Affiliation:
1Department of Cardiology, Daping Hospital, The Third Military Medical University (Army Medical University), Chongqing 400042, China
ORCID: https://orcid.org/0009-0009-9847-2885
Affiliation:
2Department of Blood Transfusion, Laboratory Medicine Center, The Second Affiliated Hospital, The Third Military Medical University (Army Medical University), Chongqing 400037, China
Email: abby_wxy666@163.com
Explor Cardiol. 2025;3:101262 DOI: https://doi.org/10.37349/ec.2025.101262
Received: April 15, 2025 Accepted: June 04, 2025 Published: July 07, 2025
Academic Editor: Karina Wierzbowska-Drabik, Medical University of Lodz, Poland
The article belongs to the special issue Cardiovascular Risk for Mothers and Offspring Resulting from Complicated Pregnancy
Peripartum cardiomyopathy (PPCM) is a rare condition characterized by heart failure secondary to left ventricular systolic dysfunction, occurring in women during the last trimester of pregnancy or within five months postpartum. Despite advancements in research, PPCM remains a life-threatening disorder associated with significant maternal morbidity and mortality. The primary clinical manifestations of PPCM result from heart failure due to impaired left ventricular systolic function. This article reviews the epidemiology, etiology, diagnostic criteria, management strategies, and outcomes of PPCM. Additionally, we present a case of a 34-year-old woman with PPCM who achieved full recovery within nine months, underscoring the importance of early recognition, prompt treatment, standardized medication, and regular follow-up.
Peripartum cardiomyopathy (PPCM) is a rare condition characterized by systolic heart failure in women occurring during the third trimester of pregnancy until 5 months after delivery. In China, PPCM has emerged as a leading non-obstetric cause of maternal death [1], with an incidence of 0.023%. It accounts for 4.25% of all pregnancy-related heart diseases and demonstrates a higher prevalence in rural and remote areas of China. Although PPCM is relatively uncommon (1 per 300 to 15,000 pregnancies), its incidence has been rising in recent years with the increasing proportion of delayed marriage/childbearing and multiple pregnancies in China [2, 3].
PPCM primarily presents as heart failure clinically with dyspnea, dizziness, chest pain, fatigue, and peripheral edema as the main symptoms. Some patients may experience arrhythmia, embolism, or acute myocardial infarction. The diagnosis of PPCM is established after exclusion of pre-existing structural heart disease and demonstration of left ventricular dysfunction by echocardiography, defined as a left ventricular ejection fraction (LVEF) < 45% and/or fractional shortening < 30% [4].
The prognosis of PPCM is highly variable. Some patients can achieve complete disappearance of clinical symptoms and normalization of clinical examination results gradually with treatment. While other patients may progress to chronic heart failure or experience sudden cardiac death [5].
The current paper highlights the importance of timely diagnosis and standardized treatment of PPCM, while enhancing the understanding of this disease.
A 34-year-old female patient was admitted to the hospital on October 24, 2021 with the main complaint of progressive shortness of breath and edema of both legs for 1 month. These symptoms were relieved in the sitting position and aggravated during daily activities, accompanied by nocturnal paroxysmal dyspnea. On admission, the vital sign measurement showed that pulse rate was 103 beats per minute, respiratory rate was 20 breaths per minute, and blood pressure was 135/72 mmHg (1 mmHg = 0.133 kPa). Edema extended from the feet to the knees. The patient denied any history of hypertension, diabetes, hepatitis and tuberculosis. She was a non-smoker and non-drinker. She had one previous vaginal delivery and a cesarean section 5 months ago. The blood tests revealed N-terminal pro-B-type natriuretic peptide (NTproBNP) 10,198 pg/mL (reference range: 0–450 pg/mL) (measured by enzyme immunoassay), albumin (ALB) 35.4 g/L (reference range: 40.0–55.0 g/L), aspartate amino transferase (AST) 169.8 U/L (reference range: 13.0–35.0 U/L), and alanine aminotransferase (ALT) 385.8 U/L (reference range: 7.0–40.0 U/L). There were no other remarkable abnormalities in her venous blood tests (Table 1).
Complete blood chemistry and hematological profile results on admission
| Test | Results/Units | Reference range |
|---|---|---|
| Complete blood count | ||
| White blood cells | 7.1 × 109/L | 3.5–9.5 × 109/L |
| Hemoglobin | 164 g/L | 115–150 g/L |
| Hematocrit | 49.0% | 35.0–45.0% |
| Platelets | 262 × 109/L | 94–268 × 109/L |
| Neutrophils | 4.53 × 109/L | 1.8–6.3 × 109/L |
| C-reactive protein | < 0.50 mg/L | 0–8 mg/L |
| Coagulation profile | ||
| Prothrombin time | 12.3 s | 9.4–13.8 s |
| International normalized ratio | 1.08 | 0.76–1.16 |
| Activated partial thromboplastin time | 26.6 s | 24–40 s |
| D-dimers | 336.69 μg/L | 0–232 μg/L |
| Urea and electrolytes | ||
| Sodium | 141.7 mmol/L | 137.0–147.0 mmol/L |
| Potassium | 4.66 mmol/L | 3.50–5.30 mmol/L |
| Urea nitrogen | 6.88 mmol/L | 2.6–7.5 mmol/L |
| Creatinine | 68.4 μmol/L | 41.0–73.0 μmol/L |
| eGFR | 111.84 mL/min/1.73 m2 | > 90 mL/min/1.73 m2 |
| Liver function tests | ||
| Alanine aminotransferase | 385.8 U/L | 7.0–40.0 U/L |
| Aspartate transaminase | 169.8 U/L | 13.0–35.0 U/L |
| Albumin | 35.4 g/L | 40.0–55.0 g/L |
| Globulin | 21.5 g/L | 20.0–40.0 g/L |
| Total protein | 56.9 g/L | 65.0–85.0 g/L |
| NTproBNP | 10,198 pg/mL | 0–450 pg/mL |
NTproBNP: N-terminal pro-B-type natriuretic peptide
Chest computed tomography (CT) showed generalized cardiomegaly, slight pericardial effusion, pulmonary venous congestion and bilateral pleural effusion (Figure 1). Electrocardiography showed sinus tachycardia, and nonspecific ST and T-wave abnormalities. Echocardiography demonstrated enlarge left atrial diameter (LAD 36 mm) and left ventricular end diastolic diameter (LVEDD 58 mm) with a reduced LVEF (27%) and left ventricular wall motion abnormality (Figure 2). Cardiac magnetic resonance (CMR) imaging showed left ventricular enlargement, mitral regurgitation, pericardial effusion and uniform signal intensity (Figure 3).
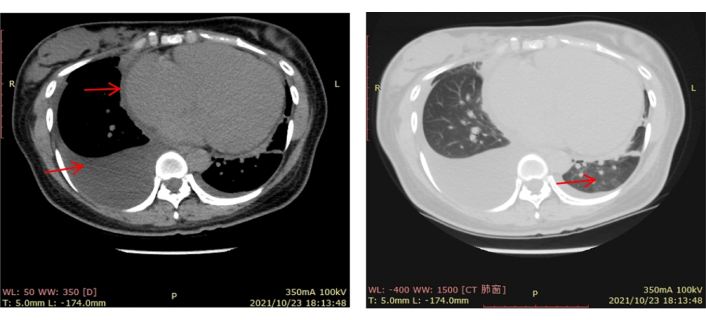
Initial CT scan of the chest. Done on October 23, 2021 and showed generalized cardiomegaly, slight pericardial effusion, pulmonary venous congestion and bilateral pleural effusion. CT: computed tomography
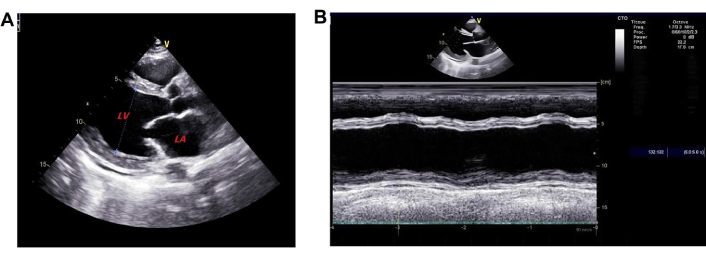
Initial echocardiogram. Done on October 25, 2021 and showed A) enlargement of left atrial and ventricle; B) left ventricular wall motion abnormality. LA: left atrium; LV: left ventricle
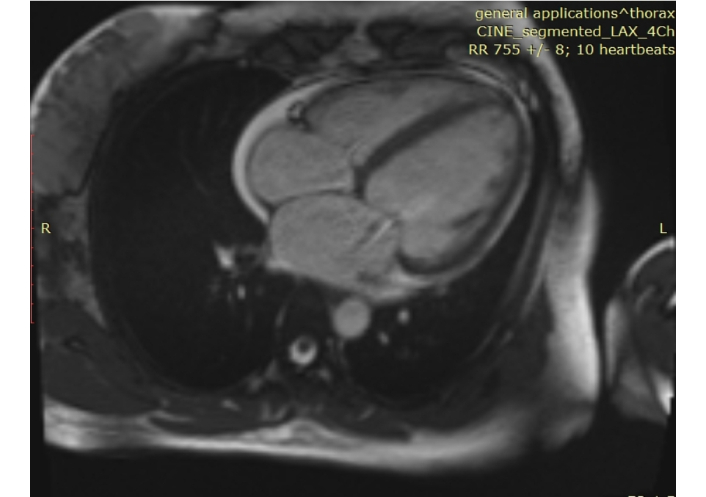
Initial MRI of the heart. Done on October 26, 2021 and showed left ventricular enlargement, mitral regurgitation, pericardial effusion and uniform signal intensity
The patient was diagnosed with PPCM based on her medical history, physical examination, and other tests. She underwent intensive monitoring, daily fluid restriction, adequate furosemide administration, and daily potassium management (blood potassium was maintained at 4.0–4.5 mmol/L). Thoracic closed drainage was promptly performed for the pleural effusion. Heart failure therapy was initiated with furosemide 20 mg once daily (qd), spironolactone 20 mg qd, and deslanoside 0.2 mg qd, with doses increased gradually later. In addition, she was given levosimendan and recombinant human brain natriuretic peptide for cardiac function, human serum ALB for edema, potassium magnesium aspartate 0.14 g:0.158 g three times a day (tid) for stabilizing electrical activity, and warfarin 2.5 mg qd for reducing risks of forming blood clots. After about 3 days of hospitalization, most symptoms resolved. The blood tests showed AST returned to normal, NTproBNP 8,935 pg/mL, and ALT 132.4 U/L. Treatment with bisoprolol 2.5 mg qd and sacubitril valsartan sodium 25 mg twice daily (bid) was initiated. The dose was up-titrated to the maximum tolerated dose to achieve ventricular reverse-remodeling benefits. Trimetazidine 20 mg tid and coenzyme Q10 10 mg tid were used to improve myocardium metabolism. CT showed the disappearance of pleural effusion on Day 5 of hospitalization (Figure 4). On the same day, edema was obviously alleviated and the retested NTproBNP dropped to 444 pg/mL, clinical symptoms disappeared, and echocardiography showed enlarged LAD (37 mm) and LVEDD (57 mm) as before, but LVEF increased to 45% (Figure 5). The patient discharged on November 04, 2021 continued treatment with warfarin (2.5 mg qd), trimetazidine (20 mg tid), sacubitril valsartan sodium (50 mg bid), bisoprolol (5 mg qd), coenzyme Q10 (10 mg tid), furosemide (20 mg qd), and spironolactone (20 mg qd) at home as maintenance therapy. The patient was followed-up regularly after discharged from the hospital. After 2 months of treatment, echocardiography demonstrated a normal size of LAD (30 mm). Two months later, BNP returned to normal. Echocardiography revealed normal LVEF (59%) 9 months after discharge. LVEDD (48 mm) was recovered to the normal state after 11 months of treatment. The patient was treated with warfarin for 6 months. Long-term treatment with bisoprolol 5 mg qd and sacubitril valsartan sodium 50 mg bid was continued. The last follow-up echocardiography in September 2024 demonstrated normal size of LAD (32 mm) and LVEDD (49 mm) with an ejection fraction of 0.69% (Figure 6). The clinical timeline is depicted in Figure 7, highlighting key events from onset to resolution.
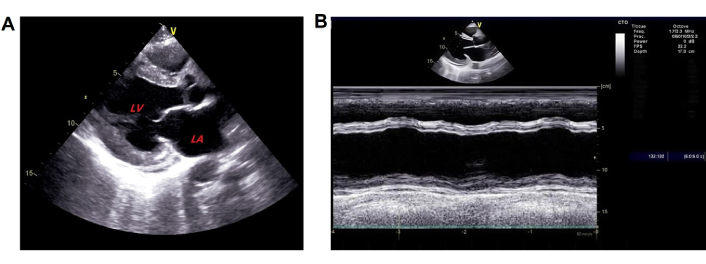
Follow-up echocardiogram. Done on November 5, 2021 and showed A) enlargement of left atrial and ventricle; B) enhanced left ventricular ejection fraction. LA: left atrium; LV: left ventricle
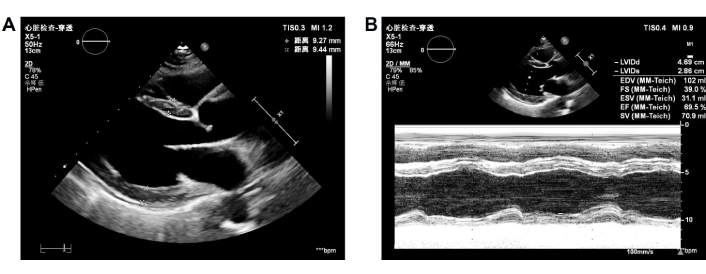
Follow-up echocardiogram. Done on September 2, 2024 and showed A) normal size of left atrial and ventricle; B) enhanced left ventricular ejection fraction
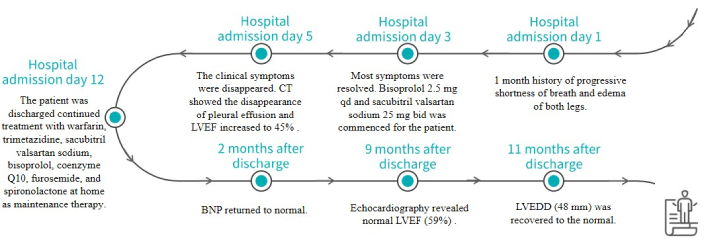
Treatment details in chronological order. CT: computed tomography; LVEF: left ventricular ejection fraction
PPCM is a secondary non-hereditary dilated cardiomyopathy characterized by heart failure occurring in the third trimester of pregnancy or in the first months post-partum [6]. With its rising incidence, misdiagnosis and delayed diagnosis are increasingly observed. Therefore, early identification of risk factors and early diagnosis of PPCM are critical for improving the prognosis of patients.
The main clinical manifestation of PPCM is congestive heart failure [7]. For patients who develop heart failure during pregnancy or the peripartum period, PPCM should be differentiated from Takotsubo (stress-induced) cardiomyopathy, worsening of pre-existing familial dilated cardiomyopathy, and myocarditis [8]. The patient was diagnosed with PPCM based on her history of cesarean delivery, increased NTproBNP, reduced LVEF (27%), long-term recovery of left ventricular systolic function, and absence of regional wall motion abnormality (RWMA), after other diseases causing heart failure were excluded.
Clinically, PPCM should be considered in patients with heart failure features in the third trimester of pregnancy or several months after delivery, and clinicians should collect detailed medical history, perform urgent echocardiography to confirm the diagnosis, and comprehensively evaluate the condition through laboratory blood tests, electrocardiograms, and other related examinations. The commonly used drug therapies for PPCM treatment include vasoactive agents, diuretics, anticoagulants, bromocriptine, and medications for heart failure. All therapies are empirical and have their limitations in clinical application. Therefore, appropriate individualized treatment is crucial for good efficacy.
The common medications for heart failure such as beta-blocker and ACEI/ATR/ARNI significantly increase LVEF and improve quality of life. The treatment should be initiated as soon as possible after acute heart failure. Scholars suggest that standard heart failure therapies should be maintained for at least 12 months [9]. And all patients should receive anticoagulant therapy and maintain it for at least 2 months after delivery or until cardiac function returns to normal [10]. Several studies have shown that PPCM patients benefited from the use of bromocriptine, including improved left ventricular function and reduced mortality because of its antioxidant and antifibrotic [11]. However, there is a controversy over the use of bromocriptine due to increasing risk of thromboembolism and its unstable therapeutic effect [12]. According to the 2018 ESC Guidelines, it is recommended to use bromocriptine in patients with an ejection fraction less than 25% [13]. For patients with cardiogenic shock, intra-aortic balloon pump (IABP), extracorporeal membrane oxygenation (ECMO), and other mechanical equipment are recommended. Pharmacotherapy should be cautious in pregnant patients or those planning to breastfeed. Excessive use of diuretics may be harmful to placental blood supply. ACEI/ATR/ARNI and sodium nitroprusside are forbidden due to teratogenicity. In this case, the patient presented symptoms of heart failure after delivery and had no breastfeeding intentions. She experienced typical symptoms of congestive heart failure without cardiogenic shock. Her ejection fraction was greater than 25%. Therefore, she received conventional medical therapy for heart failure. Intravenous diuretics and positive inotropic drugs relieved her symptoms quickly and facilitated significant cardiac recovery. Six months of standardized anticoagulation reduced the risk of venous thrombosis and hemorrhage. Long-term treatment with beta-blockers and ARNI showed significant clinical and ventricular reverse-remodeling benefits.
The prognosis of PPCM is relatively favorable with a high rate of recovery in contrast to other forms of heart failure (the ejection fraction more than 50% or 55% is a marker of myocardial function recovery [14]). Complete recovery may require several months or even several years. However, some patients may progress to chronic heart failure due to treatment failure. The Investigations of Pregnancy Associated Cardiomyopathy (IPAC) study involving 100 female patients with PPCM in the United States found that 72% of PPCM patients completely recovered within one year postpartum [15]. In this case, the myocardial function of the patient recovered within 9 months with an ejection fraction of 27% at admission. Timely treatment, accurate diagnosis, standardized use of medications, and regular follow-up contributed to the complete recovery within one year.
PPCM is one of the major causes of pregnancy-related morbidity and mortality worldwide. This case report highlights the challenges in diagnosing and managing PPCM. The management of PPCM requires a delicate balance between optimizing cardiac function and addressing associated risk factors. High-risk patients require implementation of early prevention, early diagnosis and early intervention. Once PPCM is diagnosed, timely treatment, standardized use of medications, and regular follow-up should be carried out to reduce the mortality of PPCM and improve patient outcomes.
ALB: albumin
ALT: alanine aminotransferase
AST: aspartate amino transferase
bid: twice daily
CMR: cardiac magnetic resonance
CT: computed tomography
ECMO: extracorporeal membrane oxygenation
IABP: intra-aortic balloon pump
LAD: left atrial diameter
LVEDD: left ventricular end diastolic diameter
LVEF: left ventricular ejection fraction
NTproBNP: N-terminal pro-B-type natriuretic peptide
PPCM: peripartum cardiomyopathy
qd: once daily
RWMA: regional wall motion abnormality
tid: three times a day
JG: Conceptualization, Writing—original draft, Writing—review & editing. XW: Conceptualization, Writing—original draft, Writing—review & editing, Supervision. Both authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study complies with the Declaration of Helsinki (2024 version). As it was routine treatment, ethical approval was not required for this study, according to the local committee.
Informed consent to participate in the study was obtained from all participants.
Informed consent to publication was obtained from the relevant participant.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2839
Download: 202
Times Cited: 0
Johanna A. van der Zande ... Jerome M. J. Cornette
Kazim Raza Talpur ... Muhammad Waleed Abdullah
