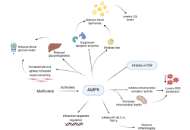


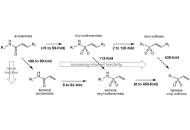







Ageing is a gradual, multifactorial process that leads to the deterioration of physical and mental health, increasing the risk of disease and eventually death. Indicators of ageing manifest at the molecular level, including genomic instability, telomere attrition, epigenetic alterations, mitochondrial dysfunction, loss of proteostasis, and dysregulation of key signalling pathways such as the mechanistic target of rapamycin (mTOR) and insulin signalling. These molecular hallmarks of ageing are interconnected, amplifying one another over time. The resulting cellular stress triggers apoptosis or drives cells into a pathological state known as cellular senescence, in which they secrete inflammatory, pro-ageing factors. Consequently, there is a progressive decline in tissue function and regenerative capacity, accompanied by atrophy and stem cell exhaustion under a chronically inflamed microenvironment. Although functional decline with age is irreversible, research indicates it can be delayed. In this review, we discuss the hallmarks of ageing, conventional pharmacological interventions with demonstrated anti-ageing effects in cellular and animal models, and emerging therapeutic strategies being explored as ageing becomes increasingly recognized as a major risk factor for disease development.
Ageing is a gradual, multifactorial process that leads to the deterioration of physical and mental health, increasing the risk of disease and eventually death. Indicators of ageing manifest at the molecular level, including genomic instability, telomere attrition, epigenetic alterations, mitochondrial dysfunction, loss of proteostasis, and dysregulation of key signalling pathways such as the mechanistic target of rapamycin (mTOR) and insulin signalling. These molecular hallmarks of ageing are interconnected, amplifying one another over time. The resulting cellular stress triggers apoptosis or drives cells into a pathological state known as cellular senescence, in which they secrete inflammatory, pro-ageing factors. Consequently, there is a progressive decline in tissue function and regenerative capacity, accompanied by atrophy and stem cell exhaustion under a chronically inflamed microenvironment. Although functional decline with age is irreversible, research indicates it can be delayed. In this review, we discuss the hallmarks of ageing, conventional pharmacological interventions with demonstrated anti-ageing effects in cellular and animal models, and emerging therapeutic strategies being explored as ageing becomes increasingly recognized as a major risk factor for disease development.
DOI: https://doi.org/10.37349/eds.2026.1008152

The significant medicinal constituents and pharmacological potential of several botanicals suggest promising therapeutic applications. Scorzonera undulata displayed a diverse phytochemical profile, with 25 volatile and 21 phenolic compounds identified, including quinic and chlorogenic acids, along with flavonoids such as kaempferol, apigenin, luteolin derivatives, quercitrin, and naringin—mostly concentrated in the aerial parts. These extracts exhibited notable antioxidant, antimicrobial, anti-inflammatory, and cytotoxic activities, especially methanolic extracts against MCF-7 breast cancer cells, indicating therapeutic relevance. Andrographis paniculata extracts, rich in andrographolide, showed clinical potential in alleviating mild COVID-19 symptoms. However, the compound’s nonlinear pharmacokinetics highlight the need for optimized delivery strategies. Morinda citrifolia fruit extracts demonstrated considerable in vitro antimicrobial effects and moderate cytotoxicity, supported by UPLC–Orbitrap MS identification of unique bioactives. These findings reinforce the need for further pharmacological and clinical validation. The antiviral efficacy of Houttuynia cordata against dengue virus type 2 was evident, with aqueous extracts showing strong virucidal action and inhibition of viral replication. Hyperoside was identified as the dominant active constituent, supported by a rich phytochemical profile including flavonoids, aristolactams, and triterpenoids. Genotoxicity assessments indicated a favorable safety profile, suggesting potential for phytotherapeutic development. Achillea millefolium (yarrow) contained essential oils enriched in camphor, 1,8-cineole, artemisia ketone, and azulene derivatives, alongside phenolic acids and flavonoids like chlorogenic acid, apigenin, luteolin, and quercetin. These contributed to its antioxidant, anti-inflammatory, antimicrobial, and hemostatic effects, validating traditional medicinal applications and warranting clinical standardization. Flavonoids such as luteolin and apigenin offered anticancer and cardiovascular benefits by inhibiting PD-L1 via STAT3 suppression and promoting autophagy to counter vascular calcification. Bryophyllum pinnatum demonstrated broad pharmacological activity attributed to bufadienolides, flavonoids, and phenolic acids, supporting its ethnomedicinal use while emphasizing the need for clinical safety validation.
The significant medicinal constituents and pharmacological potential of several botanicals suggest promising therapeutic applications. Scorzonera undulata displayed a diverse phytochemical profile, with 25 volatile and 21 phenolic compounds identified, including quinic and chlorogenic acids, along with flavonoids such as kaempferol, apigenin, luteolin derivatives, quercitrin, and naringin—mostly concentrated in the aerial parts. These extracts exhibited notable antioxidant, antimicrobial, anti-inflammatory, and cytotoxic activities, especially methanolic extracts against MCF-7 breast cancer cells, indicating therapeutic relevance. Andrographis paniculata extracts, rich in andrographolide, showed clinical potential in alleviating mild COVID-19 symptoms. However, the compound’s nonlinear pharmacokinetics highlight the need for optimized delivery strategies. Morinda citrifolia fruit extracts demonstrated considerable in vitro antimicrobial effects and moderate cytotoxicity, supported by UPLC–Orbitrap MS identification of unique bioactives. These findings reinforce the need for further pharmacological and clinical validation. The antiviral efficacy of Houttuynia cordata against dengue virus type 2 was evident, with aqueous extracts showing strong virucidal action and inhibition of viral replication. Hyperoside was identified as the dominant active constituent, supported by a rich phytochemical profile including flavonoids, aristolactams, and triterpenoids. Genotoxicity assessments indicated a favorable safety profile, suggesting potential for phytotherapeutic development. Achillea millefolium (yarrow) contained essential oils enriched in camphor, 1,8-cineole, artemisia ketone, and azulene derivatives, alongside phenolic acids and flavonoids like chlorogenic acid, apigenin, luteolin, and quercetin. These contributed to its antioxidant, anti-inflammatory, antimicrobial, and hemostatic effects, validating traditional medicinal applications and warranting clinical standardization. Flavonoids such as luteolin and apigenin offered anticancer and cardiovascular benefits by inhibiting PD-L1 via STAT3 suppression and promoting autophagy to counter vascular calcification. Bryophyllum pinnatum demonstrated broad pharmacological activity attributed to bufadienolides, flavonoids, and phenolic acids, supporting its ethnomedicinal use while emphasizing the need for clinical safety validation.
DOI: https://doi.org/10.37349/eds.2026.1008151

Aim:
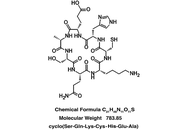
A seven amino acid cyclic peptide has been applied to human blood plasma treated with glucose metabolite methylglyoxal (MG) in “proof of concept” experiments to determine the peptide’s ability to counteract pathologies associated with hyperglycemia. Similar pathologies are evident during aging and in age-related disorders. In fact, elevated MG levels in the blood lead directly to diabetic complications and accelerated aging, including cognitive decline. These changes are attributed to oxidant stress and amyloidogenesis, the latter involving toxic accumulations of blood and tissue proteins.
Methods:
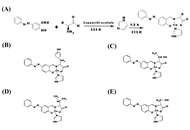
cSKE7 was redesigned from cell survival-promoting and anti-inflammatory fragments near the N-terminus of human/primate “orphan” protein DSEP/Dermcidin and incubated at low micromolar concentrations with the MG-stressed human plasma for 24 hours. The modified design of the new compound offers several practical advantages over predecessors including cyclic stability and a marked increase in aqueous solubility.
Results:
The peptide dispersed thioflavin-T-stained amyloid aggregates and reduced oxidant stress as measured by plasma levels of free thiols and of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity. Since these N-terminal fragments of DSEP/Dermcidin have been shown to bind and influence the activity of heat shock protein 70 (HSP70), HSP70 inhibitor pifithrin-μ was added to the plasma prior to peptide treatment. The inhibitor disrupted amyloid dispersion and both peptide-induced and, in some cases, normally occurring antioxidant effects, suggesting these reparative activities are HSP70 dependent.
Conclusions:
The results are discussed in terms of their potential use in new therapies for the complications of metabolic disease and disorders of aging that result from a deterioration of the quality control mechanisms of proteostasis.
Aim:
A seven amino acid cyclic peptide has been applied to human blood plasma treated with glucose metabolite methylglyoxal (MG) in “proof of concept” experiments to determine the peptide’s ability to counteract pathologies associated with hyperglycemia. Similar pathologies are evident during aging and in age-related disorders. In fact, elevated MG levels in the blood lead directly to diabetic complications and accelerated aging, including cognitive decline. These changes are attributed to oxidant stress and amyloidogenesis, the latter involving toxic accumulations of blood and tissue proteins.
Methods:
cSKE7 was redesigned from cell survival-promoting and anti-inflammatory fragments near the N-terminus of human/primate “orphan” protein DSEP/Dermcidin and incubated at low micromolar concentrations with the MG-stressed human plasma for 24 hours. The modified design of the new compound offers several practical advantages over predecessors including cyclic stability and a marked increase in aqueous solubility.
Results:
The peptide dispersed thioflavin-T-stained amyloid aggregates and reduced oxidant stress as measured by plasma levels of free thiols and of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity. Since these N-terminal fragments of DSEP/Dermcidin have been shown to bind and influence the activity of heat shock protein 70 (HSP70), HSP70 inhibitor pifithrin-μ was added to the plasma prior to peptide treatment. The inhibitor disrupted amyloid dispersion and both peptide-induced and, in some cases, normally occurring antioxidant effects, suggesting these reparative activities are HSP70 dependent.
Conclusions:
The results are discussed in terms of their potential use in new therapies for the complications of metabolic disease and disorders of aging that result from a deterioration of the quality control mechanisms of proteostasis.
DOI: https://doi.org/10.37349/eds.2026.1008150
This article belongs to the special issue Peptide Science Without Borders: Novel Insights for Drug Discovery

The s-triazine scaffold has emerged as a privileged heterocyclic nucleus/moiety in pharmaceutical discovery and development, owing to its presence in several natural products and clinically relevant therapeutic agents, including enasidenib, gedatolisib, bimiralisib, atrazine, indaziflam, and triaziflam. s-Triazine derivatives are not only economically accessible and synthetically versatile, but they also exhibit a broad spectrum of noteworthy biological activities, encompassing anticancer, anti-inflammatory, antiviral, antidiabetic, anticonvulsant, antitubercular, and antimicrobial properties. Their widespread utility is further supported by the ease of synthesis from inexpensive precursors such as amidines or the readily available 2,4,6-trichloro-1,3,5-triazine (cyanuric chloride), which enables sequential functionalization and the rapid generation of diverse analogues. The heightened reactivity and modularity of the s-triazine core have facilitated the development of structurally rich heterocyclic hybrids with enhanced potency and improved pharmacological profiles. These multitarget-directed systems offer exciting opportunities for addressing various forms of cancer. Considering the increasing pace of innovation in this field, a comprehensive overview of recent advancements in s-triazine-based hybrid molecules is both timely and necessary. This review highlights current progress, key design strategies, and emerging perspectives to inspire continued efforts toward the identification of promising s-triazine-based lead candidates for future drug development as anticancer agents.
The s-triazine scaffold has emerged as a privileged heterocyclic nucleus/moiety in pharmaceutical discovery and development, owing to its presence in several natural products and clinically relevant therapeutic agents, including enasidenib, gedatolisib, bimiralisib, atrazine, indaziflam, and triaziflam. s-Triazine derivatives are not only economically accessible and synthetically versatile, but they also exhibit a broad spectrum of noteworthy biological activities, encompassing anticancer, anti-inflammatory, antiviral, antidiabetic, anticonvulsant, antitubercular, and antimicrobial properties. Their widespread utility is further supported by the ease of synthesis from inexpensive precursors such as amidines or the readily available 2,4,6-trichloro-1,3,5-triazine (cyanuric chloride), which enables sequential functionalization and the rapid generation of diverse analogues. The heightened reactivity and modularity of the s-triazine core have facilitated the development of structurally rich heterocyclic hybrids with enhanced potency and improved pharmacological profiles. These multitarget-directed systems offer exciting opportunities for addressing various forms of cancer. Considering the increasing pace of innovation in this field, a comprehensive overview of recent advancements in s-triazine-based hybrid molecules is both timely and necessary. This review highlights current progress, key design strategies, and emerging perspectives to inspire continued efforts toward the identification of promising s-triazine-based lead candidates for future drug development as anticancer agents.
DOI: https://doi.org/10.37349/eds.2026.1008149
This article belongs to the special issue The Role of Triazine Scaffolds in Modern Drug Development

Aim:
To evaluate the real-world effectiveness of prophylactic metoclopramide in preventing opioid-induced nausea and vomiting (OINV) during the initial phase of strong opioid therapy in opioid-naïve patients with cancer-related pain.
Methods:
This retrospective, single-center observational cohort study included adult patients with pathologically confirmed malignancies who initiated strong opioid therapy between January 2023 and December 2024. Patients were categorized into a prophylactic metoclopramide group or a no-prophylaxis control group. Complete control (CC) of OINV during the first 7 days was defined as the absence of nausea, vomiting, and rescue antiemetic use. Univariate and multivariate logistic regression analyses were performed to identify factors associated with CC, adjusting for age, sex, body mass index, cancer subtype, cancer stage, comorbidity status, and morphine-equivalent daily dose (MEDD). Subgroup analyses were conducted based on age, sex, and cancer subtype.
Results:
A total of 244 patients were included, of whom 199 received prophylactic metoclopramide, and 45 received no prophylaxis. The prophylactic group achieved significantly higher CC rates than the control group (74.9% vs. 37.8%, p < 0.001). Multivariate logistic regression confirmed that prophylactic metoclopramide was independently associated with higher odds of achieving CC (adjusted OR = 0.20, 95% CI: 0.10–0.40; p < 0.001). Similar improvements were observed for nausea and vomiting control. Subgroup analyses demonstrated consistent benefits across age and sex groups, with particularly notable effects in patients with gastrointestinal cancers.
Conclusions:
Prophylactic metoclopramide significantly improves OINV control in opioid-naïve patients with cancer-related pain during the initiation of strong opioids. These findings support the rational use of early antiemetic prophylaxis in routine clinical practice. Prospective randomized trials are warranted to validate these real-world results and assess long-term safety.
Aim:
To evaluate the real-world effectiveness of prophylactic metoclopramide in preventing opioid-induced nausea and vomiting (OINV) during the initial phase of strong opioid therapy in opioid-naïve patients with cancer-related pain.
Methods:
This retrospective, single-center observational cohort study included adult patients with pathologically confirmed malignancies who initiated strong opioid therapy between January 2023 and December 2024. Patients were categorized into a prophylactic metoclopramide group or a no-prophylaxis control group. Complete control (CC) of OINV during the first 7 days was defined as the absence of nausea, vomiting, and rescue antiemetic use. Univariate and multivariate logistic regression analyses were performed to identify factors associated with CC, adjusting for age, sex, body mass index, cancer subtype, cancer stage, comorbidity status, and morphine-equivalent daily dose (MEDD). Subgroup analyses were conducted based on age, sex, and cancer subtype.
Results:
A total of 244 patients were included, of whom 199 received prophylactic metoclopramide, and 45 received no prophylaxis. The prophylactic group achieved significantly higher CC rates than the control group (74.9% vs. 37.8%, p < 0.001). Multivariate logistic regression confirmed that prophylactic metoclopramide was independently associated with higher odds of achieving CC (adjusted OR = 0.20, 95% CI: 0.10–0.40; p < 0.001). Similar improvements were observed for nausea and vomiting control. Subgroup analyses demonstrated consistent benefits across age and sex groups, with particularly notable effects in patients with gastrointestinal cancers.
Conclusions:
Prophylactic metoclopramide significantly improves OINV control in opioid-naïve patients with cancer-related pain during the initiation of strong opioids. These findings support the rational use of early antiemetic prophylaxis in routine clinical practice. Prospective randomized trials are warranted to validate these real-world results and assess long-term safety.
DOI: https://doi.org/10.37349/eds.2026.1008148

Aim:
To design, synthesize, and test small molecules and fragment-based compounds with putative selective anti-mycobacterial activity.
Methods:
Standard chemosynthetic processes were used to synthesize 42 compounds. A cell-based phenotypic screen for inhibitors of mycobacterial growth was used to identify several fragments and small molecules as representatives of urea-, carbamothioate-, and α,β-unsaturated systems (Michael acceptors) chemotypes.
Results:
All 42 compounds exhibited selective toxicity for mycobacteria as demonstrated by their lack of activity against various Gram-positive and Gram-negative bacteria and acid-fast Corynebacterium glutamicum. A thiadiazole compound, similar to (3-((5-(methylthio)-1,3,4-thiadiazol-2-yl)thio)pyrazine-2-carbonitrile), which activates the human lecitin: cholesterol acyltransferase (LCAT), exhibits growth-inhibitory activity [0.6 μg/mL in bovine serum albumin (BSA)-free media] against drug-susceptible Mycobacterium tuberculosis (Mtb). From the urea class, a 1,2,4-triazole-containing urea demonstrated anti-Mtb activity (4.7 μg/mL in BSA-free media). Several carbamothioate-based fragments demonstrated activity against Mycobacterium marinum [with a best minimum inhibitory concentration (MIC) of 6.25 μg/mL in minimal BSA-free media].
Conclusions:
This foundational study demonstrates the utility of these newly designed and synthesized low molecular-weight compounds and fragments as potential antimycobacterials.
Aim:
To design, synthesize, and test small molecules and fragment-based compounds with putative selective anti-mycobacterial activity.
Methods:
Standard chemosynthetic processes were used to synthesize 42 compounds. A cell-based phenotypic screen for inhibitors of mycobacterial growth was used to identify several fragments and small molecules as representatives of urea-, carbamothioate-, and α,β-unsaturated systems (Michael acceptors) chemotypes.
Results:
All 42 compounds exhibited selective toxicity for mycobacteria as demonstrated by their lack of activity against various Gram-positive and Gram-negative bacteria and acid-fast Corynebacterium glutamicum. A thiadiazole compound, similar to (3-((5-(methylthio)-1,3,4-thiadiazol-2-yl)thio)pyrazine-2-carbonitrile), which activates the human lecitin: cholesterol acyltransferase (LCAT), exhibits growth-inhibitory activity [0.6 μg/mL in bovine serum albumin (BSA)-free media] against drug-susceptible Mycobacterium tuberculosis (Mtb). From the urea class, a 1,2,4-triazole-containing urea demonstrated anti-Mtb activity (4.7 μg/mL in BSA-free media). Several carbamothioate-based fragments demonstrated activity against Mycobacterium marinum [with a best minimum inhibitory concentration (MIC) of 6.25 μg/mL in minimal BSA-free media].
Conclusions:
This foundational study demonstrates the utility of these newly designed and synthesized low molecular-weight compounds and fragments as potential antimycobacterials.
DOI: https://doi.org/10.37349/eds.2026.1008145
This article belongs to the special issue Discovery and development of new antibacterial compounds

Over the past several decades there has been a growing recognition of the role that covalent drug candidates have played in the drug development process. With this recognition, compounds that are capable of selectively and irreversibly inactivating their targets through covalent bond formation are now being specifically designed rather than being serendipitously identified. Until recently, vinyl sulfones comprised only a small fraction of the warheads under development as covalent drug candidates, but an increasing number of compounds containing this versatile functional group are now under development and consideration as drug candidates. Vinyl sulfones are generally more reactive than structurally-related acrylamides and vinyl sulfonamides, presenting a challenge for producing target-specific inactivators. The most progress in overcoming this challenge has been made in designing vinyl sulfones as selective inactivators of microbial and human cysteine proteases, incorporating these reactive warheads into peptide and peptide mimetic structures that utilize the substrate recognition motifs of these proteases. However, effective vinyl sulfones have also been produced against a growing range of phosphoryl-utilizing enzymes including kinases, phosphatases and metabolic enzymes. Here, target selection takes advantage of the capability of the sulfonyl group to act as a phosphoryl mimic. An example of this approach is presented for the targeting of a metabolic enzyme, fungal aspartate semialdehyde dehydrogenase, an essential microbial enzyme in amino acid metabolism. The studies conducted to date demonstrate the potential utility of designing vinyl sulfone drug candidates to achieve selectivity against challenging and new drug-resistant targets.
Over the past several decades there has been a growing recognition of the role that covalent drug candidates have played in the drug development process. With this recognition, compounds that are capable of selectively and irreversibly inactivating their targets through covalent bond formation are now being specifically designed rather than being serendipitously identified. Until recently, vinyl sulfones comprised only a small fraction of the warheads under development as covalent drug candidates, but an increasing number of compounds containing this versatile functional group are now under development and consideration as drug candidates. Vinyl sulfones are generally more reactive than structurally-related acrylamides and vinyl sulfonamides, presenting a challenge for producing target-specific inactivators. The most progress in overcoming this challenge has been made in designing vinyl sulfones as selective inactivators of microbial and human cysteine proteases, incorporating these reactive warheads into peptide and peptide mimetic structures that utilize the substrate recognition motifs of these proteases. However, effective vinyl sulfones have also been produced against a growing range of phosphoryl-utilizing enzymes including kinases, phosphatases and metabolic enzymes. Here, target selection takes advantage of the capability of the sulfonyl group to act as a phosphoryl mimic. An example of this approach is presented for the targeting of a metabolic enzyme, fungal aspartate semialdehyde dehydrogenase, an essential microbial enzyme in amino acid metabolism. The studies conducted to date demonstrate the potential utility of designing vinyl sulfone drug candidates to achieve selectivity against challenging and new drug-resistant targets.
DOI: https://doi.org/10.37349/eds.2026.1008147
This article belongs to the special issue The Rise of Targeted Covalent Inhibitors in Drug Discovery

Type 2 diabetes mellitus (T2DM) is a global health challenge often complicated by poor treatment adherence, suboptimal lifestyle habits, and progressive metabolic deterioration. Cognitive behavioral therapy (CBT) has been shown to improve adherence and psychological outcomes, yet its integration with structured lifestyle modification and pharmacotherapy in routine clinical care remains underexplored. A descriptive case series of five patients with uncontrolled T2DM (baseline HbA1c 11–14.5%) was conducted in a primary care setting in Palestine. The intervention combined CBT-inspired behavioral counseling (goal setting, problem-solving, cognitive restructuring) with a structured two-meal low-carbohydrate diet, exercise and sleep hygiene guidance, and pharmacotherapy optimization (withdrawal of insulin/sulfonylureas, initiation of metformin, DPP-4 inhibitors, and SGLT2 inhibitors as appropriate). Patients were followed for 3–6 months. All five patients achieved clinically meaningful improvements. Mean HbA1c decreased from 12.6% at baseline to 7.4% at follow-up. Weight loss ranged from 5–17 kg (mean ~10 kg). Additional benefits included reductions in blood pressure, improvements in renal function and lipid profiles, and resolution of quality-of-life issues such as fatigue and erectile dysfunction. Several patients discontinued insulin or sulfonylurea therapy while maintaining improved glycemic control. The integration of CBT-inspired counseling with structured lifestyle intervention and pharmacotherapy adjustments was associated with short-term improvements in uncontrolled T2DM, including outcomes approaching remission. Although the small sample size and uncontrolled design limit causal interpretation, the program is being done in a low-income, limited-resources area like Palestine. Patients do not have the privilege to attend and receive care from several healthcare professionals. Hence, conducting such practice in a primary care clinic and yielding such results and improvement in diabetes status is promising and provides hope to the patients with low income.
Type 2 diabetes mellitus (T2DM) is a global health challenge often complicated by poor treatment adherence, suboptimal lifestyle habits, and progressive metabolic deterioration. Cognitive behavioral therapy (CBT) has been shown to improve adherence and psychological outcomes, yet its integration with structured lifestyle modification and pharmacotherapy in routine clinical care remains underexplored. A descriptive case series of five patients with uncontrolled T2DM (baseline HbA1c 11–14.5%) was conducted in a primary care setting in Palestine. The intervention combined CBT-inspired behavioral counseling (goal setting, problem-solving, cognitive restructuring) with a structured two-meal low-carbohydrate diet, exercise and sleep hygiene guidance, and pharmacotherapy optimization (withdrawal of insulin/sulfonylureas, initiation of metformin, DPP-4 inhibitors, and SGLT2 inhibitors as appropriate). Patients were followed for 3–6 months. All five patients achieved clinically meaningful improvements. Mean HbA1c decreased from 12.6% at baseline to 7.4% at follow-up. Weight loss ranged from 5–17 kg (mean ~10 kg). Additional benefits included reductions in blood pressure, improvements in renal function and lipid profiles, and resolution of quality-of-life issues such as fatigue and erectile dysfunction. Several patients discontinued insulin or sulfonylurea therapy while maintaining improved glycemic control. The integration of CBT-inspired counseling with structured lifestyle intervention and pharmacotherapy adjustments was associated with short-term improvements in uncontrolled T2DM, including outcomes approaching remission. Although the small sample size and uncontrolled design limit causal interpretation, the program is being done in a low-income, limited-resources area like Palestine. Patients do not have the privilege to attend and receive care from several healthcare professionals. Hence, conducting such practice in a primary care clinic and yielding such results and improvement in diabetes status is promising and provides hope to the patients with low income.
DOI: https://doi.org/10.37349/eds.2026.1008146

Background:
Antimicrobial resistance (AMR) among Gram-positive bacteria has emerged as a significant global health threat, with pathogens such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VRE) exhibiting increasing resistance to conventional antibiotics. This systematic review evaluates new advances in nanomaterial-based antimicrobial agents as innovative solutions to combat AMR in Gram-positive bacteria.
Methods:
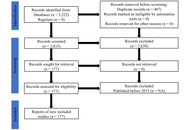
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, studies published between 2014 and 2024 were systematically screened and analysed from databases including PubMed, Scopus, Google Scholar, and HINARI. From an initial 1,405 articles, 131 experimental studies that met the inclusion criteria were systematically analysed to harness the advances in nanomaterial-based antimicrobial agents in combating AMR in Gram-positive bacteria.
Results:
The included studies demonstrated that various nanomaterials, including silver nanoparticles (AgNPs), gold nanoparticles (AuNPs), zinc oxide nanoparticles (ZnO NPs), copper and copper oxide nanoparticles (Cu/CuO NPs), as well as polymeric and hybrid systems, exhibited potent antibacterial and antibiofilm activities. Key mechanisms of action included bacterial membrane disruption, reactive oxygen species (ROS) generation, intracellular interference, and targeted drug delivery. Many nanomaterials showed enhanced efficacy and synergistic effects when combined with conventional antibiotics, effectively reducing bacterial load and inhibiting biofilm formation in resistant strains like MRSA.
Discussion:
Nanomaterials offer a multifaceted approach to overcome the evolving resistance mechanisms in Gram-positive pathogens, showing significant preclinical and clinical success. Despite these substantial preclinical results, challenges such as cytotoxicity, environmental impact, scalability, and the potential for resistance adaptation remain unaddressed. Furthermore, important translational barriers persist, most notably insufficient pharmacokinetic data and unclear regulatory pathways. Future efforts must focus on standardized manufacturing, comprehensive toxicity studies, and robust clinical trials to bridge the gap between laboratory innovation and practical therapeutic application.
Background:
Antimicrobial resistance (AMR) among Gram-positive bacteria has emerged as a significant global health threat, with pathogens such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VRE) exhibiting increasing resistance to conventional antibiotics. This systematic review evaluates new advances in nanomaterial-based antimicrobial agents as innovative solutions to combat AMR in Gram-positive bacteria.
Methods:
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, studies published between 2014 and 2024 were systematically screened and analysed from databases including PubMed, Scopus, Google Scholar, and HINARI. From an initial 1,405 articles, 131 experimental studies that met the inclusion criteria were systematically analysed to harness the advances in nanomaterial-based antimicrobial agents in combating AMR in Gram-positive bacteria.
Results:
The included studies demonstrated that various nanomaterials, including silver nanoparticles (AgNPs), gold nanoparticles (AuNPs), zinc oxide nanoparticles (ZnO NPs), copper and copper oxide nanoparticles (Cu/CuO NPs), as well as polymeric and hybrid systems, exhibited potent antibacterial and antibiofilm activities. Key mechanisms of action included bacterial membrane disruption, reactive oxygen species (ROS) generation, intracellular interference, and targeted drug delivery. Many nanomaterials showed enhanced efficacy and synergistic effects when combined with conventional antibiotics, effectively reducing bacterial load and inhibiting biofilm formation in resistant strains like MRSA.
Discussion:
Nanomaterials offer a multifaceted approach to overcome the evolving resistance mechanisms in Gram-positive pathogens, showing significant preclinical and clinical success. Despite these substantial preclinical results, challenges such as cytotoxicity, environmental impact, scalability, and the potential for resistance adaptation remain unaddressed. Furthermore, important translational barriers persist, most notably insufficient pharmacokinetic data and unclear regulatory pathways. Future efforts must focus on standardized manufacturing, comprehensive toxicity studies, and robust clinical trials to bridge the gap between laboratory innovation and practical therapeutic application.
DOI: https://doi.org/10.37349/eds.2026.1008144

Background:
Vascular aging is a major driver of cardiovascular, metabolic, and degenerative diseases, characterized by oxidative stress, mitochondrial dysfunction, endothelial senescence, and impaired proteostasis. Emerging data show that anti-infective drugs can influence these aging pathways beyond antimicrobial activity. However, their capacity to accelerate or slow vascular ageing has not been clearly defined. This review summarizes current evidence on how anti-infective agents modulate vascular ageing mechanisms.
Methods:
A systematic review was conducted following PRISMA 2020 guidelines. Studies from 2000 to 2024 were searched in major indexed databases. Eligible studies included in vitro, animal, and human research evaluating the effects of anti-infective agents on endothelial function, vascular senescence markers (p16INK4a, p21, SA-β-gal), oxidative stress, mitochondrial activity, inflammation, or proteostasis, key determinants of vascular ageing. Studies lacking mechanistic aging endpoints were excluded. Extracted data included drug class, model type, study design, and age-related outcomes. Risk of bias was assessed using SYRCLE, RoB-2, ROBINS-I, and narrative appraisal for in vitro studies.
Results:
Ninety-eight studies were identified; after removing six duplicates, ninety-two met the criteria. Macrolides, tetracyclines, and selected antivirals exerted anti-ageing effects by suppressing senescence-associated secretory phenotype (SASP), preserving mitochondrial integrity, reducing oxidative stress, and enhancing autophagy. Aminoglycosides and fluoroquinolones accelerated vascular ageing by generating reactive oxygen species, inducing DNA damage, and disrupting proteostasis. Antiviral protease inhibitors worsened endothelial dysfunction and metabolic aging. Antifungals such as itraconazole and amphotericin B impaired mitochondrial activity and angiogenesis, contributing to ageing phenotypes. Antiparasitic drugs showed mixed aging outcomes: chloroquine promoted autophagy and longevity, whereas thiabendazole impaired vascular stability. Broad-spectrum antibiotics disrupted the gut-vascular axis, increasing trimethylamine N-oxide, a mediator of inflammatory vascular aging.
Discussion:
Anti-infective drugs display diverse, class-specific effects on vascular aging. Recognizing these age-related actions is essential for safer prescribing and for repurposing anti-infective agents to target pathological vascular aging mechanisms.
Background:
Vascular aging is a major driver of cardiovascular, metabolic, and degenerative diseases, characterized by oxidative stress, mitochondrial dysfunction, endothelial senescence, and impaired proteostasis. Emerging data show that anti-infective drugs can influence these aging pathways beyond antimicrobial activity. However, their capacity to accelerate or slow vascular ageing has not been clearly defined. This review summarizes current evidence on how anti-infective agents modulate vascular ageing mechanisms.
Methods:
A systematic review was conducted following PRISMA 2020 guidelines. Studies from 2000 to 2024 were searched in major indexed databases. Eligible studies included in vitro, animal, and human research evaluating the effects of anti-infective agents on endothelial function, vascular senescence markers (p16INK4a, p21, SA-β-gal), oxidative stress, mitochondrial activity, inflammation, or proteostasis, key determinants of vascular ageing. Studies lacking mechanistic aging endpoints were excluded. Extracted data included drug class, model type, study design, and age-related outcomes. Risk of bias was assessed using SYRCLE, RoB-2, ROBINS-I, and narrative appraisal for in vitro studies.
Results:
Ninety-eight studies were identified; after removing six duplicates, ninety-two met the criteria. Macrolides, tetracyclines, and selected antivirals exerted anti-ageing effects by suppressing senescence-associated secretory phenotype (SASP), preserving mitochondrial integrity, reducing oxidative stress, and enhancing autophagy. Aminoglycosides and fluoroquinolones accelerated vascular ageing by generating reactive oxygen species, inducing DNA damage, and disrupting proteostasis. Antiviral protease inhibitors worsened endothelial dysfunction and metabolic aging. Antifungals such as itraconazole and amphotericin B impaired mitochondrial activity and angiogenesis, contributing to ageing phenotypes. Antiparasitic drugs showed mixed aging outcomes: chloroquine promoted autophagy and longevity, whereas thiabendazole impaired vascular stability. Broad-spectrum antibiotics disrupted the gut-vascular axis, increasing trimethylamine N-oxide, a mediator of inflammatory vascular aging.
Discussion:
Anti-infective drugs display diverse, class-specific effects on vascular aging. Recognizing these age-related actions is essential for safer prescribing and for repurposing anti-infective agents to target pathological vascular aging mechanisms.
DOI: https://doi.org/10.37349/eds.2026.1008143

Aim:
The prevalence of multidrug-resistant “superbugs”, particularly Acinetobacter baumannii and Klebsiella pneumoniae, is a menacing phenomenon in society, rendering last-resort antibiotics increasingly suboptimal and ineffective. Carbapenemase enzymes play a major role in this resistance by hydrolysing carbapenem antibiotics. This study aims to identify and characterize potential non-covalent carbapenemase inhibitors using multiscale computational approaches.
Methods:
A focused library of 245 compounds, comprising pharmacopeial derivatives and chemogenomic molecules, was screened using a hierarchical virtual screening workflow. Top-ranked hits were further evaluated by rescoring for thermodynamic affinity. The most promising candidate was subjected to a 100 ns molecular dynamics (MD) simulation to assess binding stability, followed by Well-Tempered Metadynamics (WTMetaD) to characterise the free energy landscape and binding behaviour. Pharmacokinetic and toxicity profiles were predicted using SwissADME and ProTox 3.0.
Results:
Three compounds, daunorubicin, doxorubicin, and EUB0000226b, emerged as potential carbapenemase inhibitors. EUB0000226b demonstrated the most favourable binding affinity and structural novelty. MD simulations showed protein stability, while ligand RMSD fluctuations (2.4–5.6 Å) suggested flexible binding. WTMetaD analysis revealed a solvent-separated metastable state that increased ligand residence time within the active site. ADME and toxicity predictions indicated acceptable drug-likeness, good gastrointestinal absorption, and a generally safe profile.
Conclusions:
Multiscale computational analysis identified EUB0000226b as a promising non-covalent carbapenemase inhibitor with favourable binding energetics, dynamic stability, and drug-like properties. These findings support its further experimental validation and potential development for combating carbapenem-resistant bacterial pathogens.
Aim:
The prevalence of multidrug-resistant “superbugs”, particularly Acinetobacter baumannii and Klebsiella pneumoniae, is a menacing phenomenon in society, rendering last-resort antibiotics increasingly suboptimal and ineffective. Carbapenemase enzymes play a major role in this resistance by hydrolysing carbapenem antibiotics. This study aims to identify and characterize potential non-covalent carbapenemase inhibitors using multiscale computational approaches.
Methods:
A focused library of 245 compounds, comprising pharmacopeial derivatives and chemogenomic molecules, was screened using a hierarchical virtual screening workflow. Top-ranked hits were further evaluated by rescoring for thermodynamic affinity. The most promising candidate was subjected to a 100 ns molecular dynamics (MD) simulation to assess binding stability, followed by Well-Tempered Metadynamics (WTMetaD) to characterise the free energy landscape and binding behaviour. Pharmacokinetic and toxicity profiles were predicted using SwissADME and ProTox 3.0.
Results:
Three compounds, daunorubicin, doxorubicin, and EUB0000226b, emerged as potential carbapenemase inhibitors. EUB0000226b demonstrated the most favourable binding affinity and structural novelty. MD simulations showed protein stability, while ligand RMSD fluctuations (2.4–5.6 Å) suggested flexible binding. WTMetaD analysis revealed a solvent-separated metastable state that increased ligand residence time within the active site. ADME and toxicity predictions indicated acceptable drug-likeness, good gastrointestinal absorption, and a generally safe profile.
Conclusions:
Multiscale computational analysis identified EUB0000226b as a promising non-covalent carbapenemase inhibitor with favourable binding energetics, dynamic stability, and drug-like properties. These findings support its further experimental validation and potential development for combating carbapenem-resistant bacterial pathogens.
DOI: https://doi.org/10.37349/eds.2026.1008140
This article belongs to the special issue Discovery and development of new antibacterial compounds

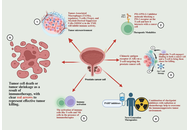
Immunotherapy has transformed oncology, yet has only been marginally effective in prostate cancer (PCa), which is a malignancy with a low mutational load and a highly immunosuppressive tumor microenvironment (TME). This critical review is a reflection on the changing position of the innovative immunotherapies in PCa that extends beyond the description stage to synthesize the synergies and constraints of immune checkpoint inhibitors (ICIs), chimeric antigen receptor (CAR) T-cell therapy, and next-generation modalities such as bispecific T-cell engagers (BiTEs). We assess the mechanistic reasoning of combination therapies, comprising androgen receptor signaling communicators, PARP communicators, and radioligand therapies, which seek to modulate the immunogenicity of the immune-cold PCa TME. Also, we combine new knowledge to novel resistance pathways, including the newly discovered thrombospondin-1-CD47 axis, in the process of T cell exhaustion through calcineurin-NFAT signaling. Although some preclinical data and initial clinical indicators in biomarker-selected subpopulations are promising, the vast majority of Phase III trials of ICIs in unselected populations with metastatic castration-resistant prostate cancer (mCRPC) have failed. This review reveals that the next generation of PCa immunotherapy would not be sequential monotherapies but rather rationally designed multimodal combinations guided by profound molecular and immune profiling to overcome inherent resistance mechanisms.
Immunotherapy has transformed oncology, yet has only been marginally effective in prostate cancer (PCa), which is a malignancy with a low mutational load and a highly immunosuppressive tumor microenvironment (TME). This critical review is a reflection on the changing position of the innovative immunotherapies in PCa that extends beyond the description stage to synthesize the synergies and constraints of immune checkpoint inhibitors (ICIs), chimeric antigen receptor (CAR) T-cell therapy, and next-generation modalities such as bispecific T-cell engagers (BiTEs). We assess the mechanistic reasoning of combination therapies, comprising androgen receptor signaling communicators, PARP communicators, and radioligand therapies, which seek to modulate the immunogenicity of the immune-cold PCa TME. Also, we combine new knowledge to novel resistance pathways, including the newly discovered thrombospondin-1-CD47 axis, in the process of T cell exhaustion through calcineurin-NFAT signaling. Although some preclinical data and initial clinical indicators in biomarker-selected subpopulations are promising, the vast majority of Phase III trials of ICIs in unselected populations with metastatic castration-resistant prostate cancer (mCRPC) have failed. This review reveals that the next generation of PCa immunotherapy would not be sequential monotherapies but rather rationally designed multimodal combinations guided by profound molecular and immune profiling to overcome inherent resistance mechanisms.
DOI: https://doi.org/10.37349/eds.2026.1008141

DOI: https://doi.org/10.37349/eds.2026.1008142

Background:
The root cause of diabetes is dysregulated pathways, including those involving AMP-activated protein kinase (AMPK), GLUT-mediated glucose transport, and the PI3K/AKT pathway. There has been a notable increase in research on phytoconstituents as pathway-specific treatments for diabetes; however, the comprehensiveness of this evidence remains unclear.
Methods:
This systematic review followed PRISMA guidelines and was registered on PROSPERO (CRD420251073083). Databases searched included PubMed, Scopus, Google Scholar, and Europe PMC for experimental studies (in vivo, in vitro, and in silico) published between 2015 and 2024. The final search was conducted in April 2025, and 2025 publications available as “early access” before this date were included. Only English-language studies were included. Animal studies (in vivo) were assessed for risk of bias using the SYRCLE tool, while in vitro studies were evaluated using the ToxRTool, based on test substance characterization, test system description, study design, and data reporting. Narrative synthesis was employed due to the heterogeneity of the data.
Results:
Out of 3,222 articles, 177 articles met the inclusion criteria. Study types included in vitro (92; 52%), in vivo (66; 37.3%), in silico (15; 8.5%), and other experimental types (4; 2.3%). Phytoconstituents predominantly targeted PI3K/AKT (44.6%), GLUT transporters (19.8%), and AMPK (14.1%) pathways. Rodent models were most used (48.02%). Primary outcomes included improved insulin sensitivity, enhanced glucose homeostasis, and reduced oxidative stress and inflammation. The risk of bias analysis revealed 68.93% of the studies carried a moderate risk, 29.94% a low risk, and 1.13% a high risk.
Discussion:
Phytoconstituent activity was consistent with the activation of diabetes-relevant signaling pathways, particularly PI3K/AKT, GLUT transporters, and AMPK cascades. However, most evidence was correlative, with limited loss-of-function validation. Methodological irregularities, moderate risk of bias, and limited translational research reduce the strength and generalizability of these findings.
Background:
The root cause of diabetes is dysregulated pathways, including those involving AMP-activated protein kinase (AMPK), GLUT-mediated glucose transport, and the PI3K/AKT pathway. There has been a notable increase in research on phytoconstituents as pathway-specific treatments for diabetes; however, the comprehensiveness of this evidence remains unclear.
Methods:
This systematic review followed PRISMA guidelines and was registered on PROSPERO (CRD420251073083). Databases searched included PubMed, Scopus, Google Scholar, and Europe PMC for experimental studies (in vivo, in vitro, and in silico) published between 2015 and 2024. The final search was conducted in April 2025, and 2025 publications available as “early access” before this date were included. Only English-language studies were included. Animal studies (in vivo) were assessed for risk of bias using the SYRCLE tool, while in vitro studies were evaluated using the ToxRTool, based on test substance characterization, test system description, study design, and data reporting. Narrative synthesis was employed due to the heterogeneity of the data.
Results:
Out of 3,222 articles, 177 articles met the inclusion criteria. Study types included in vitro (92; 52%), in vivo (66; 37.3%), in silico (15; 8.5%), and other experimental types (4; 2.3%). Phytoconstituents predominantly targeted PI3K/AKT (44.6%), GLUT transporters (19.8%), and AMPK (14.1%) pathways. Rodent models were most used (48.02%). Primary outcomes included improved insulin sensitivity, enhanced glucose homeostasis, and reduced oxidative stress and inflammation. The risk of bias analysis revealed 68.93% of the studies carried a moderate risk, 29.94% a low risk, and 1.13% a high risk.
Discussion:
Phytoconstituent activity was consistent with the activation of diabetes-relevant signaling pathways, particularly PI3K/AKT, GLUT transporters, and AMPK cascades. However, most evidence was correlative, with limited loss-of-function validation. Methodological irregularities, moderate risk of bias, and limited translational research reduce the strength and generalizability of these findings.
DOI: https://doi.org/10.37349/eds.2026.1008139

Aim:
This study aimed to establish the in vitro efficacy of chlorhexidine (CHX)-silver nanoparticles (AgNP) based preparation, against Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa (P. aeruginosa) in planktonic cultures, biofilms, and on inert stainless-steel surfaces.
Methods:
The in vitro antimicrobial activity of the CHX-AgNP formulation (Dermosedan MRSA Nano AG®) was evaluated against reference and multidrug-resistant (MDR) strains of S. aureus and P. aeruginosa by determining the minimum inhibitory and bactericidal concentrations (MIC and MBC), as well as the minimum biofilm inhibitory and eradication concentrations (MBIC and MBEC). Also, the residual bactericidal activity on inert stainless-steel surfaces was evaluated.
Results:
MIC against methicillin-resistant S. aureus (MRSA) and MDR P. aeruginosa (MDR-PA) isolates ranged between 5/2.5–20/10 µg/mL, up to 8,000 times lower than the manufacturer’s recommended concentration, while MBC ranged from 500 to 2,000 times lower. MBIC matched the MBC, while higher concentrations were needed to eradicate preformed biofilms. On stainless-steel surfaces, high antimicrobial activity was observed for both pathogens. No bacterial survival was detected even after 6 hours at 4% CHX + 2% AgNP concentration. The CHX-AgNP combination demonstrated strong antimicrobial and antibiofilm activity against both S. aureus and P. aeruginosa.
Conclusions:
These results support the potential application of Dermosedan MRSA Nano AG® as a strategy for managing topical resistant infections and for environmental disinfection in both veterinary and clinical settings.
Aim:
This study aimed to establish the in vitro efficacy of chlorhexidine (CHX)-silver nanoparticles (AgNP) based preparation, against Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa (P. aeruginosa) in planktonic cultures, biofilms, and on inert stainless-steel surfaces.
Methods:
The in vitro antimicrobial activity of the CHX-AgNP formulation (Dermosedan MRSA Nano AG®) was evaluated against reference and multidrug-resistant (MDR) strains of S. aureus and P. aeruginosa by determining the minimum inhibitory and bactericidal concentrations (MIC and MBC), as well as the minimum biofilm inhibitory and eradication concentrations (MBIC and MBEC). Also, the residual bactericidal activity on inert stainless-steel surfaces was evaluated.
Results:
MIC against methicillin-resistant S. aureus (MRSA) and MDR P. aeruginosa (MDR-PA) isolates ranged between 5/2.5–20/10 µg/mL, up to 8,000 times lower than the manufacturer’s recommended concentration, while MBC ranged from 500 to 2,000 times lower. MBIC matched the MBC, while higher concentrations were needed to eradicate preformed biofilms. On stainless-steel surfaces, high antimicrobial activity was observed for both pathogens. No bacterial survival was detected even after 6 hours at 4% CHX + 2% AgNP concentration. The CHX-AgNP combination demonstrated strong antimicrobial and antibiofilm activity against both S. aureus and P. aeruginosa.
Conclusions:
These results support the potential application of Dermosedan MRSA Nano AG® as a strategy for managing topical resistant infections and for environmental disinfection in both veterinary and clinical settings.
DOI: https://doi.org/10.37349/eds.2026.1008138
This article belongs to the special issue Discovery and development of new antibacterial compounds

Aim:
One of the causes of Alzheimer’s disease (AD) is the structural change and aggregation of target proteins due to the binding of metal ions. In this study, we investigated where copper(II) ions bound to the protein egg white lysozyme crystals in a hydrophilic buffer solution after ions were synthesized from an amino acid Schiff base copper(II) complex with a hydrophobic azobenzene group.
Methods:
X-ray crystallographic studies of the complexes and egg white lysozyme were then studied. Molecular docking studies for the binding of copper(II) ion with egg white lysozyme were also carried out.
Results:
The results suggest that the hydrophobicity of the introduced complex affected how deeply the resultant copper(II) ion penetrated into the protein. It has been revealed that when metal complexes are soaked into protein crystals, the metal complexes act as carriers, and metal ions tend to dissociate and bind to appropriate functional groups on certain specific residues of the protein. His15 and Glu35 were the more common binding residues of the protein that bound to the metal ion.
Conclusions:
An anti-Irving-Williams behaviour was observed for the interaction of the copper(II) complex with the lysozyme. Docking studies revealed various potential binding sites of copper(II) ion with the lysozyme.
Aim:
One of the causes of Alzheimer’s disease (AD) is the structural change and aggregation of target proteins due to the binding of metal ions. In this study, we investigated where copper(II) ions bound to the protein egg white lysozyme crystals in a hydrophilic buffer solution after ions were synthesized from an amino acid Schiff base copper(II) complex with a hydrophobic azobenzene group.
Methods:
X-ray crystallographic studies of the complexes and egg white lysozyme were then studied. Molecular docking studies for the binding of copper(II) ion with egg white lysozyme were also carried out.
Results:
The results suggest that the hydrophobicity of the introduced complex affected how deeply the resultant copper(II) ion penetrated into the protein. It has been revealed that when metal complexes are soaked into protein crystals, the metal complexes act as carriers, and metal ions tend to dissociate and bind to appropriate functional groups on certain specific residues of the protein. His15 and Glu35 were the more common binding residues of the protein that bound to the metal ion.
Conclusions:
An anti-Irving-Williams behaviour was observed for the interaction of the copper(II) complex with the lysozyme. Docking studies revealed various potential binding sites of copper(II) ion with the lysozyme.
DOI: https://doi.org/10.37349/eds.2025.1008137

Aim:
Diabetes mellitus is a serious public health problem, and the condition is managed using herbal medicine by many African traditional healers. This study aimed to provide scientific evidence on the effects of aqueous and ethanol extracts of Xymalos monospora (X. monospora) leaves on some biochemical parameters in diabetic rats.
Methods:
This experiment included 63 male Wistar rats. Diabetes was induced for 10 days by intraperitoneal injection of dexamethasone (16 mg/kg) in overnight fasted rats. The diabetic rats were treated with aqueous (100 and 200 mg/kg) and ethanol (100 and 200 mg/kg) extracts of X. monospora leaves and metformin (40 mg/kg) for 15 days. Fasting blood glucose, serum lipid profile, atherogenicity indices (Castelli’s Risk Index, Atherogenic Coefficient, Atherogenic Index of Plasma), tumor necrosis factor alpha, and hepatic glycogen were evaluated.
Results:
Treatment with the aqueous extracts at 100 and 200 mg/kg significantly reduced fasting blood glucose by 29.2% (p = 0.016) and 35.9% (p = 0.009), respectively. Also, the ethanol extracts at 100 and 200 mg/kg significantly reduced fasting blood glucose by 20.7% (p = 0.038) and 31.2% (p = 0.027), respectively. The aqueous extract (200 mg/kg) significantly reduced total cholesterol and triglyceride concentrations by 31.5% (p = 0.017) and 30.7% (p = 0.023), respectively. There was a significant reduction in atherogenicity indices (p < 0.05), and liver glycogen levels improved. The extracts reduced the levels of tumor necrosis factor alpha, but this was not significant (p > 0.05). However, histopathological studies were not carried out, and the above findings may not directly translate to clinical efficacy.
Conclusions:
These findings demonstrate that the oral administration of aqueous and ethanol extracts of X. monospora leaves has significant antidiabetic effects, including a decrease in fasting blood glucose, improvement of serum lipid profile, and increased glycogen storage.
Aim:
Diabetes mellitus is a serious public health problem, and the condition is managed using herbal medicine by many African traditional healers. This study aimed to provide scientific evidence on the effects of aqueous and ethanol extracts of Xymalos monospora (X. monospora) leaves on some biochemical parameters in diabetic rats.
Methods:
This experiment included 63 male Wistar rats. Diabetes was induced for 10 days by intraperitoneal injection of dexamethasone (16 mg/kg) in overnight fasted rats. The diabetic rats were treated with aqueous (100 and 200 mg/kg) and ethanol (100 and 200 mg/kg) extracts of X. monospora leaves and metformin (40 mg/kg) for 15 days. Fasting blood glucose, serum lipid profile, atherogenicity indices (Castelli’s Risk Index, Atherogenic Coefficient, Atherogenic Index of Plasma), tumor necrosis factor alpha, and hepatic glycogen were evaluated.
Results:
Treatment with the aqueous extracts at 100 and 200 mg/kg significantly reduced fasting blood glucose by 29.2% (p = 0.016) and 35.9% (p = 0.009), respectively. Also, the ethanol extracts at 100 and 200 mg/kg significantly reduced fasting blood glucose by 20.7% (p = 0.038) and 31.2% (p = 0.027), respectively. The aqueous extract (200 mg/kg) significantly reduced total cholesterol and triglyceride concentrations by 31.5% (p = 0.017) and 30.7% (p = 0.023), respectively. There was a significant reduction in atherogenicity indices (p < 0.05), and liver glycogen levels improved. The extracts reduced the levels of tumor necrosis factor alpha, but this was not significant (p > 0.05). However, histopathological studies were not carried out, and the above findings may not directly translate to clinical efficacy.
Conclusions:
These findings demonstrate that the oral administration of aqueous and ethanol extracts of X. monospora leaves has significant antidiabetic effects, including a decrease in fasting blood glucose, improvement of serum lipid profile, and increased glycogen storage.
DOI: https://doi.org/10.37349/eds.2025.1008136

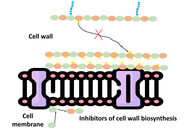
Antimicrobial peptides (AMPs) are a heterogeneous group of small, naturally occurring molecules that are an integral part of the innate immunity of nearly all life forms. Their amphiphilic nature, cationic character, and small size distinguish AMPs, which have a wide spectrum antimicrobial activity against bacteria, fungi, parasites, and viruses. Their specific ability to selectively destroy microbial membranes, without harming host cells, makes them promising contenders to treat the growing threat of antimicrobial resistance (AMR), which has undermined the effectiveness of traditional antibiotics. The action mechanisms of AMPs are multifaceted, involving both membrane-disruptive mechanisms, like barrel stave pore formation, toroidal pore induction, and carpet-like membrane degradation, and non-membrane targeting mechanisms, like inhibition of nucleic acid synthesis, protein translation, and cell wall biosynthesis. AMPs are structurally diverse, from α-helices and β-sheets to cyclic and unstructured peptides, and are distributed abundantly in nature, being derived from mammals, amphibians, insects, plants, and microorganisms. Apart from antimicrobial activity, AMPs have immunomodulatory and regenerative activities, enabling their use in many therapeutic and industrial applications. These are for the construction of new anti-infective agents, wound healing compounds, medical device coatings to inhibit biofilm growth, natural food preservatives, adjuvants for vaccines, and possible anti-cancer drugs. Although they hold great promise, stability, toxicity, and production scale issues continue to hinder translation to the clinic. This review highlights the structural variability, modes of action, and novel uses of AMPs, with a focus on their status as next-generation therapeutics against multidrug-resistant microbes and for promoting biomedical innovation.
Antimicrobial peptides (AMPs) are a heterogeneous group of small, naturally occurring molecules that are an integral part of the innate immunity of nearly all life forms. Their amphiphilic nature, cationic character, and small size distinguish AMPs, which have a wide spectrum antimicrobial activity against bacteria, fungi, parasites, and viruses. Their specific ability to selectively destroy microbial membranes, without harming host cells, makes them promising contenders to treat the growing threat of antimicrobial resistance (AMR), which has undermined the effectiveness of traditional antibiotics. The action mechanisms of AMPs are multifaceted, involving both membrane-disruptive mechanisms, like barrel stave pore formation, toroidal pore induction, and carpet-like membrane degradation, and non-membrane targeting mechanisms, like inhibition of nucleic acid synthesis, protein translation, and cell wall biosynthesis. AMPs are structurally diverse, from α-helices and β-sheets to cyclic and unstructured peptides, and are distributed abundantly in nature, being derived from mammals, amphibians, insects, plants, and microorganisms. Apart from antimicrobial activity, AMPs have immunomodulatory and regenerative activities, enabling their use in many therapeutic and industrial applications. These are for the construction of new anti-infective agents, wound healing compounds, medical device coatings to inhibit biofilm growth, natural food preservatives, adjuvants for vaccines, and possible anti-cancer drugs. Although they hold great promise, stability, toxicity, and production scale issues continue to hinder translation to the clinic. This review highlights the structural variability, modes of action, and novel uses of AMPs, with a focus on their status as next-generation therapeutics against multidrug-resistant microbes and for promoting biomedical innovation.
DOI: https://doi.org/10.37349/eds.2025.1008135
This article belongs to the special issue Bioactive Peptides: Pioneering Innovations in Latin American Research

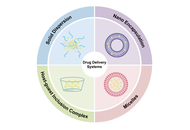
Buarque et al. (Explor Drug Sci. 2025;3:1008107. DOI: 10.37349/eds.2025.1008107) reported the synthesis of 13 biaryl hydroxy-1,2,3-triazoles and 11 fluorene-1,2,3-triazole hybrids via optimized Suzuki and telescopic one-pot reactions. Cytotoxicity evaluations against colorectal cancer (HCT-116), astrocytoma (SNB-19), triple-negative breast cancer (MDA-MB-231), and acute myeloid leukemia, FLT3-ITD mutant (MOLM-13) cell lines revealed promising antitumor activity. 1-(2-bromophenyl)-4-(9H-filoren-9-yl)-1H-1,2,3-triazole (LSO258) and 1-(4-bromophenyl)-4-(2-fluoro-9H-fluron-9-yl)-1H-1,2,3-triazole (LSO272), both being fluorene-1,2,3-triazole hybrids with bromine substituents, could selectively inhibit the activity of MOLM-13 cells, while the biaryl hydroxy-1,2,3-triazoles compounds exhibited broader antitumor activity. It is worth noting that an inevitable phenomenon is observed: The above compounds have significant aromatic structural characteristics, and their large aromatic systems lead to increased molecular hydrophobicity, resulting in poor water solubility. This critical druggability limitation will directly restrict the development of formulations. To tackle this issue, this paper proposes micelles as the optimal solution. As a carrier structure formed by the self-assembly of amphiphilic surfactants, micelles possess a unique “hydrophobic core-hydrophilic shell” configuration. Their hydrophobic core layer can efficiently encapsulate triazole compounds containing aromatic structures. Compared to other nanomedicine formulations such as solid dispersions and nanoencapsulation technology, micelles demonstrate significant advantages in terms of stability, process simplicity, and biocompatibility.
Buarque et al. (Explor Drug Sci. 2025;3:1008107. DOI: 10.37349/eds.2025.1008107) reported the synthesis of 13 biaryl hydroxy-1,2,3-triazoles and 11 fluorene-1,2,3-triazole hybrids via optimized Suzuki and telescopic one-pot reactions. Cytotoxicity evaluations against colorectal cancer (HCT-116), astrocytoma (SNB-19), triple-negative breast cancer (MDA-MB-231), and acute myeloid leukemia, FLT3-ITD mutant (MOLM-13) cell lines revealed promising antitumor activity. 1-(2-bromophenyl)-4-(9H-filoren-9-yl)-1H-1,2,3-triazole (LSO258) and 1-(4-bromophenyl)-4-(2-fluoro-9H-fluron-9-yl)-1H-1,2,3-triazole (LSO272), both being fluorene-1,2,3-triazole hybrids with bromine substituents, could selectively inhibit the activity of MOLM-13 cells, while the biaryl hydroxy-1,2,3-triazoles compounds exhibited broader antitumor activity. It is worth noting that an inevitable phenomenon is observed: The above compounds have significant aromatic structural characteristics, and their large aromatic systems lead to increased molecular hydrophobicity, resulting in poor water solubility. This critical druggability limitation will directly restrict the development of formulations. To tackle this issue, this paper proposes micelles as the optimal solution. As a carrier structure formed by the self-assembly of amphiphilic surfactants, micelles possess a unique “hydrophobic core-hydrophilic shell” configuration. Their hydrophobic core layer can efficiently encapsulate triazole compounds containing aromatic structures. Compared to other nanomedicine formulations such as solid dispersions and nanoencapsulation technology, micelles demonstrate significant advantages in terms of stability, process simplicity, and biocompatibility.
DOI: https://doi.org/10.37349/eds.2025.1008134

The escalating threat of antibiotic resistance and its advancing mechanisms for resistance development underscore the imperative need for alternative approaches to treat life-threatening infections. Consideration of bacteriophages, as well as antimicrobial peptides (AMPs) that can specifically target and eliminate particular bacteria, is gaining prominence for the improved treatment of infections. The effectiveness of bacteriophages and AMPs has been known for a long time, and their combined use is being investigated recently. Studies have shown that the use of phages or phage-derived enzymes (endolysins) in combination with AMPs has shown promising results in combating multidrug resistant bacteria. Bacteriophages lyse bacteria by hijacking the bacterial cell’s metabolic machinery, leading to the production of phage virus inside it and finally bursting the bacteria, while AMPs act by disrupting the bacterial cell membrane or affecting intracellular targets after penetration. In this review, we discuss previous studies on the combined use of both phages or phage-derived enzymes and AMPs, demonstrating their synergistic effects for combating multidrug resistant pathogens. Their mechanisms of action, and possible mechanisms of synergy and development of bacterial resistance to these, are discussed. Approaches, including genetic engineering, for improving their efficacy have been discussed. Safety and ethical issues regarding their use in human subjects are discussed. In summary, this review emphasizes the need for further research on the combined use of AMPs and bacteriophages to tap their potential effectiveness for treating antimicrobial-resistant infections.
The escalating threat of antibiotic resistance and its advancing mechanisms for resistance development underscore the imperative need for alternative approaches to treat life-threatening infections. Consideration of bacteriophages, as well as antimicrobial peptides (AMPs) that can specifically target and eliminate particular bacteria, is gaining prominence for the improved treatment of infections. The effectiveness of bacteriophages and AMPs has been known for a long time, and their combined use is being investigated recently. Studies have shown that the use of phages or phage-derived enzymes (endolysins) in combination with AMPs has shown promising results in combating multidrug resistant bacteria. Bacteriophages lyse bacteria by hijacking the bacterial cell’s metabolic machinery, leading to the production of phage virus inside it and finally bursting the bacteria, while AMPs act by disrupting the bacterial cell membrane or affecting intracellular targets after penetration. In this review, we discuss previous studies on the combined use of both phages or phage-derived enzymes and AMPs, demonstrating their synergistic effects for combating multidrug resistant pathogens. Their mechanisms of action, and possible mechanisms of synergy and development of bacterial resistance to these, are discussed. Approaches, including genetic engineering, for improving their efficacy have been discussed. Safety and ethical issues regarding their use in human subjects are discussed. In summary, this review emphasizes the need for further research on the combined use of AMPs and bacteriophages to tap their potential effectiveness for treating antimicrobial-resistant infections.
DOI: https://doi.org/10.37349/eds.2025.1008133
 Previous
Previous