Affiliation:
1Department of Internal Medicine, Federico II University, School of Medicine, 80131 Naples, Italy
Email: fazio0502@gmail.com
ORCID: https://orcid.org/0000-0002-2743-9836
Explor Cardiol. 2025;3:101268 DOI: https://doi.org/10.37349/ec.2025.101268
Received: May 26, 2025 Accepted: July 21, 2025 Published: August 08, 2025
Academic Editor: Andrea Borghini, National Research Council (IFC-CNR), Italy
Our cells and, therefore, our organism, need energy to function at their best, which is mainly produced by mitochondria. These intracellular organelles generate energy from food macromolecules across the Krebs cycle by oxidative phosphorylation. Energy is developed by converting adenosine triphosphate (ATP) to adenosine diphosphate (ADP). It is essential, for adequate mitochondrial energy production in the form of ATP, to have the right number of well-functioning mitochondria and the right amount of oxygen (O2) available. Unfortunately, the aging process and the chronic diseases that arise over the years are associated with a reduction in the number of mitochondria and their insufficient functioning. Among the chronic diseases related to significant damage of the arteries with a reduction in the supply of O2, there is atherosclerosis, where the process of atherothrombosis occurs. To keep our organs well-functioning despite aging, we must therefore protect our mitochondria and arteries. This can be achieved by intervening early in prevention with a lifestyle correction and diet integration with effective natural substances or, in some cases, with drugs. Among the many natural substances that have good scientific support, we have chosen four that have demonstrated benefits in the absence of side effects and that we know best: quercetin and pyrroloquinoline quinone to stimulate mitochondrial biogenesis and mitophagy, while L-arginine and nattokinase to protect the arteries from atherothrombosis.
In 1991, the book Evolutionary Biology of Aging offered the following definition of aging: a persistent decline in an organism’s age-specific fitness components due to internal physiological deterioration [1]. Aging must be considered a phenomenon that has pathophysiological bases that are not yet fully clarified. It is, apparently, a physiological process, but there are many pathological mechanisms that can accelerate it [2]. These mechanisms can be rapidly recognized and, possibly, interrupted by acting quickly in order to slow down the pathological process at the basis of aging. Furthermore, it is also possible to intervene on the physiological mechanisms of aging in order to slow it down and, consequently, age as healthily as possible. There is a strict relation among aging, mitochondrial dysfunction, and brain function impairment [3]. It is also known that the therapies available for cardiovascular and neurodegenerative diseases have rather poor efficacy, especially if they are instituted too late, when the damage is already irreversible. Therefore, an early approach, intervening on mitochondrial health and aging prevention, should be implemented. This is a review article intended to underline the role of mitochondria and oxygen (O2) in the production of the energy necessary for life and the correct functioning of brain and muscle cells, and the possibility of establishing the prevention of mitochondrial damage through an adequate lifestyle and the constant intake of some protective natural substances. We have selected and analyzed the manuscripts useful for the review from the most important scientific research engines, such as PubMed, Scopus, ResearchGate, etc., among those published on the topic in the last 40 years in English in impact Journals. To search for the manuscripts, the following keywords were used: mitochondria, energy production, brain, muscle, mitochondrial biogenesis, mitophagy, aging, atherosclerosis, prevention, natural substances, L-arginine, nattokinase (NK), pyrroloquinoline quinone (PQQ), quercetin (QCC).
Our cells and, consequently, our organism, need energy to live and to function at their best. Under aerobic conditions, energy is produced by intracellular organelles called mitochondria. They produce energy through cellular respiration. This is a process in which nutrients are broken down by digestion into simple sugars, amino acids, and fatty acids, which are oxidized to provide the energy necessary for adenosine triphosphate (ATP) production. This is an exothermic oxidation-reduction process in which O2 is essential as an electron acceptor. In this process, the citric acid cycle, also known as the Krebs cycle, is essential. It, through a series of biochemical reactions, releases the energy stored in nutrients by the acetyl-CoA oxidation. The Krebs cycle is used by organisms that generate energy via respiration. It has two very important functions. One is the production of intermediate compounds important for the synthesis of amino acids and fatty acids. The other is the formation of large amounts of ATP, a source of energy for many synthetic processes. The presence of O2 is essential for these reactions. Besides the mitochondria’s ATP production, the Embden-Meyeroff-Parnas (EMP) pathway (glycolysis) allows the metabolic use of glucose to generate ATP, NADH, and several biosynthetic precursors such as 3-phosphoglycerate or pyruvate. The reaction at the basis of this process, namely glycolysis, takes place in the cytoplasm and is an important source of ATP in anaerobic conditions. The total energy produced in the Krebs cycle by a single glucose molecule is 36 ATP, while glycolysis generates just 2 [4]. It is essential for adequate energy production to have a correct number of well-functioning mitochondria and the right amount of O2 available [5]. The cells that require greater quantities of O2 and energy for correct function are brain and muscle cells. Therefore, our organs, particularly the brain and muscles, require O2 and an adequate number of functional mitochondria for proper functioning. Unfortunately, the aging process and the chronic diseases that may arise over the years are associated with a reduction in the number of mitochondria and/or in the impairment of their functioning. Among the pathologies associated with age, there is atherosclerosis, which begins early in life and, if not prevented, produces significant damage to the arteries and alterations in blood flow to the organs, resulting in a reduction in the supply of O2 and nutrients and organ dysfunction. To keep the right cellular production of energy and the normal functioning of our organs despite aging, we can try to protect our mitochondria and our arteries. The cells that need large quantities of energy to work correctly, namely muscle and brain cells, are the first to suffer if the mitochondria do not function properly. The brain, in relation to its mass, is the organ that requires the greatest quantity of energy to function properly, and therefore, it is the organ whose function deteriorates more easily if mitochondrial dysfunction occurs [6].
A decline in the quality and activity of mitochondria has been associated with normal aging, but also correlated with the development of a wide range of age-related chronic diseases [7]. Beyond the nucleus, mitochondria are the only organelles in human cells to have their own mitochondrial DNA (mtDNA). Unlike the nuclear genome, the mitochondrial genome is much smaller and circular in shape. The mitochondrial genome contains 37 genes, which, among other things, code for 13 proteins involved in the oxidative phosphorylation system, which allows the mitochondria to act as the energy center of our cells. Every single cell contains numerous mitochondria, and each mitochondrion contains dozens of copies of the mitochondrial genome. Therefore, the mitochondrial genome is more likely to undergo mutations (about a hundred times more) than the nuclear genome, with a greater probability of mitochondrial dysfunctions and the development of chronic diseases related to this [8]. There is a vast scientific literature that demonstrates how mitochondrial dysfunction is associated with the aging of cells and the entire organism [9], and with numerous pathologies, such as cancer, metabolic diseases [10], in particular diabetes [11], and neurodegenerative ones [12, 13]. It is known that, as the organism ages, its mitochondria become progressively less numerous and efficient, and this alone could already explain the deterioration of the function of many organs with advancing age, such as reduction in muscle strength with easy fatigue and the progressive onset of memory loss and cognitive deficits up to real dementia (Figure 1).
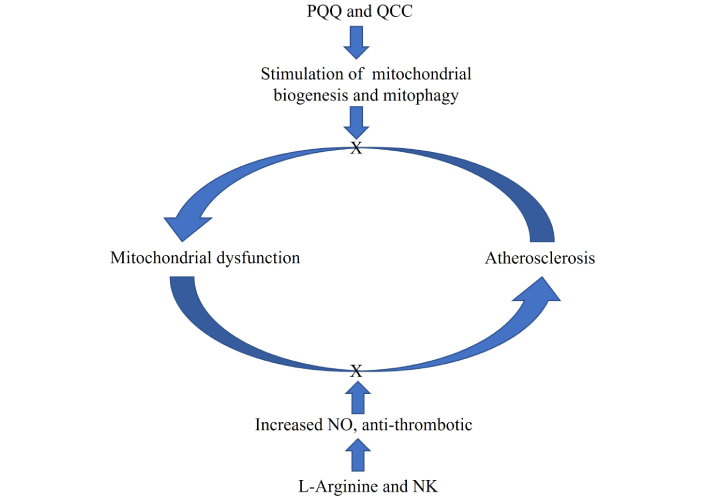
The atherosclerotic process is the downstream consequence of many factors, including mitochondrial dysfunction, although atherosclerosis itself may exacerbate and worsen mitochondrial dysfunction through various mechanisms. Preventive intervention by PQQ, QCC, L-arginine, and NK could block or slow down this vicious circle, acting as a preventive measure against the development of atherosclerosis and mitochondrial dysfunction. NK: nattokinase; PQQ: pyrroloquinoline quinone; QCC: quercetin; NO: nitric oxide; X: block
Protecting mitochondria and improving their function can lead to better energy production and, therefore, improve physical and mental health. Inside the mitochondria, there is a high production of O2 free radicals [reactive oxygen species (ROS)], and this makes mtDNA particularly exposed to oxidative damage [14]. With aging, this damage can persist and accumulate over time [15, 16]. It is hypothesized that aging and degenerative diseases may depend on the progressive accumulation of deleterious mutations at the mtDNA produced over time by ROS [17].
A vascular system in good condition is essential for the correct functioning of our organs and, obviously, of our entire organism. This is because the vascular system is the conductor of blood with nutrients and, above all, O2 for the survival and correct functioning of the cells of the various organs. The atherosclerotic process is the downstream consequence of many factors, including mitochondrial dysfunction, although atherosclerosis itself may exacerbate and worsen mitochondrial dysfunction by various mechanisms (Figure 1) [18, 19].
In particular, there are so-called noble organs, such as the brain and the heart, which require more energy and therefore greater quantities of O2 for correct functioning. The basis of the deterioration of our vascular system is mainly atherosclerosis, a progressive disease that begins at a young age and presents clinical manifestations, mostly, in adulthood. It is a pathological condition characterized by alterations in the wall of the arteries, which lose their elasticity due to the accumulation of cholesterol, calcium, inflammatory cells, and fibrotic material, with the formation of stenosing plaques [20]. Once established, atherosclerosis appears to be an irreversible and continually evolving process. An adequate lifestyle and treatments aimed at controlling common cardiovascular risk factors (physical inactivity, insulin resistance, prediabetes, diabetes, hypertension, cigarette smoking, dyslipidemia) can prevent the formation of plaques, or at least, slow down the progressive worsening of atherosclerosis. It is also commonly associated with the aging of the arteries; however, the presence of a family history of the development of atherosclerosis and the presence of one or more risk factors can facilitate its onset and worsen its evolution [20]. Endothelial dysfunction and the chronic inflammation present in the arteries at the level of the atherosclerotic plaque cause a harmful short circuit that feeds itself and the coagulation activation with the formation of fibrin and thrombi [20]. As long as the fibrinolysis/thrombolysis processes are effective and the thrombi are not bulky, our body is able to dissolve them so quickly that no ischemic damage is created, but when thrombotic phenomena increase and the fibrinolysis/thrombolysis process becomes less effective, particularly with aging, significant thrombosis occurs with ischemic damage downstream of the thrombosed artery [21, 22]. There is a large body of both experimental and clinical scientific literature demonstrating how endothelial dysfunction is encountered not only during disease states such as atherosclerosis, but also in the normal aging process. Therefore, aging is an independent factor capable of causing alterations in the vascular endothelium and thrombosis phenomena [23].
In recent years, some mitochondrial proteins encoded by nuclear genes have been reported to participate in the repair of damaged mtDNA to maintain mitochondrial genetic integrity, demonstrating the existence of mtDNA repair mechanisms in mitochondria [24]. Several mtDNA repair pathways have been reported, including base excision repair (BER), mismatch repair (MMR), direct reversal (DR), and double-strand break repair. BER is considered to be the most important mtDNA repair pathway. The basic mechanism of BER is to remove the small range of bases damaged by oxidation, alkylation, methylation, and deamination. This mechanism consists of 4 steps: 1. recognition and removal of modified bases; 2. formation of apurinic/apyrimidinic sites; 3. correction of nucleotide synthesis; 4. linking of DNA strands.
Another important mechanism for maintaining efficient mitochondria is mitochondrial biogenesis. This is a process that consists of the formation of new mitochondrial components and new mitochondria starting from existing ones, removing the damaged ones (mitophagy) [25]. Insufficient mitophagy action leads to the accumulation of damaged mitochondrial structures and molecules, which are prodromal to the development of senescence and degenerative diseases, and to the decline in organ function and health span [26]. Therefore, it is necessary to increase the number of mitochondria by stimulating the biogenesis of new mitochondria and to have the possibility of being able to intervene in mitophagy for the removal of damaged mitochondrial substances. Apart from moderate and constant physical activity and an appropriate diet, to stimulate mitochondrial biogenesis, there is also the possibility of constantly consuming natural substances such as polyphenols, which act by stimulating this process [27, 28] (Figures 1 and 2).
Formulas and structures of quercetin, pyrroloquinoline quinone, arginine, and nattokinase
There are many natural substances that are scientifically supported in their action to stimulate mitochondrial biogenesis and to protect arteries from the atherothrombotic process. Among them, we have chosen QCC, PQQ, L-arginine, and NK because we have a good knowledge of them, because they are easily obtainable in nature, and are practically free from significant side effects, if used under medical supervision (Figure 2). QCC and PQQ are widespread representatives in nature and are scientifically supported in their action to stimulate mitochondrial biogenesis [29–35].
QCC (Figure 2) is a natural active ingredient that belongs to the flavonoid family, substances widely present in the plant world, which serve to protect plants from attacks by viruses, bacteria, fungi, and UV rays [36].
Flavonoids indicate an important set of plant pigments whose chemical structure is derived from that of flavone. They are widely present in the plant world and include anthocyanins, flavones, and other pigments. In light of the studies carried out so far, they are believed to be an excellent source of antioxidant and anti-aging substances, capable of counteracting the harmful action of free radicals [29]. QCC is classified as a senolytic agent, meaning that it protects cells from aging [37, 38]. However, it is known that it is a powerful antioxidant, has anti-inflammatory properties, and reduces insulin resistance, which is a predisposing factor for cellular senescence [39, 40]. The senolytic activity of QCC has been linked to various mechanisms and, in particular, to the inhibition of phosphatidylinositol-3 kinase (PI3K) and members of the B-cell lynphoma-2 (Bcl-2) family, as well as to a modulation of microRNA-155-5p (miRNA-155-5p, involved in immune responses, inflammation and cancer development), through nuclear factor kappa B (NF-κB, a protein crucial for immune and inflammatory responses, cell growth and survival) and sirtuin1 (SIRT1) [41, 42]. A recent review on the molecular mechanisms of QCC has described interesting concepts. There is an inverse relationship between the onset and development of age-related disorders and cellular senescence and Klotho. This latter is a transmembrane protein that, in addition to other effects, provides some control over the sensitivity of the organism to insulin and appears to be involved in ageing. Senolytic medications, such as QCC, may benefit from targeting senescent cells, which enhances the protective factor α-Klotho. Additionally, other aspects of aging that could be affected by senolytics, such as limiting age-related mitochondrial dysfunction, lowering inflammation and fibrosis, blunting ROS generation, decreasing DNA damage, and reinforcing insulin sensitivity. Senolytic agents have been shown to increase adipose progenitor and cardiac progenitor cell activity in aging animals and animals with cellular senescence-related diseases, such as heart, brain, and kidney disease [43, 44].
QCC also acts on SIRT1, too. Three of the seven known mammalian sirtuins are located in the mitochondria. In particular, SIRT1 promotes mitochondrial function and regulates mitochondrial homeostasis. Studies have indicated that overexpression of SIRT1 can effectively inhibit cell death, promote cell survival, and extend the lifespan of cells [42]. It has been shown that SIRT1 produces deacetylation of peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1-α), regulating the energy metabolism through mitochondria and the mitochondrial biogenesis [45].
Mitochondrial dysfunction is considered the main causal factor in the pathogenesis of age-related Alzheimer’s disease (AD) [13]; in fact, it can cause damage to neurons, microglia, and astrocytes [44]. QCC exerts neuroprotective effects against AD by targeting SIRT1 to regulate cellular senescence and multiple aging-related cellular processes, including SIRT1/Keap1/Nrf2/HO-1 and PI3K/AKT/GSK-mediated oxidative stress-3β, SIRT1/NF-κB mediated inflammatory response, mitochondrial damage mediated by SIRT1/PGC1-α/eIF2α/ATF4/CHOP and SIRT1/FoxO-mediated autophagy [28, 46–48].
Overall, SIRT1 may serve as a promising therapeutic target in the treatment of age-related diseases through inhibition of oxidative stress, reduction of inflammatory responses, and restoration of mitochondrial dysfunction. Activated microglial cells are a major type of innate immune cells in the brain that secrete inflammatory cytokines into the extracellular environment, exert neurotoxicity on surrounding neurons, and are involved in the pathogenesis of many brain disorders. QCC prevents neuronal damage through inhibition of mtROS-mediated NLRP3 inflammasome activation in microglia, via promotion of mitophagy, which provides a potential new therapeutic strategy for neuroinflammation-related diseases [49]. QCC has also been shown to be protective against mitochondrial dysfunction and progressive dopaminergic degeneration via activation of the PKD1-AKT cell survival signaling axis in cultured cells and in the MitoPark transgenic mouse model of Parkinson’s disease [50]. Another study examined the effects of 7 days of QCC feedings in mice on markers of mitochondrial biogenesis in skeletal muscle and brain, and on endurance exercise tolerance. The study was randomized and placebo-controlled. Following 7 days of treatment, mice were killed, and the soleus muscle and brain were analyzed for mRNA expression of PGC1-α, SIRT1, mtDNA, and cytochrome c. A group of mice underwent a treadmill performance run to fatigue or were placed in voluntary activity wheel cages, and their voluntary activity (distance, time, and peak speed) was recorded. QCC increased mRNA expression of PGC1-α (P < 0.05), SIRT1 (P < 0.05), mtDNA (P < 0.05), and cytochrome c concentration (P < 0.05). These changes in markers of mitochondrial biogenesis were associated with an increase in both maximal endurance capacity (P < 0.05) and voluntary wheel-running activity (P < 0.05) [28]. Another recent experimental study, performed in cell culture (neuronal SH-SY5Y cells), has shown that QCC stimulated the expression of mitochondrial-related proteins such as SIRT1, PGC1-α, and mitochondrial transcription factor A (TFAM) and subsequently activated mitochondrial biogenesis. Additionally, QCC increased disintegrin and metalloproteinase 10 (ADAM10) expression but reduced H2O2-induced reactive O2 species production, apoptosis, β-site amyloid precursor protein cleaving enzyme 1 expression, and Aβ accumulation in the SH-SY5Y cells [51].
Although there are no clinical data in humans in the literature to support a beneficial effect of QCC in neurodegenerative diseases, the results obtained from basic research supporting a role of QCC in mitochondrial protection and a potential benefit against neurodegenerative diseases are very interesting. In addition, there are many studies, mostly experimental, which demonstrate the beneficial effects of QCC on the prevention and slowing of progression of atherosclerosis by different mechanisms, but particularly for their antioxidant and anti-inflammatory actions [52–54].
PQQ (Figure 2) is an aromatic heterocyclic orthoquinone, discovered in 1964 by Norwegian biochemist Jense G. Hauge in bacteria. However, it was not until 2003 that a working group led by Japanese neuroscientist Tadafumi Kato discovered the presence of this substance in humans. It is physiologically contained in the mitochondria, near the site of free radical formation, where it is able to intercept and inactivate them. Recent studies have shown that, just at the mitochondrial level, it is able to carry out its main functions: improving cellular energy processes and activating important mtDNA repair mechanisms [55–59]. For these reasons, most scientists who deal intensively with these topics see an indication for this molecule in preventive and anti-aging medicine. There is a vast body of scientific literature, both experimental and clinical, to support the fact that PQQ has protective effects against neurodegenerative diseases. An experimental study performed on cell culture showed that the exposure of mouse Hepa1–6 cells to 10–30 μm PQQ for 24–48 h resulted in increased citrate synthase and cytochrome c oxidase activity, Mitotracker staining, mtDNA content, and cellular O2 respiration. The induction of this process occurred through the activation of cAMP response element-binding protein (CREB) and PGC1-α, a pathway known to regulate mitochondrial biogenesis [56]. Another study concluded that the formation of mouse prion protein fibrils was dramatically prevented in the presence of PQQ, as well as the formation of β-amyloid fibrils [55].
A more recent clinical, randomized, double-blind, placebo-controlled study was carried out on a total of 62 subjects, divided into two groups of 31. One group was administered PQQ at 20 mg per day orally for 12 weeks, and the other, for the same period of time, was given the placebo. Furthermore, the treated subjects were divided into two subgroups of different ages (52.9 ± 6.6 vs. 28.8 ± 6.7, years old), in which a complete cognitive function analysis was carried out using the Cognitrax test. The results demonstrated that PQQ improved the cognitive functions of cognitive flexibility and executive speed already within 8 weeks in younger subjects, while the cognitive functions of composite and verbal memory were improved after 12 weeks of treatment only in the group of older subjects [60].
Another randomized, placebo-controlled, double-blind clinical trial was conducted in 41 healthy elderly subjects to examine the effect of PQQ on cognitive functions. Study subjects were administered PQQ at a dose of 20 mg per day for 12 weeks or a placebo. For the evaluation of cognitive functions, a Stroop test and a reverse Stroop test were carried out, and for the evaluation of visual-spatial cognitive function, a Touch Screen test was used using a laptop tablet. The results of this study suggest that PQQ can prevent reductions in brain function in older adults, especially in attention and working memory [61]. Furthermore, a preliminary experiment performed using near-infrared spectroscopy suggests that cerebral blood flow in the prefrontal cortex was increased by PQQ administration [61]. A further, very recent study evaluated the impact of 6-week treatment with PQQ on mitochondrial “biomarkers”, brain metabolism, and cognitive processes in 34 elderly subjects with mild cognitive deficits [62].
Also, in this case, the study was carried out with a parallel group design, randomized, double-blind, and placebo-controlled. The results showed an increase in brain-derived serum neurotrophic factor, an improvement in cognitive functions, and a significant increase in cerebral oxygenation saturation in subjects treated with PQQ [62].
Nitric oxide (NO) produced by endothelial cells, in addition to its well-known vasodilatory properties, also has local anti-inflammatory properties, limiting the expression of adhesion molecules; for this reason, it is currently considered a protective factor against atherosclerosis [63, 64]. It is often suggested that an alteration of the synthesis of NO from L-arginine and/or an increased production of vascular contraction factors play a relevant role in the aggravation of endothelial and parietal lesions, negatively affecting the natural history of the disease process. Furthermore, NO inhibits thrombus formation [65, 66]. It would therefore be important to maintain NO levels constant throughout life by the administration of substances that are precursors of NO. As already mentioned, both age and pathologies involving the vascular endothelium stimulate the formation of thrombi and determine vascular obstructions over time, impairing the blood circulation in various organs, leading to an acute or progressive deterioration of their function. In the normal subject, there is always a certain balance between thrombus formation and fibrinolysis/thrombolysis. Unfortunately, this homeostasis alters with age, both because the thrombosis phenomena become more accentuated and because the fibrinolysis and/or thrombolysis systems become progressively less effective [22]. Furthermore, with advancing age, chronic pro-thrombotic diseases can develop, with the formation of excessive quantities of thrombi or more resistant thrombi, which our thrombolytic systems are unable to dissolve before organ damage occurs. As it is known, thrombi are mostly formed by fibrin containing platelets, red and white blood cells, and are formed by blood coagulation within the uninterrupted cardiovascular system, which distinguishes them from clots, which instead form outside the cardiovascular system when there are interruptions, such as in wounds. Fibrin plays an important role in thrombus formation. However, there are drugs and natural substances that can help us prevent or rebalance these alterations at the vascular level, protecting us from thrombosis phenomena. L-Arginine is an amino acid that is converted at a vascular level by NO synthetase into citrulline and subsequently into NO (Figures 1 and 2). It has been shown that it has many beneficial effects for the human organism [67–70]. One of the most important actions of L-arginine is to maintain vascular homeostasis through the synthesis of NO, which is a powerful vasodilator and which has a protective function against the development of atherosclerosis. It is known that a reduced availability of NO leads to the development of endothelial dysfunction, which, in turn, plays a relevant role in the development of cardiovascular diseases [63–65]. It was verified that, after the administration of 0.9 g of L-arginine, endothelium-dependent vasodilation, assessed by cutaneous Laser Doppler flow, was significantly increased [71]. Furthermore, it seems that the increased ingestion of this amino acid produces an improvement in the alterations of vascular reactivity and reduces the intimal thickness in atherosclerosis, and can also reduce blood pressure and the excessive proliferation of vascular smooth muscle cells in hypertension [72, 73]. Hyperinsulinemia associated with insulin resistance facilitates the development of atherosclerosis both due to a reduction in NO production at the vascular level and due to its action as a growth factor at the level of vascular smooth muscle cells and endothelial cells, determining, over time, endothelial dysfunction, vascular stiffness, and atherosclerosis [74]. The administration of L-arginine can counteract the development of these pathological events both by stimulating the formation of NO and by reducing insulin resistance. In fact, while in normal conditions there is a balance between the secretion of NO and endothelin-1 (ET-1), in conditions of insulin resistance/hyperinsulinemia there is an alteration of vascular homeostasis with prevalent vasoconstriction, determined by the fact that hyperinsulinemia, in this case, determines a reduction in vascular secretion of NO and a relative/absolute increase in ET-1 [74]. The verified action of L-arginine at the endothelial level determines an increase in NO production by endothelial synthetase and a reduction in insulin resistance at the endothelial cell level. Furthermore, it has also been shown that a potential mechanism responsible for the favorable effect of L-arginine supplementation on insulin resistance may be due to the increase in adiponectin concentrations. In fact, it has been seen that L-arginine is an effective stimulus for the release of adiponectin. Adiponectin is a hormone produced by adipose tissue that produces insulin-sensitizing effects through binding to its receptors, which determines the activation of various pathways, known (AMPK and PPAR-α) and others still unknown, which improve insulin sensitivity. In insulin resistance, both adiponectin and its receptors are “down” regulated. Therefore, a therapy that determines an “up” regulation of adiponectin and its receptors, or the increase in the functioning of adiponectin receptors, may be a good option for the treatment of insulin resistance [75, 76]. A constant intake of substances that help keep NO levels constant over time, such as L-arginine, should protect our vascular system by slowing down the deterioration due to aging and disease, without producing particular side effects.
NK is a fibrinolytic enzyme derived from natto, a food widely used in Japanese cuisine, obtained through the fermentation of soybeans using a specific bacterium, Bacillus subtilis (Figure 2). It exerts lipid-lowering and anti-atherosclerotic effects by activating hormone-sensitive lipase, inhibiting hydroxymethylglutaryl monoacyl coenzyme A reductase, and enhancing lipoprotein lipase activity. Large-scale clinical studies have confirmed that NK significantly improves the lipid profile and reduces atherosclerotic plaque area and intima-media thickness, with a favorable safety profile [77–79].
High natto consumption has been linked to the longest average lifespan and lowest rate of death from heart disease in the Japanese population [77]. Fibrin is a fundamental part of the formation of thrombi. Physiologically, in our body, there is a perfect balance between the formation of microthrombi and their destruction, so that long-lasting vascular occlusions do not occur. Unfortunately, however, in some situations, including aging and many chronic diseases, this balance is altered in favor of an increase in thrombotic phenomena rather than a deficiency of thrombolytic processes, so that in old age we are more likely to form thrombi, which can cause vascular occlusions in important organs and produce, for example, heart attacks and cerebral strokes. Many studies show that NK helps dissolve blood clots, which makes it easier to maintain good blood vessel structure, improves blood flow, and reduces the risk of cardiovascular disease. It can also help lower blood pressure, reducing strain on the heart, brain, and kidney vessels, which can lead to significant damage over time [80]. A considerable number of studies have been performed to evaluate the thrombolytic effects of NK in vitro and animal models [81–85]. Plasminogen activator inhibitor 1 (PAI-1) is the major inhibitor of tissue polypeptide antigen (tPA) and regulates fibrinolytic activity in the fibrinolytic cascade [86, 87]. In a study investigating the mechanism by which NK exerted its fibrinolytic effect, NK enhanced fibrinolysis through cleavage and inactivation of PAI-1 [88]. The enhanced fibrinolytic activity observed in the absence of PAI-1 appears to be induced through direct dissolution of fibrin by NK [89, 90]. NK also enhanced the production of clot-dissolving agents, such as urokinase, through the conversion of pro-urokinase to urokinase [91, 92]. Furthermore, it has been shown that NK is able to block the formation of thromboxane with consequent inhibition of platelet aggregation without producing the side effect of bleeding [93]. Therefore, NK has been found to be a potent antithrombotic agent that, in addition to reducing thrombus formation, is also able to slow down the progression of plaque formation and reverse the evolution of atherosclerotic lesions [79].
Data from human studies also strongly support NK as a potent and promising fibrinolytic agent. In a first-in-human study, oral administration of NK was shown to produce a gradual increase in fibrinolytic activity in plasma, as indicated by euglobin lysis time (plasma ELT) and tPA production. After administration of Bacillus natto (100 mg/kg) to healthy adult volunteers, ELT was reduced and tPA activity was significantly increased (P < 0.05) [86, 87]. In an open-label, self-controlled clinical study, Hsia and collaborators [94], after two months of oral NK administration in humans, found that fibrinogen, factor VII, and factor VIII levels were significantly decreased, implying a promising cardiovascular benefit from NK administration. Even after a single dose of oral NK at 2,000 FU, the levels of fibrin/fibrinogen degradation products in the blood were significantly increased 4 h after NK administration (P < 0.05), confirming the improvement of thrombolysis and anticoagulation profiles. The results of this study support NK as a useful fibrinolytic/anticoagulant agent to reduce the risk of thrombosis and cardiovascular disease in humans [94].
It is known from scientific literature and clinical practice that, despite the progress made in the last decades in prevention and therapy, cardiovascular and neurodegenerative diseases continue to be a major cause of death and high health costs. In particular, the results obtained from the therapy of cardiovascular diseases and dementia, when started in the already symptomatic phases of the diseases, are rather unsatisfactory. Cardiovascular and neurodegenerative diseases are strictly associated with the aging process. We should, therefore, intervene much earlier, in prevention, trying to slow the aging process, protecting the mitochondria, our principal energy source, from the oxidative damage that accumulates over the years, by anti-oxidative substances and stimulating mitochondrial biogenesis and mitophagy. An important intervention to protect mitochondria should also be made on the arteries, which bring O2 and substrates to cells necessary for energy production, protecting them from atherothrombosis processes. QCC and PQQ are natural substances that have been shown to have great anti-oxidant properties and anti-aging effects, also acting directly on mitochondrial biogenesis, protecting the most sensitive organs, with greater energy needs, from damage related to aging. In addition, L-arginine and NK are two other natural substances, which, together, have synergistic mechanisms to protect the vascular system from atherothrombosis. All the above-mentioned counteract the leading causes of mitochondrial dysfunction, and can represent a key strategy for successful aging.
AD: Alzheimer’s disease
ATP: adenosine triphosphate
BER: base excision repair
ELT: euglobin lysis time
ET-1: endothelin-1
mtDNA: mitochondrial DNA
NF-κB: nuclear factor kappa B
NK: nattokinase
NO: nitric oxide
O2: oxygen
PAI-1: plasminogen activator inhibitor 1
PGC1-α: peroxisome proliferator-activated receptor gamma coactivator 1α
PI3K: phosphatidylinositol-3 kinase
PQQ: pyrroloquinoline quinone
QCC: quercetin
ROS: reactive oxygen species
SIRT1: sirtuin1
tPA: tissue polypeptide antigen
SF: Conceptualization, Investigation, Writing—original draft, Supervision, Validation. FA: Conceptualization, Writing—review & editing, Validation. VF: Writing—review & editing, Validation. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2382
Download: 110
Times Cited: 0
