Affiliation:
Department of Internal Medicine, Mission Hospital, Asheville, NC 28801, USA
Email: jas.virk@mahec.net
ORCID: https://orcid.org/0000-0001-9938-6991
Explor Cardiol. 2025;3:101266 DOI: https://doi.org/10.37349/ec.2025.101266
Received: May 14, 2025 Accepted: July 08, 2025 Published: July 24, 2025
Academic Editor: Karina Wierzbowska-Drabik, Medical University of Lodz, Poland
We report a rare case of non-ischemic cardiomyopathy in a 62-year-old female with mucous membrane pemphigoid following rituximab therapy. The patient presented with dyspnea and chest pain, and cardiac evaluation-including transthoracic echocardiogram and nuclear medicine stress testing-revealed left ventricular dysfunction without evidence of ischemia. Given the temporal association and absence of other etiologies, rituximab was suspected as the causative agent. This case emphasizes the importance of recognizing the potential cardiotoxic effects of biological agents used in autoimmune disease management and supports the consideration of cardiac surveillance in at-risk patients receiving rituximab.
Mucous membrane pemphigoid (MMP) is a rare, chronic, autoimmune disease presenting with subepithelial blistering and erosive lesions on the mucosal surface of the mouth, eyes, and occasionally the pharynx (Figure 1) [1]. While initial treatment of mild pemphigoid of the oral cavity can be treated with topical steroid therapy, refractory treatment can involve the utilization of chronic oral steroid therapy and biologic therapies in severe disease. While these treatments can be effective in treating autoimmune conditions, many are associated with secondary effects on other organs, including the heart. Rituximab is a chimeric monoclonal antibody directed against CD20 on pre-B and B lymphocytes that has shown success in treating refractory MMP [2]. It is often used in combination with a systemic glucocorticoid, such as prednisone [3].
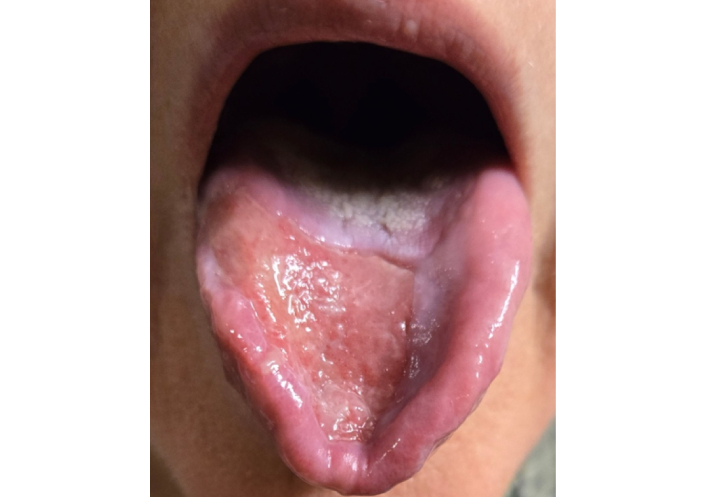
Mucous membrane pemphigoid of the tongue. Mucous membrane pemphigoid lesions on the oral mucosa and lingual surface, with post-biopsy depression noted
Despite its overall safe medication profile, infusion-related side effects of rituximab have been reported, in addition to more serious side effects, which include the development of progressive multifocal leukoencephalopathy, acute respiratory distress syndrome, myocardial infarction, angioedema, and cardiogenic shock [4]. We report a case of a 62-year-old female who developed non-ischemic cardiomyopathy shortly after receiving her first infusion of rituximab for MMP.
A 62-year-old female with a past medical history of MMP, essential hypertension, and inflammatory arthritis presented to the emergency department on 10/21/24 due to generalized weakness and chest pain. These symptoms occurred approximately one week after her first infusion of rituximab for biopsy proven MMP of the oral cavity. She was also being treated with chronic prednisone for her condition. Her only other chronic medications included metoprolol for hypertension. Upon arrival at the emergency department, she was noted to have an elevated D-dimer (2,299 ng/mL), pro-B-type natriuretic peptide (pro-BNP) (3,530 pg/mL), and troponin I (0.097 ng/mL). A CT angiogram was negative for pulmonary embolism, and the elevated troponins were likely a type II non-ST segment elevated myocardial infarction (NSTEMI) event in the setting of her acute illness, therefore, no anticoagulation was initiated. She was admitted to the observation unit for further monitoring of her weakness and dyspnea.
A 2D echocardiogram was obtained, which revealed an ejection fraction (EF) of 35% with moderate mitral regurgitation (Figure 2). Of note, a stress echocardiogram obtained three years prior revealed an EF of 50%. Subsequently, a Lexiscan stress test was obtained to better evaluate her new-onset cardiomyopathy. The EF was calculated at 38% with no evidence of ischemia (Figures 3 and 4). Due to these findings of new-onset non-ischemic cardiomyopathy, the cardiology service was consulted for further recommendations. Given the time course of the patient’s findings, there was concern that she had developed rituximab-induced cardiomyopathy. She was subsequently started on guideline-directed medical therapy with dapagliflozin and losartan, with recommendations to continue close follow-up with the cardiology service in the outpatient setting.
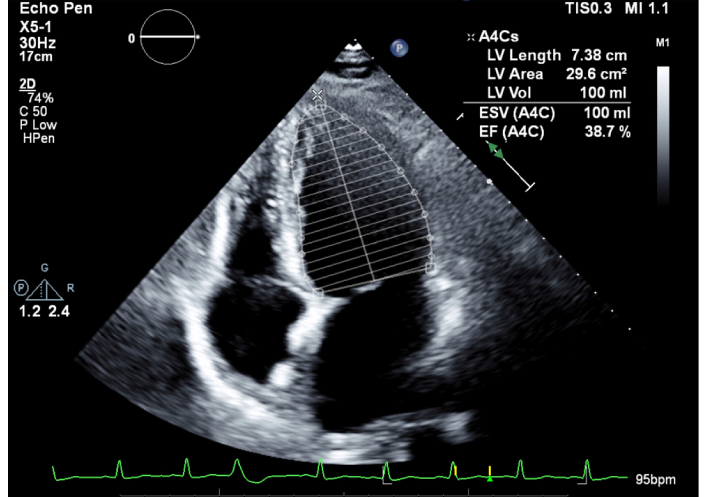
2D echocardiogram during hospitalization. LV volume, length, and area noted in legend. LV: left ventricle; EF: ejection fraction
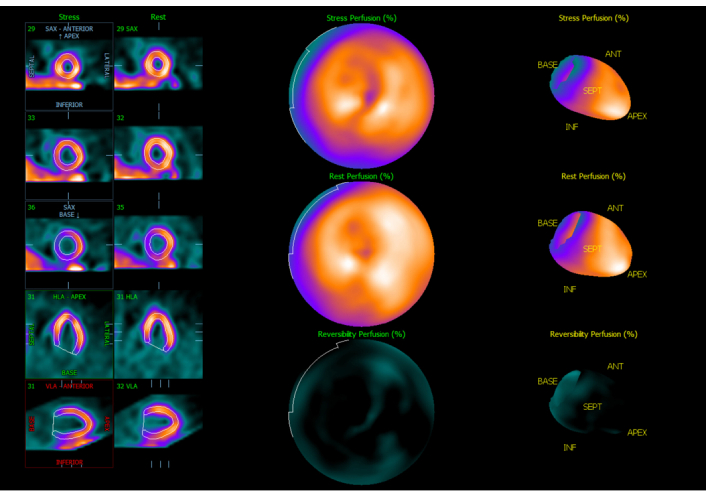
Perfusion imaging of LV. Normal LV size and perfusion at rest and with stress. LV: left ventricle
Rituximab is a highly effective monoclonal antibody with uses as both an immunological agent and an antineoplastic agent. It has been shown to be effective in numerous conditions, including non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, and myasthenia gravis [4, 5]. Its mechanism of action involves binding to CD20 cells, a marker expressed on pre-B cells and mature B cells. This binding leads to depletion of CD20+ B cells via a complement-dependent, apoptotic, or antibody-dependent cell-mediated cytotoxicity [6]. Despite the overall safety of this medication, it does carry a boxed warning in the United States. Infusion-related side effects, such as fevers, chills, and rigors, have been reported to be as high as 90% [4]. Caution is advised in patients with underlying heart disease due to reports linking rituximab to the development of arrhythmias, angina, and acute coronary syndrome [7].
The mechanism of rituximab-induced cardiac injury has not been well elucidated, but multiple hypotheses exist. A phase II study in Europe detected cardiac arrhythmias in 8.3% of patients treated with rituximab for mantle-cell lymphoma. These included atrial fibrillation and tachyarrhythmia. It is thought that these arrhythmias occur due to the release of cytokines in response to cell lysis, most prominently interleukin-6 and tumor necrosis factor α [8]. Similarly, a report of cytokine release leading to vasoconstriction, platelet activation, and plaque rupture in coronary arteries leading to myocardial infarction has also been reported after rituximab treatment [9, 10].
The pathophysiology of rituximab-induced cardiotoxicity when treating non-Hodgkin’s lymphoma has been further analyzed. Researchers noted that after rituximab infusion, tissue analysis revealed diffuse reticulin fibers in the cardiac muscle. Transforming growth factor-β was also elevated after administration of rituximab. They hypothesized that elevation of transforming growth factor-β may promote growth of reticulin fibers in cardiac muscle, leading to a reduction in cardiac function [11]. This pathophysiology is thought to be contributing to the development of non-ischemic cardiomyopathy in our patient by driving maladaptive cardiac fibrosis. Similarly, this pathophysiology was also suspected to play a role in the development of Takotsubo’s cardiomyopathy in a patient treated with rituximab for chronic lymphocytic leukemia [12].
The development of non-ischemic cardiomyopathy in our patient’s case appears temporally related to rituximab administration, with symptom onset following treatment and no prior history of cardiac dysfunction. A thorough diagnostic workup to exclude other common etiologies, including coronary artery disease, infiltrative myocarditis, and endocrine abnormalities was carefully considered and evaluated (Figure 5). While generally well tolerated, there is emerging evidence of its role in the development of various cardiovascular complications. While causality cannot be definitively established, the exclusion of other diagnoses and temporal correlation in our case supports the likely presence of drug-related adverse cardiac effects.
In conclusion, patients undergoing treatment for any chronic condition should have a thorough cardiac workup, with caution used in patients with underlying cardiac disease. Pretreatment and posttreatment 2D echocardiograms should be considered in all patients. Additionally, lab markers such as N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) and troponin I have been shown to be important when predicting left ventricular EF (LVEF) reduction during therapy [13]. Multiple reports of cardiotoxicity have been reported in patients receiving rituximab, including arrhythmias, cardiogenic shock, and myocardial infarction. Limitations to our case include that some comparison studies utilized more than one chemotherapy agent, thus extrapolating the cardiac effects to just rituximab was not possible. In addition, the principle of causality could not be determined as other factors may have also been at play, affecting the patient’s cardiomyopathy development. Our case highlights the development of non-ischemic cardiomyopathy after rituximab treatment for MMP and subsequent initiation of guideline directed medical therapy. Additional studies, especially randomized controlled trials, will be beneficial to further elucidate the relationship between rituximab and cardiotoxicity.
EF: ejection fraction
LV: left ventricle
LVEF: left ventricular ejection fraction
MMP: mucous membrane pemphigoid
NSTEMI: non-ST segment elevated myocardial infarction
NT-pro-BNP: N-terminal pro-B-type natriuretic peptide
pro-BNP: pro-B-type natriuretic peptide
The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
This research was supported (in whole or in part, non-financial support) by HCA Healthcare and/or an HCA Healthcare affiliated entity.
JV: Conceptualization, Investigation, Writing—original draft. DN: Investigation, Conceptualization, Writing—original draft. VT: Investigation, Writing—review & editing, Validation, Supervision. All authors read and approved the submitted version.
The authors declare that this research received non-financial support from HCA Healthcare and/or an HCA Healthcare affiliated entity, as acknowledged in the Acknowledgments section. HCA Healthcare is a regulatory organization overseeing Mission Hospital and approximately 200 hospitals across the United States. The authors have no personal, financial, or professional relationships with HCA Healthcare or its affiliated entities beyond the aforementioned non-financial support for this study. No other conflicts of interest are declared.
The study complies with the Declaration of Helsinki (2024 version). Ethical approval for the case report was not required according to the requirements of Mountain Area Health Education Center Ethics and Research Department.
Informed consent to participate for the study was obtained from the participant.
Informed consent to publication was obtained from the participant.
The data for this study could be available from the corresponding author upon reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3102
Download: 195
Times Cited: 0
