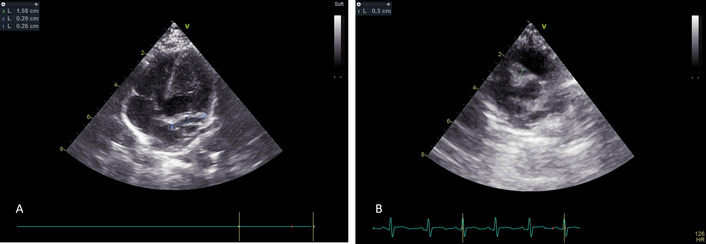
Echocardiogram on day 23. (A) Dilation of the left coronary artery and left anterior descending branch. (B) Remaining right coronary artery dilation.
He continued to be stable until discharge and follow-up.
One year later, the repeat echocardiogram showed improvement of the aneurysms with the LCA measuring 1.72 mm (z score 1.07) and the RCA measuring 1 mm (z score 0.53) (Figure 3).
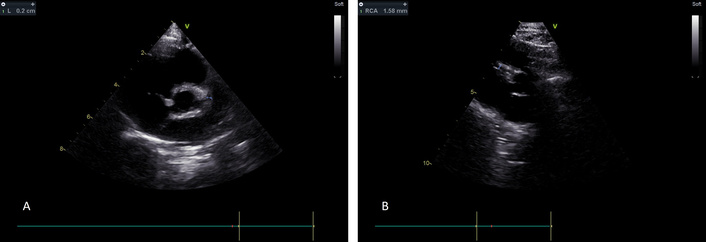
Echocardiogram one year later showing full resolution of the aneurysms. (A) The LCA aneurysm has fully resolved. (B) Similarly, the RCA now has a normal diameter. LCA: left coronary artery; RCA: right coronary artery.
Kawasaki disease and multisystem inflammatory syndrome in children (MIS-C) appear to share some of the host immunological responses to a viral infection and subsequently clinical patterns. However, they also diverge in epidemiology, clinical presentation, and laboratory characteristics [5, 6].
Initial observations mentioned the decreased incidence of thrombocytosis in MIS-C [7, 8]. MIS-C patients typically have lower lymphocyte, platelet, and albumin levels but higher C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), ferritin, D-dimers, and cardiac markers than patients with Kawasaki disease [9].
Moreover, there are differences in epidemiology and clinical presentation of these two conditions. Both conditions have a predilection for boys. Kawasaki disease is more prevalent in Asians, whereas MIS-C is more frequent in Hispanic/African children [10].
Although both conditions may result in coronary aneurysms, they are more frequent in Kawasaki disease than in MIS-C. On the contrary, cardiac dysfunction such as ventricular dilation and need for vasopressors and intensive care admission are more likely to be associated with MIS-C. That is confirmed in laboratory indices such as high levels of NT proBNP and troponin levels [9, 10]. Moreover, multiorgan dysfunction is more likely in MIS-C. Pulmonary dysfunction is usually absent in Kawasaki but is frequently present in MIS-C [11]. Gastrointestinal complaints are more persistent in MIS-C [11–13].
Both conditions are characterized by increased CRP and ESR. However, increased white cell count, thrombocytosis, and eosinophilia are often observed in Kawasaki disease. In MIS-C, however, bone marrow suppression results in leucopenia and thrombocytopenia. Fibrinogen and ferritin levels are also elevated [11–13].
Due to the high titers of SARS-CoV-2 antibodies and the absence of any other virus, the diagnosis of MIS-C associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19 was considered. Although fever was present, apart from the skin changes, there was no other notable multisystem involvement that would contradict the definitions of MIS-C set by the Centers for Disease Control, Royal College of Pediatric and Child Health, and the World Health Organization, respectively [14–17]. Moreover, the age of the patient is more in keeping with Kawasaki disease rather than MIS-C. He did not fulfill the criteria for neonatal MIS [18]. The mucocutaneous symptoms of the baby were reminiscent of the classically described Kawasaki disease [19–21]. Finally, our patient showed a white cell count and high lymphocyte counts. There was thrombocytosis. Although the inflammatory markers were raised, the cardiac function markers were not significantly raised. Also, the fibrinogen was normal (Table 2). Resistance to immunoglobulins is associated with the development of coronary aneurysms. Therefore, adjunct therapies are being evaluated [22]. Interleukin 1 (IL-1) receptor blockade has proven to be beneficial in preventing myocardial dysfunction [23].
That is not unprecedented, given the pivotal role that IL-1α plays in the proinflammatory cycle of many tissues [24]. IL-1 blockade has been used in polygenic autoinflammatory disorders such as systemic juvenile idiopathic arthritis, adult-onset Still’s disease, idiopathic recurrent pericarditis, Behcet syndrome, and gout [25]. Anakinra, an IL-1α receptor antagonist, has been used as a second-line therapy in giant coronary artery aneurysms in Kawasaki disease [26]. Anakinra has been used as an off-label therapy for the treatment of IVIG-resistant Kawasaki disease in doses of 1–9 mg/kg/day [27]. Similarly, other studies report dosing schedules from 1–8 mg/kg/day [28]. There is less information about neonatal dosing. However, off-label anakinra has been used in neonates with Kawasaki disease from 1 to 6–9 mg/kg/day [29].
Indeed, anakinra has been used in IVIG-resistant Kawasaki to promote apyrexia and prevent the appearance of coronary aneurysms [30]. More relevant, anakinra has been used as a second-line treatment in the case of infants with resistant Kawasaki and giant aneurysms. The result was improvement and stability, but not complete resolution [31, 32]. In older infants, however, with resistant Kawasaki and medium to giant aneurysms treated with anakinra, there was complete resolution of the aneurysms [33–35]. Recurrent Kawasaki has been successfully treated with adjunctive anakinra, with full resolution of medium-sized aneurysms [36]. Treatment with anakinra has also resulted in the resolution of medium-sized aneurysms in older children as well [37, 38]. In a case series of 11 children with ages from 4 months to 9 years, all patients exhibited improvement with variable regression of the coronary aneurysms, with one exception, who died from complications of coronary artery rupture [39]. A trial of anakinra in refractory Kawasaki has shown some improvement in the dilation of coronary arteries and improvement of fever [40] (Table 3).
Literature review.
| Reference number | Study | Year | Study type | Number of patients | Sex | Age | Condition | Reason | Treatment | Outcome | Follow up | Max z score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [30] | Sánchez-Manubens et al. | 2017 | Case report | 1 | Female | 3 years | KD | Resistant | 2 doses IVIG + glucocorticosteroids + aspirin + anakinra 2 mg/kg OD for 14 days | No CAA | 16 weeks | No CAA |
| [31] | Shafferman et al. | 2014 | Case report | 1 | Female | 11 weeks | KD + MAS | Aneurysms | 3 doses IVIG + aspirin + glucocorticosteroids + infliximabAnakinra 3 mg/kg BD for 3 days, then 3 mg/kg TID | Mild dilation RCA | 8 months | 11 (giant) |
| [32] | Walser et al. | 2020 | Case report | 1 | Male | 3 months | KD resistant | Resistant + aneurysms | 2 doses IVIG + aspirin + etanercept + anakinra 6–8 mg/kg/day | Improvement | 2 years | 11 (giant) |
| [33] | Lind-Holst et al. | 2019 | Case report | 1 | Male | 12 weeks | KD + MAS | Aneurysms | 2 doses IVIG + aspirin + anakinra 10 mg/kg/day | CAA stable | 19 months | 12.68 (giant) |
| [34] | Gambacorta et al. | 2020 | Case report | 1 | Male | 9 months | KD + MAS | Resistant + aneurysms | 2 doses IVIG + clopidogrel | Normalization of CAA | 1 year | 11.49 (giant) |
| [35] | Barbara et al. | 2021 | Case report | 1 | Male | 12 months | KD + Salmonella so | Resistant + aneurysms | 2 doses IVIG + aspirin + anakinra 8 mg/kg/day | Normalization of CAA | 19 days | 3.27 (small) |
| [36] | Guillaume et al. | 2018 | Case report | 1 | Male | 18 months | KD | Resistant + aneurysms | 2 doses IVIG + glucocorticosteroids + aspirin + anakinra 6 mg/kg/day | Improvement | 7 months | 9.94 (large) |
| [37] | Bossi et al. | 2022 | Case report | 1 | Male | 2 months | Recurrent KD | Aneurysms | IVIG + corticosteroids + aspirin + anakinra 2 mg/kg/day | Normalization of CAA | 2 years | 4.89 (medium) |
| [38] | Blonz et al. | 2020 | Case report | 1 | Female | 16 years | KD | Resistant + aneurysms | IVIG + heparin + anakinra 100 mg SC | Improvement | 6 months | Aneurysmal dilation 9 mm |
| [39] | Kone-Paut et al. | 2018 | Retrospective case series | 11 | 8 male, 3 female | 4 months–9 years | KD resistant | Resistant IVIG | IVIG + corticosteroids + aspirin + anakinra 2–6 mg/kg/day | 1 died, 10 improved | Variable | Variable |
| [40] | Kone-Paut et al. | 2020 | Prospective open-label study | 16 | 14 male, 2 female | 3 months–83 months | KD resistant | Resistant | Variable | Improvement | 45 days | Variable |
BD: bis die; CAA: coronary artery abnormalities; IVIG: intravenous immunoglobulin; KD: Kawasaki disease; MAS: macrophage activation syndrome; OD: once daily; RCA: right coronary artery; SC: subcutaneous; so: status post; TID: three times daily.
Future and existing trials will help clarify the potential role of prompt treatment to prevent coronary aneurysm formation.
CRP: C-reactive protein
ESR: erythrocyte sedimentation rate
IL-1: interleukin 1
IVIG: intravenous immunoglobulin
LAD: left anterior descending
LCA: left coronary artery
MIS-C: multisystem inflammatory syndrome in children
RCA: right coronary artery
AA and NA: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. ET: Resources, Supervision. MT: Resources, Writing—review & editing. NGE: Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study was approved by the Ethics Committee of Agia Sofia Children’s Hospital Reference Number 5893/2022, and complies with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from the participant’s guardians.
Informed consent to publication was obtained from relevant participant guardians.
The data of this manuscript could be available from the corresponding authors upon reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2751
Download: 109
Times Cited: 0
