Affiliation:
1Division of Cardiology, San Luca Hospital, 55100 Lucca, Italy
Email: lacortig@tin.it
ORCID: https://orcid.org/0000-0002-1804-4939
Affiliation:
2Cardiology Department, Parma University Hospital, 43126 Parma, Italy
ORCID: https://orcid.org/0000-0002-7895-2840
Explor Cardiol. 2025;3:101263 DOI: https://doi.org/10.37349/ec.2025.101263
Received: May 17, 2025 Accepted: July 04, 2025 Published: July 13, 2025
Academic Editor: Dimitrois Tousoulis, Athens University Medical School, Greece
We report the case of a 58-year-old patient with chronic ischemic syndrome who underwent dipyridamole stress echocardiography for the evaluation of chest pain. The stress test was negative with normal coronary flow velocity reserve, preserved chronotropic competence, and no inducible wall motion abnormalities. However, upon administration of aminophylline at the end of the test, the patient developed acute chest pain, ST-segment elevation, and severe apical wall motion abnormalities. These findings resolved with intravenous nitrates. Subsequent coronary angiography revealed a significant vasospastic stenosis in the left anterior descending artery superimposed on a mild organic stenosis of around 50% after intracoronary nitrates. The coronary stenosis was successfully treated with a drug-eluting stent. This case highlights the potential for vasospastic angina to be unmasked following vasodilator reversal, even after a negative stress echocardiography.
Vasospastic angina (VSA), characterized by transient coronary artery spasm leading to myocardial ischemia, is a known but often underrecognized cause of chest pain, particularly when angiographically significant stenosis is absent or minimal [1]. Dipyridamole stress echocardiography is a commonly used non-invasive test to assess myocardial ischemia. Typically, a negative test suggests low likelihood of flow-limiting coronary disease. However, rare paradoxical responses, particularly after the administration of aminophylline, have been reported to provoke coronary spasm and reveal underlying VSA [2–5]. This case demonstrates the rare occurrence of coronary vasospasm triggered by aminophylline after a negative dipyridamole stress echocardiography. It adds to the literature by highlighting a paradoxical response during vasodilator reversal.
A 58-year-old female with a history of typical chest pain at rest that had been onset for approximately 4 weeks and whose attacks had become increasingly frequent (2–3 per day) was referred for dipyridamole stress echocardiography. Risk factors included hypertension and smoking.
Baseline echocardiography showed preserved left ventricular ejection fraction and no regional wall motion abnormalities. Stress was performed with the recommended methodology and standards [6]. During dipyridamole infusion, coronary flow velocity reserve (CFVR) in the mid-distal left anterior descending (LAD) coronary artery was within normal limits (> 2.0) (Figure 1), chronotropic reserve was preserved, defined as stress heart rate/rest heart rate > 1.22, and no inducible ischemia or wall motion abnormalities were observed. The test was considered negative for ischemia.
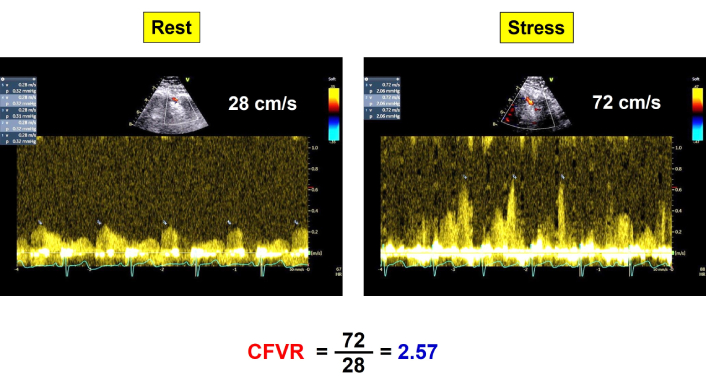
CFVR in LAD. Normal increase of diastolic coronary flow velocity (CFVR = 2.57) from rest (left) to peak stress (right). CFVR: coronary flow velocity reserve; LAD: left anterior descending
To reverse the vasodilatory effects of dipyridamole, aminophylline was administered. Immediately following its infusion, the patient experienced:
- Acute substernal chest pain;
- ST-segment elevation on electrocardiogram (ECG) (antero-apical and lateral leads) (Figure 2);
- Development of apical regional wall motion abnormality on echocardiography (Figure 3).
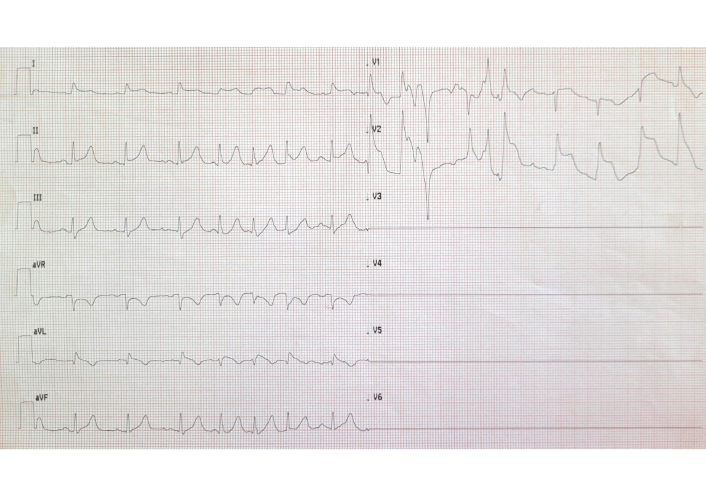
ST-segment elevation in anterolateral leads after aminophylline and chest pain. The 12-lead ECG was not technically satisfactory since the patient was immediately rushed to the catheterization laboratory. ECG: electrocardiogram
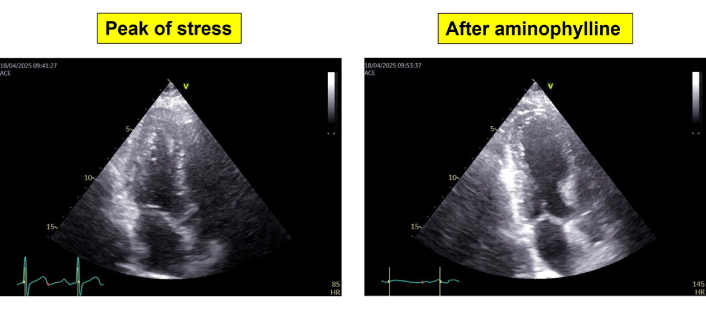
End-systolic frames from apical 5-chamber view showing a small left ventricle with normal regional wall motion at peak stress (left panel) and severe cavity dilation with apical akinesia after aminophylline (right panel)
Prompt administration of intravenous nitrates led to rapid resolution of symptoms, normalization of ECG changes, and recovery of apical wall motion.
The patient underwent urgent coronary angiography, which revealed a spontaneous focal, tight vasospastic stenosis in the mid-LAD segment (Figure 4) which was partially relieved by intracoronary nitrates, confirming the diagnosis of VSA. After nitrates an organic stenosis of around 50% was observed and treated with a drug-eluting stent (DES) to prevent recurrent spasm and ensure vessel patency.
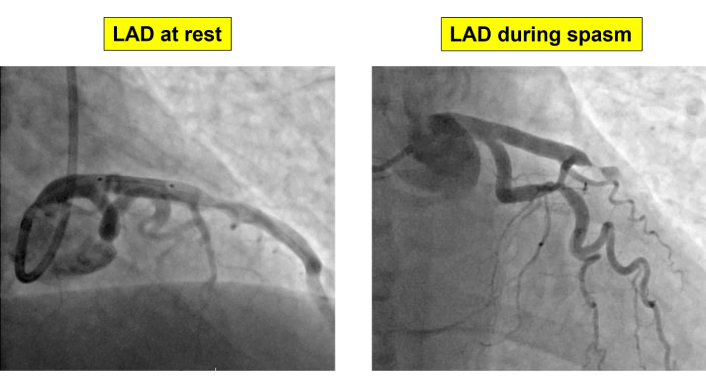
Coronary angiographic findings. Coronary vasospasm of proximal LAD resolved after intracoronary administration of nitrates, showing a residual mild 50% stenosis. LAD: left anterior descending
The patient reported feeling well after the procedure and did not experience further symptoms. Figure 5 summarizes the timeline of the events.
This case illustrates a rare but clinically significant phenomenon: the unmasking of VSA following aminophylline administration at the end of a dipyridamole stress test. While dipyridamole typically induces coronary vasodilation via adenosine-mediated pathways, its reversal with aminophylline—an adenosine receptor antagonist—may paradoxically lead to coronary vasoconstriction in susceptible individuals [7].
The development of symptoms and ischemic ECG changes in this context, despite a negative stress test, suggests that the patient’s symptoms were due to dynamic coronary spasm rather than fixed atherosclerotic obstruction. This supports the concept that normal CFVR and wall motion during pharmacologic stress testing do not exclude coronary vasomotor disorders.
Coronary vasospasm has been reported in association with various pharmacologic provocations, and in some cases, the administration of aminophylline or even the withdrawal of vasodilators can induce spasm. By blocking vasodilatory A2A receptors in coronary vessels, aminophylline removes adenosine’s vasodilatory effect, potentially resulting in unopposed vasoconstriction, especially in vessels with impaired endothelial function or spasm-prone segments. Aminophylline is a methylxanthine (like caffeine and theophylline). It also increases catecholamine release (e.g., norepinephrine) by antagonizing presynaptic adenosine A1 receptors. This sympathetic activation can result in vasoconstriction through alpha-1 receptor activation by norepinephrine, but this is an indirect effect, not due to aminophylline acting on alpha-1 receptors itself [8]. Management typically includes calcium channel blockers and nitrates [9], and the decision to stent the underlying organic coronary lesion can be associated with recurrence of symptoms due to persisting vasospasm not treated by stenting [10].
In this case, the decision to stent the culprit segment was driven by the severity of the stenosis and the clinical presentation, as symptoms in these patients were refractory to medical therapy and persisted despite intensive treatment. As reported in the literature, stenting mild-to-moderate coronary stenoses can be safe and effective in selected patients [11]. However, we recommend that the decision be individualized, taking into account the clinical presentation, response to medical therapy, and anatomical accessibility of the coronary stenosis.
In conclusion, clinicians should be aware of the potential for coronary vasospasm, particularly after pharmacologic stress testing. A negative dipyridamole test does not exclude VSA, and aminophylline can, in rare cases, precipitate coronary spasm.
CFVR: coronary flow velocity reserve
DES: drug-eluting stent
ECG: electrocardiogram
LAD: left anterior descending
VSA: vasospastic angina
LC: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. DD: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. FB: Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The Declaration of Helsinki (2024 version) was adequately addressed, and the study was approved by the institutional ethics committees in its latest versions as part of the more comprehensive Stress Echo 2030 study 291/294/295 Comitato Etico Lazio-1, March 8, 2021; Clinical trials (https://clinicaltrials.gov/). Gov Identifier NCT05081115.
Informed consent to participate in the study was obtained from the participant.
Informed consent to publication was obtained from relevant participant.
Data will not be shared as it involves patients’ privacy, but may be available from the corresponding author upon reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2654
Download: 199
Times Cited: 0
