Affiliation:
State Key Laboratory of Bioactive Molecules and Druggability Assessment, Guangdong Basic Research Center of Excellence for Natural Bioactive Molecules and Discovery of Innovative Drugs, College of Pharmacy, Jinan University, Guangzhou 511443, Guangdong, China.
†These authors contributed equally to this work.
ORCID: https://orcid.org/0009-0006-5291-5095
Affiliation:
State Key Laboratory of Bioactive Molecules and Druggability Assessment, Guangdong Basic Research Center of Excellence for Natural Bioactive Molecules and Discovery of Innovative Drugs, College of Pharmacy, Jinan University, Guangzhou 511443, Guangdong, China.
†These authors contributed equally to this work.
ORCID: https://orcid.org/0009-0003-6728-4466
Affiliation:
State Key Laboratory of Bioactive Molecules and Druggability Assessment, Guangdong Basic Research Center of Excellence for Natural Bioactive Molecules and Discovery of Innovative Drugs, College of Pharmacy, Jinan University, Guangzhou 511443, Guangdong, China.
Email: huangzhengw@jnu.edu.cn
ORCID: https://orcid.org/0000-0003-2351-7347
Explor Drug Sci. 2025;3:1008134 DOI: https://doi.org/10.37349/eds.2025.1008134
Received: August 13, 2025 Accepted: October 12, 2025 Published: November 20, 2025
Academic Editor: Rangasamy Jayakumar, Amrita Vishwa Vidyapeetham University, India; Fernando Albericio, University of KwaZulu-Natal, South Africa, Universidad de Barcelona, Spain
Buarque et al. (Explor Drug Sci. 2025;3:1008107. DOI: 10.37349/eds.2025.1008107) reported the synthesis of 13 biaryl hydroxy-1,2,3-triazoles and 11 fluorene-1,2,3-triazole hybrids via optimized Suzuki and telescopic one-pot reactions. Cytotoxicity evaluations against colorectal cancer (HCT-116), astrocytoma (SNB-19), triple-negative breast cancer (MDA-MB-231), and acute myeloid leukemia, FLT3-ITD mutant (MOLM-13) cell lines revealed promising antitumor activity. 1-(2-bromophenyl)-4-(9H-filoren-9-yl)-1H-1,2,3-triazole (LSO258) and 1-(4-bromophenyl)-4-(2-fluoro-9H-fluron-9-yl)-1H-1,2,3-triazole (LSO272), both being fluorene-1,2,3-triazole hybrids with bromine substituents, could selectively inhibit the activity of MOLM-13 cells, while the biaryl hydroxy-1,2,3-triazoles compounds exhibited broader antitumor activity. It is worth noting that an inevitable phenomenon is observed: The above compounds have significant aromatic structural characteristics, and their large aromatic systems lead to increased molecular hydrophobicity, resulting in poor water solubility. This critical druggability limitation will directly restrict the development of formulations. To tackle this issue, this paper proposes micelles as the optimal solution. As a carrier structure formed by the self-assembly of amphiphilic surfactants, micelles possess a unique “hydrophobic core-hydrophilic shell” configuration. Their hydrophobic core layer can efficiently encapsulate triazole compounds containing aromatic structures. Compared to other nanomedicine formulations such as solid dispersions and nanoencapsulation technology, micelles demonstrate significant advantages in terms of stability, process simplicity, and biocompatibility.
We are delighted to read the impressive article contributed by Buarque et al. [1]. The study mainly focused on synthesizing novel biaryl hydroxy-triazole and fluorene-triazole hybrids and evaluating their antitumor activity to identify potential cancer therapy leads. There were 13 biaryl hydroxy-1,2,3-triazoles and 11 fluorene-1,2,3-triazole hybrids synthesized via optimized Suzuki and telescopic one-pot reactions, with yields of 16–97%. Cytotoxicity tests against colorectal cancer (HCT-116), astrocytoma (SNB-19), triple-negative breast cancer (MDA-MB-231), and acute myeloid leukemia, FLT3-ITD mutant (MOLM-13) cell lines showed 1-(2-bromophenyl)-4-(9H-filoren-9-yl)-1H-1,2,3-triazole (LSO258) and 1-(4-bromophenyl)-4-(2-fluoro-9H-fluron-9-yl)-1H-1,2,3-triazole (LSO272) selectively inhibited FLT3-ITD mutant leukemia cell line MOLM-13, with half-maximal inhibitory concentration (IC50) of 25.5 μM and 12.5 μM, respectively, with a selectivity index ≥ 2. In contrast, [1,1'-biphenyl]-2-yl(1-(2,5-dibromophenyl)-1H-1,2,3-triazol-4-yl) methanol (LSO278) exhibited broad-spectrum activity, with IC50 values of 18.7–34.3 μM against three of the tested cell lines. It is believed that the findings can provide valuable insights into the design of antitumor agents by highlighting the critical role of bromine substitution and rigid fluorene scaffolds in modulating selective cytotoxicity, thereby offering promising lead structures for the development of targeted therapies against FLT3-mutant leukemia and other cancers. We sincerely appreciated the authors’ contribution.
Although the synthesized compounds may serve as candidate anticancer drugs, there remain some unnegligible druggability issues. Regarding the molecular skeleton of the new entities (Figure 1), they possess large aromatic (benzene) structures, which are highly hydrophobic. As such, the water solubility of them is predicted to be very low, compromising the degree of druggability. A piece of indirect evidence comes from the original study, where the designed compounds should be dissolved in dimethyl sulfoxide (DMSO) to be administered to the cancer cell lines.
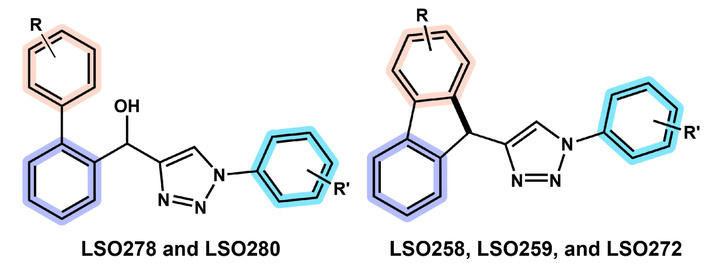
Backbone of the biaryl hydroxy-triazole and fluorene-triazole hybrids. Adapted from [1]. © The Author(s) 2025. Licensed under a Creative Commons Attribution 4.0 International License.
The widely applied and accepted dosage forms for anticancer drugs are oral formulations (like tablets and capsules) and injections. However, the poor water solubility may probably limit the bioavailability for oral formulations; it is literally infeasible to develop aqueous injections owing to the low solubility. Certainly, it is also improper to establish DMSO-based injections for clinical use, since the toxicity of DMSO will provoke much safety concern [2]. In this scenario, regardless of the potential antitumor activity, the druggability is far from satisfactory. Strategies should be employed to enhance the aqueous solubility of the new entities and render them druggable.
It is well documented that drug delivery systems like solid dispersion, host-guest inclusion complex, nanoencapsulation, and micelles have been established to solubilize the water-insoluble drugs [3] (Figure 2). However, solid dispersion suffers from issues of drug aging and poor stability: The drug may precipitate from the carrier as time passes, leading to decreased drug dissolution and compromised efficacy [4]. Host-guest inclusion complex involves a complex preparation process, requiring precise control over host-guest molar ratios and reaction conditions. Their limited encapsulation efficiency further hinders large-scale production [5]. Nanoencapsulation faces problems of nanoparticle aggregation and agglomeration, which cause increased particle size, affect their in vivo distribution and efficacy, and raise concerns about the safety of nanoparticles [6]. For the highly hydrophobic and poorly water-soluble potential anticancer compounds, such as the biaryl hydroxy-triazoles and fluorene-triazoles synthesized by Buarque et al. [1], the micellar encapsulation strategy exhibits significant advantages. Micelles are colloidal aggregates self-assembled from amphiphilic molecules (e.g., surfactants). By definition, such amphiphilic molecules consist of two distinct segments: polar/hydrophilic moieties (e.g., the polyoxyethylene chains) and nonpolar/hydrophobic moieties (e.g., the alkyl chains). In aqueous solutions, the formation of micelles is strictly dependent on the critical micellar concentration (CMC). Surfactant concentrations above the CMC trigger spontaneous self-assembly of amphiphilic molecules into micellar aggregates with a typical “hydrophobic core-hydrophilic shell” structure: Hydrophobic groups aggregate inward to form the core, while hydrophilic groups extend outward to form the shell, thereby forming a thermodynamically stable dispersion system in aqueous solutions [7]. This stable structure is an essential property for their role in solubilizing hydrophobic drugs (e.g., biaryl hydroxy-triazole and fluorene-triazole hybrids).
Compared with solid dispersions, host-guest inclusion complexes, and nanoencapsulation, micelles stabilize drug encapsulation through hydrophobic interactions, reducing the risk of drug precipitation and improving formulation stability. Relevant studies have confirmed that hydrophobic drugs encapsulated in micelles maintain high solubility and dispersibility during long-term storage [8], with significantly better stability than solid dispersions. In terms of preparation processes, micelle synthesis is characterized by simplicity and controllability. It merely requires mixing surfactants with drugs in a suitable solvent, followed by self-assembly via simple physical methods such as stirring or sonication [8]. This process is simple, controllable, and suitable for industrial scaling-up. Additionally, the particle size of micelles can be precisely controlled by adjusting surfactant type and concentration. Their amphiphilic structures can minimize nonspecific in vivo interaction, ensuring good biocompatibility and lowering in vivo toxicity [9]. Consequently, micellar nanocarriers demonstrate significant advantages in terms of stability, process simplicity, and biocompatibility. Furthermore, they exhibit strong application potential for encapsulating and delivering compounds synthesized by Buarque et al. [1].
Of note, surfactants approved by the Food and Drug Administration (FDA) for use in injectable formulations include polysorbate 80, poloxamer 188, etc. [10], with relevant products such as paclitaxel injections using polysorbate 80 as a solubilizer. The hydrophilic segments of polysorbate 80 can enhance the water solubility of the micellar shell, while its hydrophobic segments tightly bind to the aromatic structures of biaryl hydroxy-triazoles and fluorene-triazoles through hydrophobic interactions, significantly improving drug solubility. Meanwhile, its good biocompatibility and low toxicity can reduce adverse reactions after administration, and the stable micellar structure can effectively prevent drug aggregation and precipitation, further ensuring the efficacy of the formulation. Nevertheless, micelles formed by small-molecule surfactants like polysorbate 80 have inherent limitations: their relatively high CMC may cause dissociation upon in vivo dilution, and their small hydrophobic core leads to low drug-loading capacity, restricting high-dose delivery. For LSO-analogs, however, these limitations are manageable—given the compounds’ moderate effective doses and the primary challenge being poor solubility rather than high-dose delivery needs.
For solubilizing and delivering LSO-analogs, micelles still stand as the optimal approach, among which polysorbate 80 may be preferably selected as the surfactant, to solubilize and deliver LSO-analogs. But this strategy should be combined with molecular structure optimization to improve intrinsic activity (e.g., reducing IC50). Additionally, further exploration of formulation bioavailability and cytochrome P450 (CYP450) enzyme/transporter interactions—alongside pharmacokinetic properties, long-term stability, and in vivo distribution is required to confirm their safety and effectiveness, laying a solid foundation for clinical applications.
In conclusion, we have proposed an improvement strategy to address the poor water solubility of triazole hybrid compounds developed by the Buarque’s group, which is a limitation in their druggability. Specifically, amphiphilic surfactants can spontaneously assemble through physical interactions to form nanomicelles with a “hydrophobic core-hydrophilic shell” structure. The hydrophobic core layer within these micelles can efficiently encapsulate hydrophobic triazole compounds, thereby significantly enhancing the water solubility of the drug. This micelle-based strategy demonstrates superior advantages in terms of formulation stability, ease of preparation, and biocompatibility. Polysorbate 80, which has been approved by the FDA, possesses validated biosafety and serves as an optimal material for preparing such micelles. However, to advance this strategy toward clinical application, further systematic evaluation of the pharmacokinetic characteristics (such as absorption, distribution, metabolism, and excretion processes) and long-term stability of the formulations is still required to comprehensively validate their clinical applicability and reliability.
CMC: critical micellar concentration
DMSO: dimethyl sulfoxide
FDA: Food and Drug Administration
IC50: half-maximal inhibitory concentration
ZL: Conceptualization, Investigation, Writing—original draft. MZ: Investigation, Writing—original draft, Writing—review & editing. ZH: Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
We appreciated the support from the College Students’ Innovation and Entrepreneurship Competition of Jinan University (Project Number: [S202410559120]). The sponsor provided financial funding for this study, offered guidance on study design, supported literature acquisition and key data verification related to drug delivery systems. The sponsor had no role in the final decision to submit the manuscript for publication.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1078
Download: 28
Times Cited: 0
