Affiliation:
1UHasselt, Faculty of Medicine and Life Sciences, Hasselt University, 3590 Diepenbeek, Belgium
2Department of Cardiology and Jessa & Science, Jessa Hospital, 3500 Hasselt, Belgium
Email: ruben.hoffmann@jessazh.be
ORCID: https://orcid.org/0009-0004-6569-4629
Affiliation:
2Department of Cardiology and Jessa & Science, Jessa Hospital, 3500 Hasselt, Belgium
ORCID: https://orcid.org/0000-0003-4928-4836
Affiliation:
1UHasselt, Faculty of Medicine and Life Sciences, Hasselt University, 3590 Diepenbeek, Belgium
Affiliation:
1UHasselt, Faculty of Medicine and Life Sciences, Hasselt University, 3590 Diepenbeek, Belgium
Affiliation:
1UHasselt, Faculty of Medicine and Life Sciences, Hasselt University, 3590 Diepenbeek, Belgium
Affiliation:
2Department of Cardiology and Jessa & Science, Jessa Hospital, 3500 Hasselt, Belgium
ORCID: https://orcid.org/0000-0002-4303-9226
Affiliation:
1UHasselt, Faculty of Medicine and Life Sciences, Hasselt University, 3590 Diepenbeek, Belgium
2Department of Cardiology and Jessa & Science, Jessa Hospital, 3500 Hasselt, Belgium
ORCID: https://orcid.org/0000-0002-6373-180X
Explor Cardiol. 2025;3:101281 DOI: https://doi.org/10.37349/ec.2025.101281
Received: October 14, 2025 Accepted: November 10, 2025 Published: November 13, 2025
Academic Editor: Ji-min Cao, Shanxi Medical University, China
Aim: Pulmonary vein isolation (PVI) is a widely accepted and effective treatment for atrial fibrillation (AF). Even though success rates have been climbing, some patients experience AF recurrence after ablation. This study aimed to identify predictors of AF recurrence, with a focus on the potential role of premature atrial contractions (PAC).
Methods: A retrospective single-center analysis was conducted on 185 patients with AF who underwent primo PVI at a single center between 07/2014 and 01/2017. Patients underwent AF ablation using radiofrequency ablation (n = 61), by the CARTO (n = 50) and EnSite (n = 11) mapping systems, and the endoscopic laser balloon (n = 124). Exclusion criteria were combined procedures or the absence of a 24-hour Holter recording three months post-ablation. The primary endpoint was freedom from atrial arrhythmia 12 months after ablation with an application of a 90-day blanking period.
Results: Survival analysis revealed a significant difference in AF recurrence rates between low and high PAC burden groups (log-rank test, p = 0.004). ROC-analysis identified an optimal PAC burden cut-off of 57 PAC’s over 24 hours (AUC 0.69). This association remained significant in multivariable Cox proportional hazards analysis, with a hazard ratio of 3.38 (p = 0.021).
Conclusions: PAC burden measured on 24-hour Holter monitoring at three months proved to be an independent predictor of AF recurrence following PVI. Multivariable analysis confirmed a significant hazard ratio of 3.38 for AF recurrence within one year. An optimal predictive threshold of 57 PAC demonstrated high negative predictive value for AF recurrence.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is associated with significant morbidity, including stroke, heart failure, and reduced quality of life. Pulmonary vein isolation (PVI) has become a cornerstone in the management of AF, particularly for patients who are refractory to antiarrhythmic drugs [1, 2]. Despite its proven efficacy, AF recurrence after PVI remains a significant challenge, with reported recurrence rates ranging from 25% to 40% within the first-year post-ablation [1].
The mechanisms underlying AF recurrence after ablation are multifactorial, involving a combination of patient-specific factors, procedural variables, and atrial substrate characteristics [1]. Among these, premature atrial contractions (PAC) have been implicated as potential triggers for AF, and its association with AF recurrence after ablation has been of recent interest [3–6]. PAC may reflect a diseased atrial substrate, characterized by increased fibrosis, electrical remodeling, or persistent ectopic activity, which predisposes patients to arrhythmia recurrence [3].
While previous studies have examined predictors of AF recurrence, the role of PAC burden following PVI has not been comprehensively evaluated. These PAC can reflect an underlying atrial substrate and potentially trigger arrhythmias. While smaller studies suggest that PAC burden correlates with AF recurrence, robust evidence remains limited, and optimal thresholds for prediction vary widely [4–8]. Furthermore, in these studies, the time of Holter monitoring ranges from the day of procedure to 6 months post ablation [4–8]. This study aims to investigate the predictive value of PAC burden for AF recurrence, as measured by 24-hour Holter monitoring at three months post-ablation, based on the 2017 guideline, leaving a blanking period for 3 months to allow dissipation of acute inflammatory effects [9]. Additionally, the study evaluates whether patient demographics, procedural details, or other clinical variables contribute to recurrence risk. Understanding these factors could help refine risk stratification and guide post-ablation management strategies.
This single-center, retrospective observational study analyzed data from patients who underwent PVI at Jessa Hospital, Hasselt, Belgium, between July 2014 and January 2017. Initially, 416 patients were reviewed. Patients were included if they underwent their first endocardial AF ablation with PVI as the sole lesion set. Exclusion criteria were combined procedures, redo ablations, or the absence of a 24-hour Holter recording three months post-ablation. Lastly, patients with a presence of AF or atrial tachycardia on 24-hour Holter lasting more than 30 s were also excluded. The final analysis included 185 patients. Data was obtained from the C2M online patient database. This study was approved by the ethics committee of Jessa Hospital.
All procedures were performed under general anesthesia. An activated clotting time of ≥ 300 s was maintained throughout the procedure. Catheter choice depended on operator preference and patient-specific factors. Three distinct ablation techniques, all targeting PVI, were employed:
CARTO 3 Mapping System: Used to guide point-by-point radiofrequency ablation via detailed electro-anatomical mapping by making use of the ThermoCool SmartTouch catheter using standard radiofrequency energy settings, typically between 35 and 45 W in a power-controlled mode (Biosense Webster, Inc., Diamond Bar, CA, USA) [10, 11].
EnSite NavX Velocity Mapping System: Facilitated point-by-point radiofrequency ablation with precise mapping also by using the ThermoCool SmartTouch catheter (St. Jude Medical, Inc., St. Paul, MN, USA) [10, 11].
Laser Balloon Ablation: Utilized endoscopic laser energy for circumferential PVI (Cardiofocus, Inc., Marlborough, MA, USA) [12].
Anticoagulation was continued post-procedure and antiarrhythmic therapy was tapered, attempting discontinuation when clinically feasible. However, decisions were individualized according to the underlying substrate and patient tolerance. Follow-up was standardized according to current protocol with follow-up after three and twelve months with 24-hour Holter. Earlier consultations with electrocardiography were possible if the patient reported symptoms.
Demographic, clinical, procedural, and follow-up data were extracted from medical records. The PAC burden was assessed via 24-hour Holter monitoring, three months (±30 days) post-ablation. PAC were quantified by an automatic algorithm, which was verified by an experienced cardiac technician or cardiologist. The primary endpoint was freedom from atrial arrhythmia 12 months after ablation. Recurrence of arrhythmia was defined as any documented episode of AF or atrial tachycardia lasting at least 30 s after a 90-day blanking period, confirmed by electrocardiogram, Holter monitoring, or event recorders during follow-up [1, 9].
Continuous variables were summarized as means ± standard deviations or medians with interquartile ranges, depending on distribution. Categorical variables were expressed as frequencies and percentages. Comparisons between groups were conducted using t-tests, Mann-Whitney U tests, chi-squared tests, or Fisher’s exact tests, as appropriate. Receiver operating characteristic (ROC) analysis determined the optimal PAC threshold for predicting recurrence. Kaplan-Meier survival analysis estimated recurrence-free survival, with differences tested via the log-rank test. Multivariable Cox-regression analysis was performed to control for pre-defined potential confounders (PAC load, type AF, ablation system used, rhythm post-catheterisation, gender, age, left atrial volume, presence of obesity, presence of arterial hypertension). This was preferred to propensity score matching as Cox regression usually performs better in survival analysis [13–15]. All analyses were conducted using R software (The R Foundation for Statistical Computing, Vienna, Austria; version 4.3.2), with statistical significance set at p < 0.05.
The median PAC burden three months post-ablation was 50 (16–145) beats/24 hours. ROC analysis established an optimal cut-off of 57 PAC/24 hours, yielding a sensitivity of 0.80, specificity of 0.59, positive predictive value (PPV) of 0.19, and negative predictive value (NPV) of 0.96 (Figure 1). Based on this cutoff, patients were classified into low (≤ 57 PAC/24 hours) and high (> 57 PAC/24 hours) PAC burden groups.
Baseline characteristics can be found in Table 1. Patients with high PAC burden were older and had larger left atrial diameters compared to those with low burden. Other patient characteristics were not significantly different. Procedural parameters were similar along both high and low PAC burden, with no significant difference in mapping method (p = 0.16). Kaplan-Meier analysis demonstrated significantly lower recurrence-free survival in the high PAC burden group (95% [95% confidence interval (CI): 0.91–0.99] vs. 80% (CI: 0.72–0.89), log-rank p = 0.004) (Figure 2). No significant differences in recurrence rates were observed across ablation techniques (p = 0.76) (Figure 3). Multivariable Cox proportional hazards analysis confirmed PAC burden as an independent predictor of AF recurrence [hazard ratio (HR) 3.38, CI: 1.20–9.50, p = 0.021], as illustrated in Figure 4. Patients who remained in AF immediately post-ablation and had an electrical reconversion demonstrated a borderline non-significant increase in risk of recurrence (HR 2.38, CI: 0.85–6.60, p = 0.099).
Baseline characteristics
| Baseline characteristics | PAC low(n = 102) | SD/%/IQR | PAC high(n = 83) | SD/%/IQR | p-value |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Continuous | |||||
| Age (years) | 61 | 55–68 | 64 | 59–69.5 | 0.035* |
| BMI | 26.8 | 24.6–30.0 | 28.1 | 24.7–30.7 | 0.82 |
| LV ejection fraction (%) | 60 | 60–60 | 60 | 60–60 | 0.699 |
| Left atrial diameter (mm) | 38.5 | 36–41 | 40 | 37–44 | 0.049* |
| GFR (mL/min) | 79.44 | 18.81 | 78.27 | 16.76 | 0.66 |
| Categorical | |||||
| Gender, male (%) | 76 | 74.5% | 61 | 73.5% | 0.875 |
| AF type paroxysmal (%) | 69 | 67.6% | 51 | 61.4% | 0.38 |
| Arterial hypertension (%) | 89 | 87.3% | 66 | 79.5% | 0.156 |
| Procedural parameters | |||||
| Continuous | |||||
| Procedural time (min) | 138 | 120–179 | 145 | 115–172 | 0.659 |
| Fluoroscopy time (min) | 13.3 | 9.4–19 | 13.2 | 8.5–17.8 | 0.49 |
| DAP (mGy/cm2) | 6,640 | 3,934–11,111 | 6,250 | 4,170–10,640 | 0.694 |
| Categorical | |||||
| Rotational angiography (%) | 92 | 90.2% | 71 | 85.5% | 0.331 |
Parametric continuous variables are given as mean ± SD; non-parametric continuous variables are given as the median [interquartile range]; categorical variables are given as n (%); *: p < 0.05. PAC: premature atrial contractions; LV: left ventricle; GFR: glomerular filtration rate; DAP: dose area product.
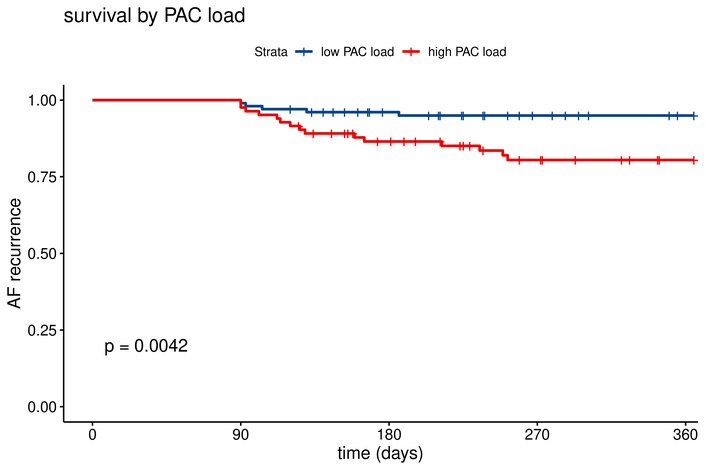
Kaplan-Meier curve by PAC load for recurrence rate of atrial arrhythmia. Low PAC load: 95% vs. high PAC load: 80.4%. AF: atrial fibrillation; PAC: premature atrial contractions.
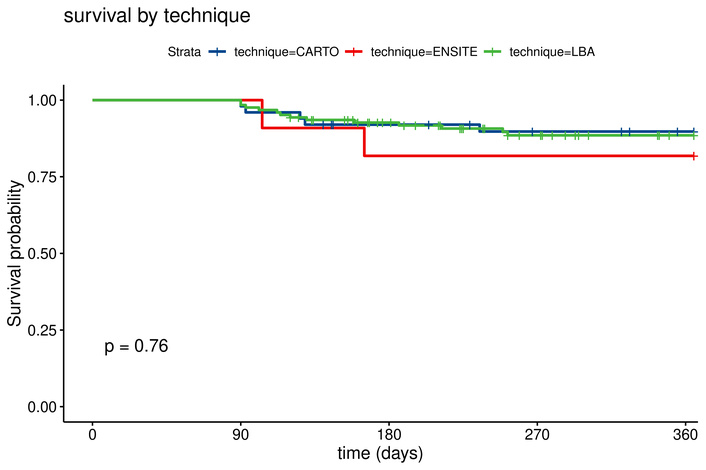
Kaplan-Meier curve by ablation technique for recurrence rate of atrial arrhythmia. CARTO: 90% vs. EnSite: 82% vs. laser balloon: 86%. LBA: Laser Balloon Ablation.
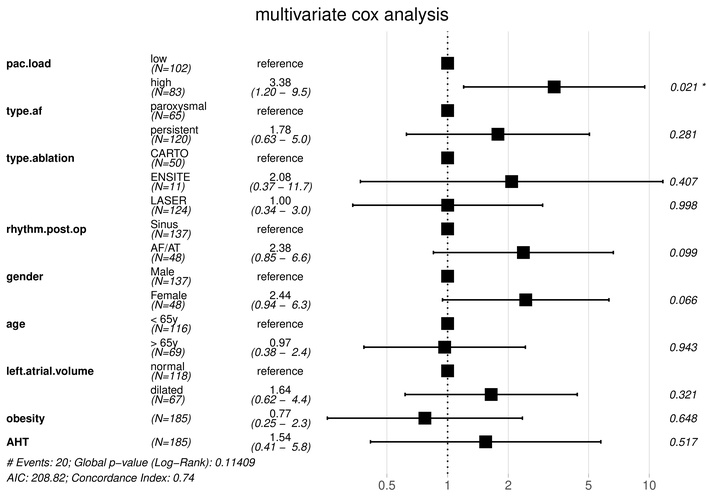
Multivariable Cox analysis evaluating predictors of AF recurrence after PVI. Variables included in the model were selected based on clinical relevance. Hazard ratios > 1 indicate increased risk of AF recurrence. Confidence intervals represent 95% bounds. AF: atrial fibrillation; AHT: arterial hypertension; PAC: premature atrial contractions; PVI: pulmonary vein isolation.
No significant differences in AF recurrence were observed between the three ablation techniques (CARTO®, EnSite NavX™, and Laser Balloon Ablation). Similarly, other known risk factors, including left atrial size and type of AF, did not show statistical significance in this cohort.
This study evaluated clinical and procedural predictors of AF recurrence after PVI, focusing on the burden of PAC three months post-ablation. PAC burden, as measured by 24-hour Holter monitoring, was a significant predictor of AF recurrence, with an optimal threshold of 57 PAC/24 hours. Patients with a PAC burden above this threshold demonstrated a higher risk of recurrence, whereas a low PAC burden was associated with a high NPV, suggesting a low likelihood of recurrence. PAC burden may reflect a more diseased atrial substrate, characterized by increased fibrosis or electrical remodeling, which predisposes to AF recurrence. In this study, a PAC burden > 57 was associated with an HR of 3.38 for recurrence, even after adjusting for known confounders.
The role of PAC in predicting AF recurrence aligns with previous studies highlighting the arrhythmogenic potential of these PAC [4–8]. However, cutoff points varied, most likely due to variance in timing and evaluation methods. For example, Fujisawa et al. [4] and Alhede et al. [6] both had monitoring post-procedure, while Gang et al. [5] and Inoue et al. [7] had monitoring after 6 months. Coutiño et al. [8] timed their monitoring after 1 month. When looking at the respective cutoff points, one finds that the cutoff point for early monitoring (783–1,431) is much higher than that with late monitoring (58–142). This is to be expected, as in catheter ablation, a blanking period is in place, as early recurrence may happen but does not necessarily equal a late recurrence. Furthermore, the PAC burden may be higher in the blanking period compared to post-blanking. This is based on the inflammatory and autonomic responses of the heart caused by radiofrequency ablation [16–18]. Optimal evaluation of PAC might therefore be planned after the blanking period, such that the inflammatory response has been reduced.
While statistically significant in both univariate and multivariable analysis, sensitivity (0.80) and specificity (0.59) remained low with an AUC of 0.69. Inoue et al. [7] found an AUC of 0.63, similar to that of Fujisawa et al. [4] with an AUC of 0.61. In the current study, this low AUC translated also to a very low PPV of 0.19, which on first sight might suggest very low clinical significance. However, the NPV was very high (0.96), which may be relevant for risk stratification in follow-up. This pattern indicates that a low PAC burden effectively rules out likely recurrence (useful to de-escalate follow-up), whereas a high PAC burden does not reliably predict recurrence for an individual (as over 80% of high-PAC patients did not have documented recurrence). Clinically, a high PAC burden should prompt closer surveillance (e.g., extended monitoring, earlier clinic review) rather than immediate re-intervention.
The relationship between PAC burden and AF recurrence may also indicate that a successful PVI effectively suppresses ectopic triggers, reducing PAC activity. A higher PAC burden, however, could signal the presence of a pulmonary vein reconnection or a more advanced atrial substrate, which PVI alone may not adequately address.
Our analysis revealed no significant differences in recurrence rates between the three ablation techniques (CARTO®, EnSite NavX™, and Laser Balloon Ablation). This finding aligns with previous studies showing comparable success rates among these technologies when the endpoint of durable PVI is achieved [11, 12, 19]. While procedural nuances may vary, the choice of ablation technique did not influence long-term outcomes in this cohort, suggesting that factors such as patient selection may be more critical determinants of success.
Interestingly, several variables commonly associated with AF recurrence, including left atrial size and AF type (paroxysmal vs. persistent), were not significant in this study. This may be explained by the relatively small sample size and potential collinearity between these variables and PAC burden. For example, both persistent AF and larger left atrial size often indicate more extensive atrial remodeling, which could also manifest as an elevated PAC burden.
Post-procedural AF presence demonstrated a borderline significant HR of 2.38 for recurrence. This finding suggests that patients in AF immediately post-ablation may have underlying arrhythmogenic substrates beyond the pulmonary veins, increasing the likelihood of recurrence. However, this variable did not reach statistical significance in multivariable analysis, likely due to the limited sample size.
Left atrial diameter was higher in the high-PAC group in univariate comparisons (Table 1). However, left atrial volume was not independently significant in the multivariable model (HR 1.64, p = 0.321), potentially due to collinearity with PAC burden or limited power. This suggests PAC burden may encapsulate functional aspects of atrial remodeling not fully captured by diameter alone.
This study has several limitations that warrant consideration. First, as a single-center, retrospective analysis, the findings may not be generalizable to other populations or settings. Second, the reliance on 24-hour Holter monitoring may have underestimated AF recurrence, particularly asymptomatic episodes. Continuous monitoring over extended periods could provide more accurate recurrence rates. Furthermore, pre-procedural PAC burden was not available in a standardized manner for the full cohort. Therefore, we could not analyze relative change in PACs pre- versus post-ablation. Third, the lack of a standardized protocol for antiarrhythmic drug use during follow-up may have introduced variability in outcomes. Finally, the small sample size limits the ability to detect statistically significant associations for certain variables.
In summary, this study underscores the importance of PAC burden as an independent predictor of AF recurrence after PVI. A PAC threshold of 57 beats on 24-hour Holter monitoring provides a valuable tool for risk stratification, enabling clinicians to identify high-risk patients who may benefit from closer follow-up or additional interventions. Conversely, patients with a low PAC burden can be reassured of a low likelihood of recurrence. Further prospective studies with larger cohorts are needed to validate these findings and explore the mechanistic links between PAC burden and atrial substrate pathology.
AF: atrial fibrillation
CI: confidence interval
HR: hazard ratio
NPV: negative predictive value
PAC: premature atrial contractions
PPV: positive predictive value
PVI: pulmonary vein isolation
ROC: receiver operating characteristic
This study is part of Limburg Clinical Research Center, supported by Hasselt University, Jessa Hospital, and Ziekenhuis Oost-Limburg. The support provided to this manuscript did not involve financial funding, and the institutions had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
RH: Formal analysis, Data curation, Writing—original draft. JV: Writing—review & editing. ZA: Investigation, Writing—original draft. HVE: Investigation, Writing—original draft. PVDL: Investigation, Writing—original draft. TP: Writing—review & editing. PK: Conceptualization, Investigation, Writing—original draft. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study was approved by the Jessa Hospital Ethics Committee (2023/161) and complies with the Declaration of Helsinki.
As this study is a retrospective study, informed consent, specific to this study, is not obligated as retrospective studies fall outside the scope of the Belgian Law of 7 May 2004 concerning experiments on the human person. However, following Belgian privacy law, the patient is informed about the possible processing of his personal hospital data through a signed document when the patient is admitted.
Not applicable.
The data that support the findings of this study are available from the corresponding author upon reasonable request. Due to privacy and ethical restrictions, the data are not publicly available, as they contain information that could compromise the confidentiality of research participants.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3048
Download: 101
Times Cited: 0
