Affiliation:
Faculty of Life and Medical Sciences, Business Management School (BMS) Campus, Colombo 00600, Sri Lanka
Email: mathi@bms.ac.lk
ORCID: https://orcid.org/0000-0003-2590-6637
Affiliation:
Faculty of Life and Medical Sciences, Business Management School (BMS) Campus, Colombo 00600, Sri Lanka
Affiliation:
Faculty of Life and Medical Sciences, Business Management School (BMS) Campus, Colombo 00600, Sri Lanka
ORCID: https://orcid.org/0009-0000-6425-7225
Affiliation:
Faculty of Life and Medical Sciences, Business Management School (BMS) Campus, Colombo 00600, Sri Lanka
Explor Drug Sci. 2025;3:1008123 DOI: https://doi.org/10.37349/eds.2025.1008123
Received: May 22, 2025 Accepted: July 09, 2025 Published: August 05, 2025
Academic Editor: Alessandra Tolomelli, University of Bologna, Italy
Aim: The science of manipulating matter at almost atomic scales to create new structures and devices that function at nanoscale dimensions is known as nanotechnology, which is essential to many sciences, such as medicine and environment. This field of study has been reported to investigate better alternatives for the advancement of medicine; one such alternative is the use of plants, which contain substantial amounts of essential phytochemicals. This study aims to utilize such a plant species, Canna indica (C. indica) leaves, known as traditional medicinal plants or commonly grown plants, to synthesize silver nanoparticles (AgNPs) and evaluate their potential in green medicine.
Methods: The synthesis was carried out using five varieties of leaf water extracts: Pink red, Yellow, Pink, Yellow red, and Red, under different conditions, to which scanning electron microscopy was performed. The antioxidant capacity was evaluated by total flavonoid content, total phenolic content, total antioxidant capacity, and 2,2-diphenyl-1-picrylhydrazyl radical scavenging assay. The antibacterial activity of AgNPs and water extracts was evaluated against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli). Finally, the cytotoxicity of AgNP is evaluated using the brine shrimp lethality assay.
Results: The optimum condition for AgNP synthesis was determined to be room temperature, and Pink_AgNPs were observed as spherical with a size of 27–48 nm in scanning electron microscopy. The antioxidant assays concluded that AgNPs show significantly higher antioxidant capacity and exhibit higher scavenging activity. This study’s findings showed the efficiency of AgNPs against both strains, and higher efficiency against S. aureus. It was observed that with 240 ppm of AgNPs, 100% viability is obtained.
Conclusions: These novel findings emphasize the significance of C. indica AgNPs, their promise in the medical field, and their application in manufacturing green medicine for environmentally friendly healthcare.
Natural or synthetic polymers can be combined to form spherical, polymeric particles known as nanoparticles, which range from 1 to 100 nm in size [1]. In a variety of applications, significant advancements are being achieved, making the subject of nanotechnology one of the fastest growing fields of scientific study and development. Silver nanoparticles (AgNPs) are a type of metallic colloidal nanoparticles [2]. Due to their unique physical and chemical characteristics, AgNPs are being used more in a variety of industries, including healthcare, food consumer, and industrial sectors. In addition, they are currently in use in a variety of medical device coatings, cosmetics, optical sensors, pharmaceuticals, antibacterial agents, and household products. Also, they have developed the tumor-killing capabilities of anticancer drugs [3].
AgNPs have unique physical and chemical properties and are tiny in size, which makes them suitable for a wide range of environmental applications [4]. AgNPs are employed in several biological and therapeutic applications, like anti-cancer, antibacterial, cosmetics, bio sensing, anti-inflammatory, textiles, and many more [5]. Synthesis of nanoparticles is divided into two methods, which are the bottom-up approach and top-down approach [6]. The top-down method starts with breaking bulk material into small fragments, which eventually develops into nanoparticles. The bottom-up method is where different types of nanoparticles are created by gathering of atoms and molecules [7]. The top-down method consists of physical methods such as mechanical milling and sputtering. The bottom-up method consists of biological and chemical methods. Chemical methods include chemical reduction and supercritical fluid synthesis. The biological method is the most prominently used method of synthesis, which uses microorganisms or plant materials [8]. This method is a sustainable and environmentally friendly process of producing AgNPs without the use of harmful solvents or hazardous substances, which is reported to be non-toxic. However, using microbes for synthesis of AgNPs has many disadvantages, such as easy contamination, need for specific growth conditions, for example warm and moist environment, neutral or slightly acidic pH [9]. Furthermore, there may be environmental issues with the disposal of microbial by-products during synthesis process.
The physical method often produces pure nanoparticles with exact control over size and shape, but due to the extensive energy and costly equipment, this method is not frequently selected [10]. Chemical methods also pose disadvantages, such as using hazardous chemicals, which may be dangerous to both humans and the environment. The chemical waste from synthesis processes must also be disposed of carefully, if not, it can pollute the environment [11, 12]. Due to these disadvantages, the widely used technique is biological synthesis using plant materials. In recent years, the section on plant extracts is due to the understanding of phytochemicals’ significance in the synthesis of AgNPs. They have the ability to reduce and stabilize the AgNP, which gives it the acquired antioxidant and antibacterial properties. The use of plant materials has another advantage: it can reduce the wastage of important components in the widely available plant species. Therefore, the biological approach can address the problems of waste reduction while simultaneously developing efficient nanoparticles [13].
Canna indica (C. indica), which belongs to the family Cannaceae is one such plant species. C. indica is native to tropical regions of America, known as an ornamental plant, and later established in countries such as India and Sri Lanka. Although this plant is recognized as an ornamental plant grown in central highlands of Sri Lanka, this species is not extensively researched [14, 15]. C. indica leaves have long been used to treat wound healing, malaria, and diarrhea [16], and flowers may also be used to cure several eye conditions. However, the roots can cure gonorrhea, and a powdered blend of leaves and seeds can cure dermatosis [17]. This use in various medical purposes is due to its phytochemical profile, which includes important phytochemicals such as carbohydrates, steroids, saponins, alkaloids, and proteins [18].
Numerous health advantages, including increased antioxidant capacity and antibacterial activity, have been found with the manufacture of AgNPs utilizing plant resources. Given these characteristics, AgNPs can be added to medications and exhibit increased efficacy.
The antioxidant capacity of AgNP is governed by the concept of free radical scavenging and neutralization of reactive oxygen species (ROS). Humans are exposed to multiple routes of free radical production, including exposure to ionizing radiation, photosensitizer drugs, and redox cycling of xenobiotic [19, 20]. These radicals are unstable and harmful molecules since they have the ability to take electrons from normal neighboring cells of the human body. This ultimately leads to a state known as oxidative stress: an imbalance between antioxidants and oxidants that interferes with regular cellular functions. To combat this rising health issue of oxidative stress, antioxidants are being used. Antioxidants are molecules with the capacity to donate their electrons to free radicals using single electron transfer or hydrogen transfer mechanisms [21]. For a molecule to act as an antioxidant, it should show characteristics of low concentration, neutralize target molecules (oxygen or nitrogen free radicals), and produce less toxic products [22]. Antioxidants help prevent chronic illnesses that are caused by free radicals, such as cancer, heart disease, and malignant modifications [23]. Vegetables and fruits like citrus fruits and leafy greens are reservoirs for natural antioxidants; however, research has shown better antioxidant capacity in AgNPs produced through plants [24, 25].
Along with oxidative stress-induced disease, the emergence of antibiotic-resistant pathogenic bacteria is a health concern. The widespread administration of antibiotics for infection and improper disposal of these drugs are the main causes of resistance. In addition, resistance to novel antibiotics has led researchers to investigate a different approach to fighting microbes [26]. The discovery of nanotechnology and its vast application has helped develop nanoparticles as viable alternatives against antibiotic-resistant bacteria [27]. Silver has been widely used in the medical field, and its incorporation with nanoparticles has been shown to enhance antibacterial activity. The mechanism of action is related to its morphology, particularly the high surface-to-volume ratio, and its interaction with microorganism cell structure [28]. Studies have shown that chemically synthesized AgNPs have a higher efficiency than AgNPs synthesized using the green approach. However, green AgNPs are more beneficial because they can bind to the bacterial deoxyribonucleic acid (DNA) and directly influence bacterial replication. For these reasons, AgNPs are also being used as alternatives in water purification and medical applications [29].
The increased capability of nanoparticles has led to a significant demand for their products. Long-term use of these medications or products, however, has been linked to health problems. As a result, before incorporating these nanoparticles into medications, their ambient exposure and safety levels should be considered [30]. A cytotoxicity assay should be carried out to evaluate the toxicity of AgNPs. The preliminary bioassay for toxicity testing uses Artemia salina (A. salina), also known as brine shrimp, because of its easy adaptability to harsh conditions, rapid hatching, and cost-effectiveness. The other assays used in cytotoxicity assays are the trypan blue exclusion assay, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, and the ATP-based luminescence cell viability assay, which has its drawbacks [31].
The aim of this study is to synthesize AgNPs using five different varieties of C. indica leaves and determine their size and shape using scanning electron microscopy (SEM) analysis. The antioxidant capacity of these AgNPs will be determined using assays such as total flavonoid content (TFC), total phenolic content (TPC), total antioxidant capacity (TAC), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. Antibacterial activity will be tested using Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) using a well diffusion method. A. salina will be used to test toxicity of AgNPs. Thereby, these results can be used to highlight the potential of C. indica leaf AgNPs in the medical industry.
Five different types of C. indica leaves (Figure 1) were collected from different home gardens in Colombo, Sri Lanka, in September 2024.

Five varieties of C. indica leaves were selected, named based on the flower color: (a) Pink red, (b) Yellow, (c) Pink, (d) Yellow red, and (e) Red
The C. indica leaves were shade dried for a week and crushed using a mortar and pestle. Each sample was weighed using an analytical balance (model-PR423, specification: capacity 210 g, readability 0.0001 g, OHAUS Corporation, USA) for 2 g and 50 mL of distilled water (DW) was added. These solutions were incubated at 50°C for 30 min in the hot air oven [model-DHG-9053A, specification: temperature range: room temperature (RT) + 10°C to 200°C, volume: 30 L, Meditry Instrument Co., Ltd., China]. After incubation, samples were allowed to cool and filtered using Whatman filter paper (Cat No 1001 110) to obtain the water extracts (WEs) [32]. It was stored at 4°C for future use.
Phytochemical tests were performed for the WE (Table 1).
| Phytochemical | Methodology |
|---|---|
| Tannins | Few drops of 5% ferric chloride (CAS-7758-94-3) were added to 0.5 mL of WE. The color change to greenish black was observed. |
| Saponins | Into 0.5 mL of WE, 2 mL of DW was added, shaken vigorously for a few minutes. The formation of foam was observed. |
| Amino acid | Into a test containing 0.5 mL of WE, 0.5 mL of ninhydrin solution (CAS-485-47-2) was added. The mixture was heated in the water bath for 20 min, and purple color change was observed. |
| Alkaloid | Into 0.5 mL of WE, 3 drops of Mayer’s reagent were added (CAS-7789-99-3). The formation of yellowish precipitate was observed. |
| Reducing sugar | Into 0.5 mL of WEs, 1 mL of Benedict’s reagent (CAS-63126-89-6) was added and boiled in a water bath. The color change from green to red was observed. |
| Anthocyanin | Into 0.5 mL WEs, 0.5 mL of concentrated hydrochloric acid (CAS-7647-01-0) was added. The change of color to red/purple was observed. |
| Phenols | Into 0.5 mL of WEs, few drops of 10% ferric chloride (CAS-7758-94-3) were added. The color change to a green-blue or violet was observed. |
| Phlobatannins | Into 0.5 mL of WEs, 0.333 µL of hydrochloric acid (CAS-7758-94-3) was added. The formation of white precipitate was observed. |
| Starch | Into 0.5 mL of WEs, few drops of iodine reagent (CAS-7553-56-2) were added. The color change to blue-black was observed. |
| Flavonoids | Into 0.5 mL of WEs,125 µL of aluminum chloride (CAS-7446-70-0) was added. The color change to yellow was observed. |
DW: distilled water; WE: water extract
One millilitre of each WE was added with 9 mL of 3 mM silver nitrate (CAS-7761-88-8). Each solution was incubated at different temperatures and time intervals: 90°C and 60°C for 60 min, 45 min, 30 min, and 15 min, and RT for 24 hours. The absorbance was then measured with the ultraviolet (UV)-visible spectrophotometer (model-6305, specification: wavelength range: 198–1,000 nm, bandwidth: 8 nm, Bibby Scientific Ltd) from 320 nm to 520 nm, using the DW as a blank [40]. The synthesized AgNPs were stored at 4°C for future use.
Pink_AgNP sample was centrifuged at 13,000 rpm using a High-speed mini centrifuge [model-Microspin 12, specification: max speed: 6,000 rpm, capacity: 12 × 1.5/2.0 mL tubes, Grant-Instruments (Cambridge) Ltd]. This step was repeated several times while discarding the supernatant until a prominent pellet was obtained. The pellet was left dry at 40°C for 24 hours. The dried pellet was sent to the Sri Lanka Institute of Nanotechnology, Sri Lanka, for analysis using Field-Emission SEM (model-SU6600, specification: high-resolution imaging, secondary and backscattered electron detectors, Hitachi High-Technologies Corporation, Japan).
The WEs and optimized AgNPs were diluted 15 times and used for the following assays.
One millilitre samples was added to respective test tubes. Then, 0.2 mL of 10% aluminum chloride (CAS-7446-70-0) and 0.2 mL 1 M potassium acetate (CAS-127-08-2) were added to each test tubes and were incubated in dark for 30 min at RT. The absorbance was taken at 420 nm in triplicate [36] using DW as the blank. The TFC was calculated using quercetin (CAS-117-39-5) standard curve and expressed in µg/quercetin/100 g.
One millilitre samples was added with 2.5 mL of 10% Folin-Ciocalteu reagent (CAS-12111-13-6) and 2 mL of 2% sodium carbonate (CAS-497-19-8) in test tubes. It was incubated in dark for 60 min at RT. The absorbance was taken at 765 nm in triplicate [36] and using DW as the blank. The TPC was calculated using gallic acid (CAS-149-91-7) standard curve and expressed in g/gallic acid/100 g.
Phosphomolybdenum reagent of 28 mM of sodium phosphate (CAS-7558-80-7), 4 mM of ammonium molybdate (CAS-12054-85-82) and 0.6 M sulfuric acid (CAS-7664-93-9) was prepared in 1:1:1 ratio. In each test tube, 100 μL of sample was added, and 1 mL of phosphomolybdenum reagent was added and incubated at 95°C for 90 min. The absorbance was measured at 685 nm in triplicate [41] and using DW as the blank. The TAC was calculated using ascorbic acid (CAS-50-81-7) standard curve and expressed in g/ascorbic acid/100 g.
The absorbance of 3 mL of the 0.1 M DPPH (CAS-1898-66-4) solution was measured using methanol (CAS-67-56-1) as the blank at 517 nm. Two millilitres of 0.1 mM of DPPH solution were added to 1 mL of each sample concentration, ranging from 1, 0.8, 0.6, 0.4, 0.2 mg/L. The absorbance was taken at 0th min using methanol as the blank at 517 nm. Scavenging activity was calculated using percentage activity equation [42] and the values of inhibition concentration (IC50) were obtained using the linear regression equation.
Mueller Hinton agar was prepared and sterilized using the autoclave (model-YXQ-LS-50SII, specification: capacity 50 L, temperature 121°C, pressure 15 psi, time 15 min, Meditry Instrument Co., Ltd., China). The agar was left to cool, and it was poured into a petri dish and allowed to solidify. E. coli (ATCC-25922) and S. aureus (ATCC-25923) were streaked on respective plates, and wells were made for the S1, S2, and negative sample (Figure 2). Gentamycin (30 mcg) (Himedia) was used as the positive control, and 0.9% saline was used as the negative control. All plates were incubated in the carbon dioxide incubator (Thermo SCIENTIFIC BB 15) at 37°C for 24 hours, and the zone of inhibition (ZOI) was measured using a ruler.
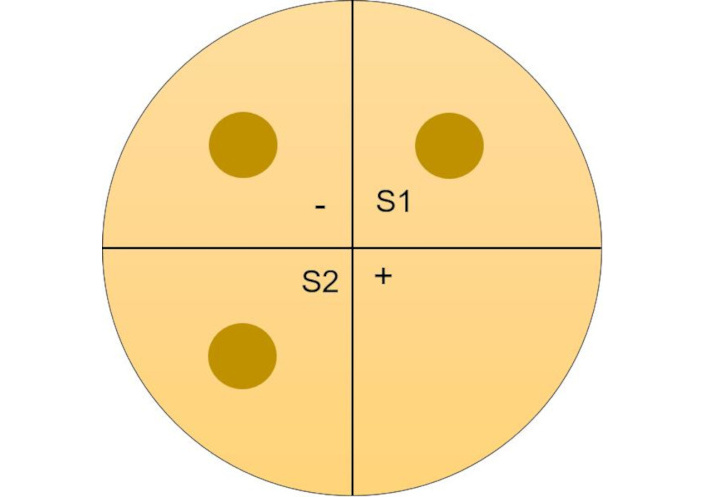
Visual representation of Mueller Hinton agar plate. (-) Negative control; S1 and S2: sample duplicates; (+) positive control
Decapsulated Brine shrimp eggs (American Eagle) were left to hatch overnight under yellow lamp and aeration. Into 96 well plate (Biologix Europe), AgNP at 240 ppm was added in triplicate and two hatchlings were introduced to each well. Filtered sea water with a pair of hatchlings was used as control and the apparatus was kept for 24 hours [43]. Percentage (%) viability was calculated using Equation 1.
One-way ANOVA test was performed using MS Excel 2013, and Pearson correlation was performed using SPSS software [version: 28.0.0.0 (190)] for results of antioxidant assays.
Phytochemical tests conducted on WEs of C. indica showed the presence of starch, amino acids, phenols, tannins, and reducing sugars (Table 2).
Phytochemical reaction results
| Phytochemical tests | Pink red | Pink | Red | Yellow | Yellow red | Color change |
|---|---|---|---|---|---|---|
| Starch | √ | √ | √ | √ | √ |  |
| Amino acid | √ | √ | √ | √ | √ | 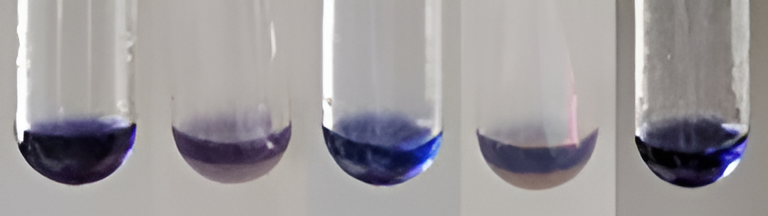 |
| Phenols | √ | √ | √ | √ | √ |  |
| Alkaloid | × | × | × | × | × |  |
| Tannins | √ | √ | √ | √ | √ |  |
| Saponins | × | × | × | × | × |  |
| Phlobatannins | × | × | × | × | × |  |
| Reducing sugars | √ | √ | √ | √ | √ |  |
| Flavonoids | × | √ | √ | × | √ |  |
| Anthocyanin | × | × | × | × | × |  |
√ indicates as present; × indicates as not present
All samples of C. indica leaves showed synthesis at all temperatures and time durations except for Pink red and Yellow red at 60°C for 15 min (Table 3). However, the optimized AgNP conditions were selected as RT for 24 hours due to the presence of significant peaks at 440 nm for all samples (Figure 3) and it showed a prominent color change from colorless to brown after completion of synthesis compared to other conditions (Figure 4).
Optimization of C. indica leaf silver nanoparticle at 60°C, 90°C, and RT
| Sample ID | 60°C, 15 min | 60°C, 30 min | 60°C, 45 min | 60°C, 60 min | 90°C, 15 min | 90°C, 30 min | 90°C, 45 min | 90°C, 60 min | RT, 24 hours |
|---|---|---|---|---|---|---|---|---|---|
| Pink | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Red | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Pink red | × | √ | √ | √ | √ | √ | √ | √ | √ |
| Yellow red | × | √ | √ | √ | √ | √ | √ | √ | √ |
| Yellow | √ | √ | √ | √ | √ | √ | √ | √ | √ |
√ indicates as synthesized; × indicates as not synthesized. RT: room temperature
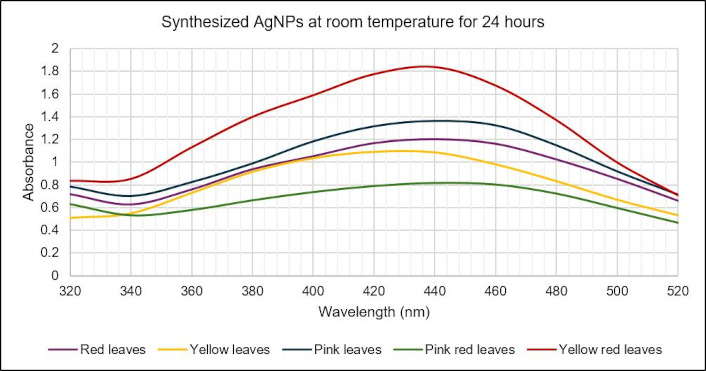
UV-visible spectrum of C. indica leaf AgNPs synthesized at an optimized condition of room temperature for 24 hours. AgNPs: silver nanoparticles; UV: ultraviolet
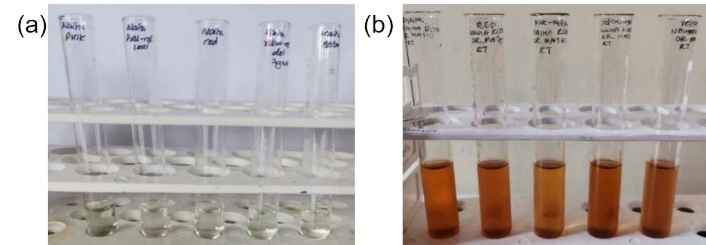
A distinctive color shift from pale yellow before incubation (a) to dark yellow after incubation (b), confirming the formation of silver nanoparticles
SEM analysis shows the presence of spherical Pink_AgNPs with a size range of 27 nm to 48 nm (Figure 5).

SEM image of Pink_AgNP at 15 kV 10.5 mm × 90.0 k/scale-500 nm. Magnification: 90.0 k. SEM: scanning electron microscopy
TFC levels are higher in AgNPs compared to WE (Figure 6a and 6b) and the P value, 1.34404E–05 (< 0.05) shows a significant difference between TFC values of AgNPs and WEs. TPC levels are higher in AgNPs compared to WE (Figure 6c) and the P value, 1.32595E–06 (< 0.05) shows a significant difference between TPC values of AgNPs and WEs. TAC levels are higher in AgNPs compared to WE (Figure 6d and 6e) and the P value, 5.45511E–16 (< 0.05) shows a significant difference between TAC values of AgNPs and WEs.
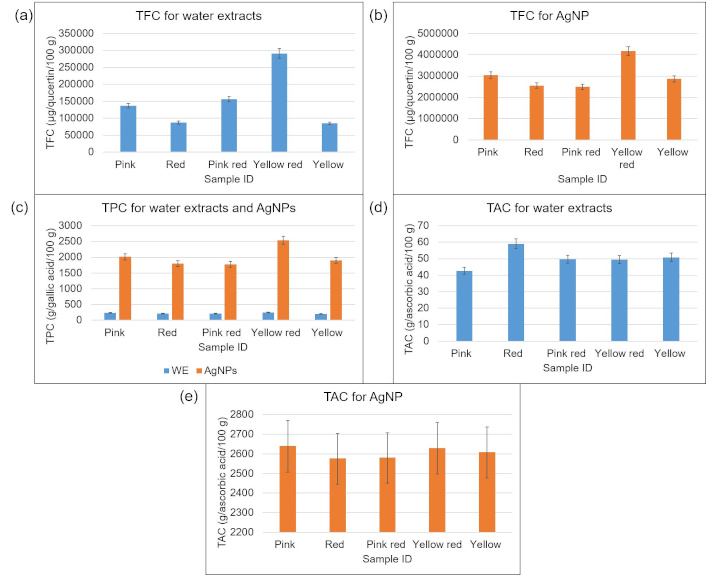
Antioxidant assay results. (a) TFC for C. indica leaf WEs, (b) TFC for C. indica leaf AgNPs, (c) TPC of C. indica leaf WEs and their respective AgNPs, (d) total antioxidant content for C. indica leaf WEs, and (e) total antioxidant content for C. indica leaf AgNPs. AgNPs: silver nanoparticles; TAC: total antioxidant capacity; TFC: total flavonoid content; TPC: total phenolic content; WE: water extract
DPPH assay shows scavenging activity (%) is higher in AgNPs than in WEs (Figure 7), and the calculated IC50 values show higher values for WE compared to AgNPs, except for Pink_AgNP and Red_AgNP (Table 4).
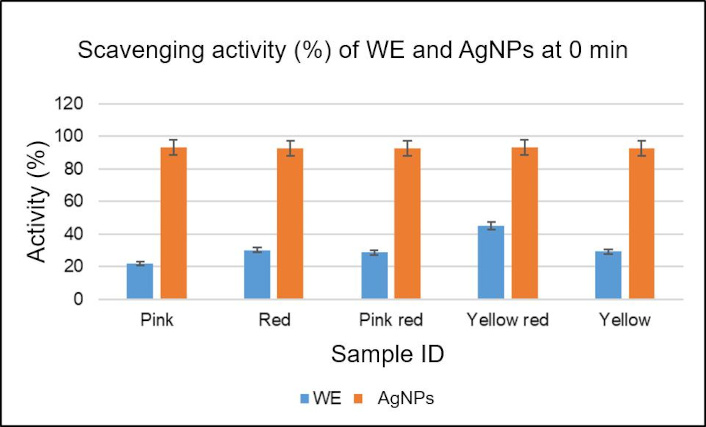
Graph representing the percentage of DPPH scavenging activity of C. indica leaf AgNPs and WEs. AgNPs: silver nanoparticles; DPPH: 2,2-diphenyl-1-picrylhydrazyl; WE: water extract
IC50 values in DPPH assay for AgNPs and WEs
| Sample ID | WE (mg/L) | AgNP (mg/L) |
|---|---|---|
| Pink | 1.71 | 5.35 |
| Red | 0.52 | 1.94 |
| Pink red | 31.55 | 0.56 |
| Yellow red | 0.91 | 0.53 |
| Yellow | 0.49 | 0.40 |
AgNP: silver nanoparticle; DPPH: 2,2-diphenyl-1-picrylhydrazyl; WEs: water extracts; IC50: inhibition concentration
All AgNPs have shown antibacterial activity against E. coli and S. aureus (Tables 5 and 6), with no activity present in the WEs. The activity is higher against S. aureus.
ZOI against E.coli for AgNPs and their respective WEs
| Sample ID | Average ZOI/cm | ZOI of positive control/cm |
|---|---|---|
| Red_AgNP | 1.2 | 2.6 |
| Pink red_AgNP | 1.2 | 2.6 |
| Pink_AgNP | 1.05 | 2.6 |
| Yellow_AgNP | 1.0 | 2.5 |
| Yellow red_AgNP | 1.2 | 2.8 |
| Red WE | - | 2.6 |
| Pink red WE | - | 2.5 |
| Pink WE | - | 2.5 |
| Yellow WE | - | 2.6 |
| Yellow red WE | - | 2.6 |
-: no data. AgNP: silver nanoparticle; WEs: water extracts; ZOI: zone of inhibition
ZOI against S. aureus for AgNPs and their respective WEs
| Sample ID | Average ZOI/cm | ZOI of positive control/cm |
|---|---|---|
| Red_AgNP | 1.6 | 4.2 |
| Pink red_AgNP | 1.65 | 3.8 |
| Pink_AgNP | 1.25 | 3.5 |
| Yellow_AgNP | 1.45 | 3.5 |
| Yellow red_AgNP | 1.85 | 3.6 |
| Red WE | - | 3.5 |
| Pink red WE | - | 4.0 |
| Pink WE | - | 4.1 |
| Yellow WE | - | 3.7 |
| Yellow red WE | - | 3.0 |
-: no data. AgNPs: silver nanoparticles; WEs: water extracts; ZOI: zone of inhibition
The microscopic images (Figure 8) and percentage viability data (Table 7) show 100% viability of Brine shrimps at exposure to 240 ppm of AgNP samples.
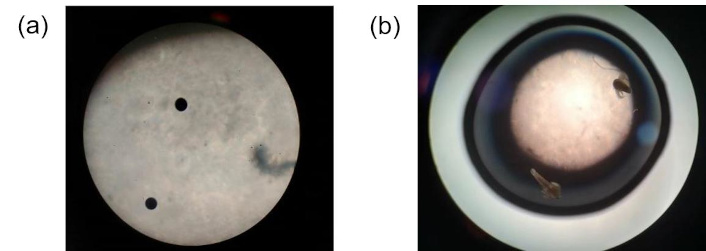
Microscopic images at different stages of the assay. (a) Brine shrimp eggs and (b) Brine shrimp hatchlings after exposure to 240 ppm of C. indica leaf silver nanoparticles
Viability percentage in cytotoxicity studies at 240 ppm AgNP exposure
| Sample ID | Well 1 | Well 2 | Well 3 | Viability |
|---|---|---|---|---|
| Pink_AgNP | 2/2 | 2/2 | 2/2 | 100% |
| Red_AgNP | 2/2 | 2/2 | 2/2 | 100% |
| Pink red_AgNP | 2/2 | 2/2 | 2/2 | 100% |
| Yellow red_AgNP | 2/2 | 2/2 | 2/2 | 100% |
| Yellow_AgNP | 2/2 | 2/2 | 2/2 | 100% |
| Control | 2/2 | 2/2 | 2/2 | 100% |
AgNP: silver nanoparticle
Nanoparticles have become important factors for modern medicine in recent years [44]. Over the past few decades, the application of nanoparticles in the field of analysis has grown drastically. Because of their distinct properties, nanoparticles may be applied to a variety of analytical tasks. This study was conducted using five varieties of C. indica leaves.
Phytochemical tests were performed for the WE (Table 1), which showed the presence of various phytochemicals (Table 2). These results are verified by previous research conducted on the phytochemical profile of C. indica [18]. The presence of phytochemicals mainly contributes to reduction of silver and nucleation process in AgNP formation [45]. The presence and absence of tested phytochemicals can change depending on the extraction solvent. It has been reported that ethanolic extracts showed the presence of a broad spectrum of phytochemicals, whereas extracts made from methanol, chloroform, and DW varied in this regard [46]. This occurs due to the different nature of solvents used, such as polarity and chemical properties, which allows selective phytochemicals to be dissolved [47]. AgNP synthesis involves several steps such as harvesting the plant species and shade drying it, preparation of the extract using the selected solvent, followed by a suitable incubation, and finally addition of silver precursor (silver nitrate) to the filtered extracts to form the AgNPs [48, 49]. The effects of ethanol, DW, and dimethyl sulfoxide as solvents in the synthesis process have all been studied. According to reports, these solvents are appropriate for synthesis. However, DW is less toxic and more eco-friendly. Compared to other solvents, DW has demonstrated greater success in the synthesis [50]. During synthesis, Ag+ ions are converted into Ag0 with the help of capping agents [51], phytochemicals present in the WE, followed by subsequent nucleation process that accumulates the Ag0 atoms, forming the final AgNP structure [52, 53].
In this study, confirmation of AgNP synthesis was done by color change observation and peak absorbance data [54]. Based on the peak absorbance values obtained, the optimum condition for C. indica leaf AgNP was selected as RT for 24 hours (Table 3) among all the synthesis conditions used, confirmed by a peak absorbance (Figure 3) and a distinct color change (Figure 4). This is supported by previous research, which showed similar results [55], and research has found that higher temperatures can lead to undesirable side reactions or formation of by-products [56]. The surface plasmon resonance effect is the phenomenon that explains the mechanism of AgNP synthesis. This explains the oscillation of outer free electrons of AgNP at a certain frequency after the interaction with light photons [57], which causes a color change from clear to dark orange/brown [58].
The analysis of size and shape of AgNP is performed by SEM [59]. Pink_AgNP were observed as spherical in shape with sizes 27 nm to 48 nm (Figure 5). These observations are supported by previous research performed on C. indica leaf showing spherical to oval AgNP with a size of 25 nm to 30 nm [32]. The factors that directly influence the size of the synthesized AgNP include the concentration of extract and silver precursor used [60].
In this study, the synthesized AgNPs were further classified based on their band gap energies. This is the energy level that separates a distinct set of electrons from the other group in an atom between valence band and conduction band [61], classifying AgNPs as semiconductors (< 3 eV) and insulators (> 4 eV) [62, 63]. Using the Tauc equation, C. indica leaf AgNPs was classified as semiconductors (Table 8).
Bandgap energies of the optimized C. indica AgNPs
| Sample ID | Band gap energy (eV) |
|---|---|
| Red_AgNP | 2.82 |
| Pink_AgNP | 2.95 |
| Pink red_AgNP | 2.82 |
| Yellow_AgNP | 2.82 |
| Yellow red_AgNP | 2.82 |
AgNPs: silver nanoparticles
The antioxidant capacity of the optimized C. indica leaf AgNPs and WEs was evaluated using TFC, TPC, TAC, and DPPH assay. These experiments are conducted in order to further understand the potential of AgNPs as antioxidants.
The principle of TFC states that C-4 keto group and the C-3 or C-5 hydroxyl groups of flavones and flavanols combine with aluminum chloride to generate acid stable complexes, which are detected at 420 nm [64]. According to the results (Figure 6a and 6b), AgNPs showed significantly higher TFC levels. Among the AgNPs, Yellow red _AgNP was the highest, and Red_AgNP and Pink red_AgNP were the lowest.
The principle of TPC shows that in the presence of phenolic, the Folin-Ciocalteu reagent is reduced and produces a blue molybdenum-tungsten complex, which can be detected at 760 nm [65]. According to this study, TPC level is significantly higher in AgNPs than in WE (Figure 6c). The highest TPC level was recorded in Yellow red_AgNP, and the lowest in Yellow WE.
The principle of TAC states that phosphate-molybdenum (VI) is converted to phosphate-molybdenum (V) in the presence of antioxidants, which gives a greenish blue color detected at 695 nm [66]. Significantly higher TAC levels are observed in AgNPs (Figure 6d and 6e), with the highest for Pink_AgNP and lowest for Pink WE.
However, these results cannot be supported by research, due to the unavailability of supporting research conducted on antioxidant levels of C. indica AgNPs synthesized using water as the solvent. Pearson’s correlation coefficient was performed on the antioxidant results to understand the linear relationship between two variables’ strength and their contribution to antioxidant capacity of the optimized C. indica AgNPs. A strong positive correlation between TFC-TAC, TPC-TAC, and TFC-TPC is observed (Figure 9). This is due to the antioxidants found in the leaves, such as flavonoids, phenols, and other phytochemicals, that enhance the antioxidant activity with resistance to ROS [67].
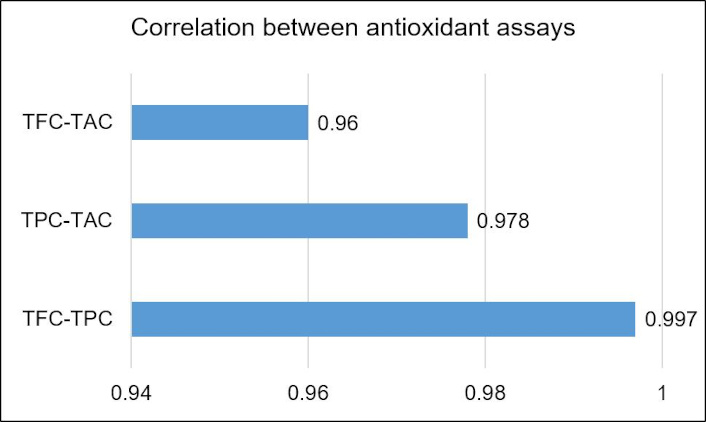
Correlation between antioxidant assay: TFC-TAC, TPC-TAC, and TFC-TPC. TAC: total antioxidant capacity; TFC: total flavonoid content; TPC: total phenolic content
The DPPH method uses free radicals to determine if a chemical can act as a hydrogen donor or scavenger of free radicals. The WE and AgNPs’ capacity to scavenge free radicals can be evaluated using DPPH analysis. When DPPH interacts with an antioxidant, it loses its ability to function as a free radical, and the color changes from violet to yellow [68].
The evaluation of this activity is by the percentage scavenging activity and IC50, which is the concentration of sample needed to quench 50% of DPPH [69]. Studies state that AgNPs show higher potential and lower IC50, proving them as stronger DPPH scavengers than WE [70, 71]. This is due to phytochemicals and silver ions serving as antioxidants, thereby allowing AgNPs to act as singlet oxygen quenchers. Spherical shaped AgNPs are reported to have enhanced scavenging ability caused by bioactive compound absorption from their respective WEs [72–74]. However, AgNPs can have a lower scavenging ability at a certain instance. AgNPs can use the antioxidant capacity of the phytochemicals that help form the AgNPs. This leads to the formation of superoxide radical that ultimately reduces the overall antioxidant capacity of the AgNP [75].
This study showed higher percentage of scavenging capacity in all AgNPs at similar levels (Figure 7). The IC50 values were higher in WEs except for Pink and Red samples (Table 4). These novel results prove that the synthesized C. indica AgNPs have a strong radical scavenging potential.
The comprehensive mechanism of the antibacterial mechanism of nanomaterials against gram-positive and gram-negative bacteria is not completely understood. However, several possible mechanisms have been documented. The primary factor is the interaction of negative bacterial cell membranes and positive nanomaterials. Literature shows that direct contact between bacterial cell membranes and nanomaterials with very sharp edges leads to cell membrane damage and increased permeability, internalization of nanomaterials, and inhibition of respiratory enzymes, leading to oxidative stress by ROS production. Research also documents the preferred binding between nanomaterials and sulfur- or phosphorus-containing molecules, such as membrane proteins or DNA, that leads to their subsequent damage [76–81]. Nanomaterials release their respective ions (for example, silver or zinc ions) into the bacterial cell, that has led inhibition of DNA replication and cellular damages [82]. Morphological changes are reported after the effects of nanomaterials on bacteria, which were viewed using SEM. These changes include decomposed bacterial shape, leaked bacterial cytoplasm and nucleotide, and membrane collapse [83].
To evaluate the action of C. indica leaf AgNP against both gram-negative and gram-positive bacteria, E. coli and S. aureus strains were utilized. This study showed ZOI against both bacterial strains by AgNPs and not by WEs (Tables 5 and 6).
Based on the structure of gram-negative and gram-positive bacteria, the efficiency of AgNPs’ antibacterial activity differs. Gram-negative bacteria consist of a thin peptidoglycan layer and an outer membrane while gram-positive bacteria consists of only a think peptidoglycan layer. This additional barrier in gram-negative bacteria keeps the AgNPs from entering the cell wall and causing damage [84], whereas in gram-positive bacteria, the lack of outer membrane allows substances to flow through with ease, and it is relatively porous [85]. Research shows that AgNPs are more active against S. aureus than E. coli [86]. This observation is also seen in this study of C. indica leaf AgNPs: higher average ZOI of AgNP against S. aureus (Table 6). Additional factors that influence the antibacterial activity of nanomaterials are their size. Smaller nanomaterials have a high surface-to-volume ratio, which gives them higher antibacterial potential [87]. Previous study using Canna edulis leaf AgNP showed similar behavior against the different bacteria strains [88] and another study conducted using Canna lily flower AgNP reported high antibacterial activity against Ralstonia solanacearum strain YY06 compared to the WEs [89]. The findings of this research emphasize the possible potential of C. indica leaf AgNPs in combating the antibiotic resistant microorganisms. However, further evaluation by SEM study on the morphology of tested bacteria after their exposure to C. indica leaf AgNPs should be conducted to completely understand the specific mechanism of action.
This research has proved novel findings of antioxidant capacity and potential antibacterial activity of the synthesized C. indica leaf AgNPs. To evaluate the safety of these AgNPs, brine shrimp lethality assay was conducted. Similar to the action of AgNP against bacterial cells, AgNPs can enter other cells through diffusion, phagocytosis, and endocytosis. Silver ions within the cell can generate ROS, which leads to oxidative stress and can cause protein denaturation, DNA damage, and antioxidant defenses. It can also cause mitochondrial dysfunction, which can lead to the apoptosis of the cell [90, 91]. Due to these actions, there is necessity to understand the safety of the produced AgNPs. The cytotoxicity studies using A. salina rely on the interaction of silver ions with the chitin on the cuticle layer [92]. These ions have the ability to impair biological processes and induce oxidative stress, which can result in cell damage and death [93].
Percentage viability equation was utilized to estimate the viability after exposure. The synthesized C. indica leaf AgNPs showed 100% viability at the tested concentration of 240 ppm (Table 7), and observations under the microscope confirm the viability after 24-hour exposure (Figure 8). This observation concludes that at 240 ppm, C. indica leaf AgNPs are non-toxic. A study of cytotoxicity of C. indica L. Leaf AgNPs synthesized using methanol and Petroleum ether soluble also used Brine shrimp lethality assay. These findings presented a certain percentage of lethality against A. salina [94].
In conclusion, this study explores a novel and green synthesis approach to synthesize AgNPs using C. indica leaf extract. This species is reported to be a commonly grown plant in regions of Sri Lanka, and researchers have primarily focused on its phytochemical profile and its potential in traditional medicines. The current research proves that WEs obtained from five varieties of C. indica leaves can be useful in AgNP synthesis, although they have not been utilized extensively for this purpose. The optimized synthesis was observed at RT for 24 hours, with an optimum peak at 440 nm. The morphological structure confirmed spherical Pink_AgNP at a size of 27 nm to 48 nm. The conductivity study showed AgNPs as semiconductors (E less than 3.0 eV). The phytochemical analysis revealed the presence of starch, amino acids, phenols, tannins, and reducing sugars in WEs. The antioxidant activity analysis demonstrated a greater antioxidant capacity in AgNPs than in the WEs. The free radical scavenging activity analysis demonstrated greater scavenging activity along with lower IC50 compared to the WEs. The non-toxicity of the 240 ppm AgNPs was validated by the viability of A. salina after a 24-hour exposure. However, further analysis on the safety of these AgNPs should be performed along with their effects on other animal models to fully evaluate their non-toxicity. These results can allow C. indica leaves AgNPs to be used as treatment options for multiple diseases. The current study displayed a significant effect on bacterial growth inhibition exhibited by S. aureus and E. coli. The findings on antioxidant capacity, antibacterial capacity, and non-toxicity highlight the potential of C. indica leaf AgNPs for green medicine alternatives. The synthesis of AgNP predominantly uses phytochemicals, and the alteration of synthesis conditions results in the formation of distinct AgNPs with varied activity. Therefore, to completely comprehend its potential in the medical industry, a thorough and comprehensive investigation should be carried out on its different properties and methods for improving them.
AgNPs: silver nanoparticles
DNA: deoxyribonucleic acid
DPPH: 2,2-diphenyl-1-picrylhydrazyl
DW: distilled water
IC50: inhibition concentration
ROS: reactive oxygen species
RT: room temperature
SEM: scanning electron microscopy
TAC: total antioxidant capacity
TFC: total flavonoid content
TPC: total phenolic content
WEs: water extracts
ZOI: zone of inhibition
The authors acknowledge the support provided by Institute of Nanotechnology (SLINTEC) for SEM analysis using Hitachi SU6600 SEM.
MK: Conceptualization, Methodology, Funding acquisition, Project administration, Supervision, Validation, Writing—original draft, Writing—review & editing. NR: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing—original draft. BG: Investigation, Supervision, Validation, Visualization, Writing—review & editing. OP: Supervision, Validation, Visualization, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Authors thank BMS Campus for funding. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2719
Download: 132
Times Cited: 0
