Affiliation:
1Department of Biochemistry and General Chemistry, Faculty of Medicine, Medicum Collegium, University of Rzeszów, 35-959 Rzeszów, Poland
Email: dbartusikaebisher@ur.edu.pl
ORCID: https://orcid.org/0000-0002-5557-5464
Affiliation:
2Student Scientific Club URCell, Faculty of Medicine, Medicum Collegium, University of Rzeszów, 35-959 Rzeszów, Poland
Affiliation:
2Student Scientific Club URCell, Faculty of Medicine, Medicum Collegium, University of Rzeszów, 35-959 Rzeszów, Poland
Affiliation:
3Department of Photomedicine and Physical Chemistry, Faculty of Medicine, Medicum Collegium, University of Rzeszów, 35-959 Rzeszów, Poland
Email: daebisher@ur.edu.pl
ORCID: https://orcid.org/0000-0002-2661-6570
Explor Drug Sci. 2025;3:1008126 DOI: https://doi.org/10.37349/eds.2025.1008126
Received: April 22, 2025 Accepted: July 15, 2025 Published: August 19, 2025
Academic Editor: Xiqun Jiang, Nanjing University, China
Nanotechnology is a relatively young field of science that has found wide application in medicine, especially in oncology. It focuses on studying molecules at the atomic, molecular, and supramolecular levels, enabling the development of innovative therapeutic solutions. Thanks to research in this field, it has become possible to introduce nanoparticles (NPs) into therapy, specially designed molecules that release the drug in a precisely defined place. This approach allows for maintaining the appropriate therapeutic concentration of the drug substance in the body for a longer period of time. The use of NPs in the treatment of cancer diseases helps to overcome the limitations of traditional chemotherapy, such as systemic, toxic effects of drugs, lack of specificity towards cancer cells, and limited bioavailability. NPs can be used not only as drug carriers, but also as contrast agents enabling imaging at the molecular level. More accurate visualization of diseased tissues is possible thanks to the small size of NPs, optical properties, and the ability to accumulate in the tumor area. Additionally, the use of specific ligands allows detection of pathological changes at the cellular level, allowing for earlier detection of changes, which in turn increases the probability of complete recovery of the patient.
Cancer is a serious social, health, and economic problem of the 21st century. Despite significant medical progress in diagnostic and therapeutic methods, as indicated by the latest statistics (Eurostat), cancer is the second most common cause of death in the general population [1]. Data based on the latest estimates from GLOBOCAN (Global Cancer Observatory) and updated by the International Agency for Research on Cancer (IARC) indicate that in 2022, nearly 20 million new cases of cancer will be detected, and 9.7 million people worldwide will die from it. Based on the projected changes in population growth and aging, it is predicted that in 2050, the number of new cancer diagnoses will exceed 35 million, which is a 77% increase compared to 2022 [2]. These data indicate a significant problem that modern medicine is and will be facing, and at the same time prove the need to search for new and more effective methods of treating cancer. Classic chemotherapy is based on the use of highly cytotoxic drugs, the task of which is to destroy cancer cells. It is most often used in combination with other oncological treatment methods (surgery, radiotherapy, targeted drugs, and immunotherapy), which results from the fact that the exclusive use of cytostatics allows for the cure of only a small percentage of patients (approx. 5%) [3]. With the advancement of medicine, new substances with greater cytotoxicity and effectiveness in destroying abnormal cells are being discovered, but despite this, conventional chemotherapy often does not bring the expected treatment effect. The main reason for the low effectiveness of the therapy is limited bioavailability, development of drug resistance, and lack of specificity towards cancer cells, which contributes to the destruction of not only abnormal cells but also healthy ones, consequently leading to serious adverse effects [4].
In recent years, special attention has been paid to the use of nanotechnology in medicine, oncology, and molecular diagnostics. Nanotechnology is an interdisciplinary branch of science and technology that deals with the design and creation of structures called nanoparticles (NPs), the size of which is in the range of 1–100 nm [5]. The use of nanotechnology in medicine, referred to as nanomedicine, enables the introduction of a number of NPs with different chemical compositions and diverse structures in order to facilitate imaging and diagnostics of cancers, as well as to increase the effectiveness of their treatment [6]. These new methods based on drug carriers [NPs, liposomes, polymer micelles, polymer drug conjugates, and carbon nanotubes (CNTs)] show the potential to overcome the problems with traditional chemotherapy. Their use allows for an increase in the bioavailability of therapeutic compounds, and also increases the accumulation of the drug in the tumor area, which allows for the delivery of drugs to cancer cells in a concentration high enough to destroy them. Additionally, coating NPs with tumor-specific ligands allows for targeted therapy with greater effectiveness and, at the same time, limited side effects resulting from cytotoxicity towards normal cells [7].
NPs are characterized by a diverse structure and can take forms such as liposomes, block copolymer micelles, albumin nanocarriers (NCs), polymer NPs, dendrimers, and CNTs. Depending on the material used, NPs can act synergistically, enhancing the anticancer effects of drugs, which is why selecting the right type of NP is crucial and requires taking into account the physicochemical and functional properties of the materials used. In recent years, the focus has also been on the mechanism of drug co-delivery, combining molecules with different physicochemical and pharmacological properties using NDCDS (nano drug co-delivery system) nanotechnology. This allows minimizing the side effects of therapy and the toxicity of individual molecules.
In this paper, we reviewed classical chemotherapy and its limitations, nanotechnology in medicine, passive drug transport by NPs, targeted drug delivery in low enhanced permeability and retention (EPR) tumors, active drug transport to the tumor, lipid-based NPs, albumin NCs, and dendrimers. For carrying out this study, relevant sources from Scopus, Google Scholar, ResearchGate, and other research platforms were identified.
Chemotherapy, along with surgical resection and radiotherapy, is still the most common and considered the most effective method of treating cancer. Anticancer drugs, cytotoxic drugs, have been used in medicine since the 1940s. With the advancement of medicine, substances with greater cytotoxicity and therefore stronger anticancer effects are being discovered [8]. Currently, there are over 200 chemotherapeutic agents with different mechanisms of action and efficacy. Depending on the chemical structure and mechanism of action, conventional cytotoxic drugs can be divided into 5 main groups:
Antimetabolites (methotrexate, fludarabine, and cytarabine).
Alkylating cytostatics (cyclophosphamide, chlorambucil, and melphalan).
Natural preparations (vinblastine, etoposide, and bleomycin).
Hormones (estrogens, aromatase inhibitors, and gonadotropin-releasing hormone analogues).
Others (compounds not classified in the above groups: mitoxantrone, mitotane, and procarbazine) [9].
Structures of selected drugs are presented below (Figure 1).
The mechanism of action of these drugs is complex and includes, among others, the influence on the structure of DNA, inhibition of nucleic acid synthesis, influence on nucleic acid transcription and DNA replication, consequently leading to cell death. However, due to the systemic action of chemotherapeutics, normal cells are also damaged, especially those with high mitotic activity (cells of the bone marrow, gastrointestinal tract, and hair follicles), which may pose a threat to the health or even life of the treated patient. The lack of specificity towards cancer cells and limited bioavailability are common causes of low effectiveness of standard anticancer therapy regimens. Currently, low accumulation/retention of the drug in the tumor is considered to be the main factor in the failure of anticancer therapy [10]. Therefore, searching for and developing methods that can improve the effectiveness and safety of chemotherapy is currently an important direction of research.
Nanotechnology defines areas of science and engineering in which phenomena occur at nanoscale dimensions. Nanotechnology is a field that studies molecules at the atomic, molecular, and supramolecular levels. It allows for the implementation of modern drug production that improves the scheme of their delivery to individual organs, and also enables imaging diagnostics of changes previously unavailable using traditional imaging methods. An example of such progress is the introduction of NPs—specially designed molecules that can release the drug in a specific place, thus ensuring the maintenance of a therapeutic concentration in the body for a longer period of time [11]. Thanks to this, it has become possible to conduct targeted therapy at the molecular level with the elimination of adverse effects caused by classical chemotherapy, which is particularly important when using very strong cytotoxic drugs [12]. In the case of carrier NPs, their size should be less than 0.1 μm, thanks to which it is possible for these particles to penetrate tissues. They consist of a core—a molecule acting as a carrier and one or more functional groups connected to the core. Drug NPs, such as liposomes, micelles, polymeric nanomaterials, or polymer-drug conjugates, can be designed to reach the desired site in the body or even in the cell. The composition of carriers can be very diverse, as are the starting materials. They can contain biological lipids, dextran, lactic acid, phospholipids, chitosan, or chemical substances such as silica, carbon, metals, and various polymers [13]. The latest intelligent materials can respond to changes in the body, such as pH or temperature fluctuations, and in response release the transported compound, which allows for precise control of drug release and can improve the efficacy of treatment [14, 15]. NPs are generally non-toxic, chemically stable, and biocompatible materials, which allows for their wide use in targeted cancer therapy. Depending on the material, they can act synergistically, increasing the anticancer properties of drugs, therefore, the type of NP should be selected taking into account the physicochemical and functional properties of the material it is composed of [16]. Their main advantages are: more effective drug delivery, especially for those insoluble in water, targeted delivery, co-delivery of two or more drugs as part of a combination therapy, and visualization of the drug delivery site by combining an imaging system and the therapeutic substance [17]. Targeted therapy using NPs can be based on two mechanisms of drug transport: passive and active.
Passive transport of NPs was first used in 1995 with the approval of PEGylated liposomal doxorubicin (Doxil) [18]. Passive drug transport to the tumor site is possible due to the specific characteristics of the tumor microenvironment. As a result of the rapid increase in the volume of tumor tissue, abnormal vascular proliferation occurs in its area, and the resulting vessels are leaky, which, together with defective and poorly developed lymphatic drainage, leads to preferential accumulation of the drug in the tumor [19]. This phenomenon, called the EPR effect, was discovered and described over 30 years ago. In recent times, the existence of this effect and its significance have been the subject of intense discussion. Currently, the occurrence of NC accumulation within the tumor has been proven, but attention has been drawn to the different extent of this phenomenon depending on the patient and the type of cancer they suffer from [20]. Therefore, it is recommended to quantitatively assess the EPR effect using non-invasive imaging in order to correctly qualify the patient for treatment using nanotechnology. The size of NPs plays a significant role in the effectiveness of EPR, but interestingly, it is not true that the smaller their diameter, the greater the penetration into the tumor microenvironment. In a study conducted by Tang et al. [21], the biological profiles of silica nanoconjugates of three different sizes (20 nm, 50 nm, and 200 nm) were compared. It was proven that the highest degree of penetration, uptake by tumor cells, and retention in the tumor, and therefore the highest efficacy against primary and metastatic tumors in vivo, was obtained by NPs of 50 nm [21]. The effect of NPs is also influenced by other physicochemical properties, such as shape, which is a key factor determining blood circulation and the level of uptake by cancer cells and macrophages [22]. Flexibility determines the length of NP circulation in the tumor area. Research results show that softer particles (10 kPa) are characterized by better circulation and biodistribution compared to harder NPs in in vivo studies. Additionally, in vitro experiments show that softer particles show significantly lower uptake in immune system cells, endothelial cells, and also in cancer cells [23]. The above information indicates that physicochemical properties are of great importance in the accumulation, retention, and penetration of NPs into the tumor environment, and additionally, the building material of NCs should be optimized, taking into account the pathophysiology of the tumor, its type and stage, which allows for maximizing the quality of therapy [24]. Recent meta-analyses of preclinical data on cancer therapy using nanotechnology over a 10-year period have shown that an average of 0.7% of the injected drug dose reaches the tumor microenvironment [25]. This percentage seems small and initially questioned the usefulness of EPR-based therapy, but it turns out that this result is much greater and statistically significant compared to most conventional chemotherapy methods, including paclitaxel, doxorubicin (DOX), and docetaxel [26, 27]. Currently, research is underway on methods that could enhance the EPR effect by preparing the tumor microenvironment to receive as many NPs as possible. Among the physical methods considered in the studies, the use of ultrasound, hyperthermia, micro/nanobubbles (to enhance the thermal effect), acoustic jet, and ultrasound cavitation mechanisms are distinguished [28, 29]. The enhancement of the EPR effect can also be achieved by angiotensin II-induced pressure increase or by vasodilation using heat, but both of these methods can complicate the clinical application of NCs. Angiotensin II-induced pressure increase can lead to systemic side effects, and the application of heat requires precise control of the area of action to avoid damage to healthy tissues.
In the case of low EPR tumors, targeted drug delivery methods based on techniques using a different approach are recommended. An example of such a solution is the use of an injectable NP generator (iNPG). iNPG is a micrometer-sized discoidal, nanoporous silicon particle that can be loaded with drug-polymer conjugates. This system uses the natural tropism and increased dynamics of blood vessels to accumulate in tumors. Once in the tumor microenvironment, iNPG releases drug-polymer conjugates, which then self-assemble into NPs transported to the perinuclear region of cells, bypassing efflux systems, i.e. pumps responsible for resistance to anticancer therapy. Thanks to the development of modern drug delivery systems, such as iNPG, which focuses on the use of natural mechanisms, but does not rely solely on the EPR effect, the efficiency of NP delivery can be significantly improved, especially in tumors with a low level of this effect [30]. Another approach that is independent of the level of the EPR effect may be the use of certain types of cells that show tropism for the tumor environment and migrate and then settle in its area. In studies conducted on mice by Huang et al. [31], T lymphocytes were used, on whose surface NPs containing the drug, SN-38 (topoisomerase I inhibitor) were placed. Such modified cells were introduced into a mouse model of Burkitt’s lymphoma. Delivery of the drug via cells caused a 90-fold increase in the concentration of SN-38 in the lymph nodes compared to free drug administered systemically at a dose 10-fold higher, which allowed the research group to survive longer without noted greater toxicity resulting from the treatment [31]. These studies, in addition to the benefits of treatment effectiveness, give hope for discovering new treatment options for cancers in the disseminated stage, with multiple multi-organ metastases. However, this approach is limited to therapeutic agents with low toxicity to carrier cells.
Active drug transport to the tumor involves the targeted targeting of NPs to receptors or surface proteins that are overexpressed on the surface of cancer cells. Due to the presence of a ligand on the NP, it is possible to deliver it to previously identified cells or even to subcellular sites, such as individual cell organelles [32]. The aim of developing this method was to increase the effectiveness of targeted therapy by increasing the retention of NPs, and therefore the drug, within the tumor microenvironment. However, it has been proven that the incorporation of ligands targeting cancer cells into NP increases their internalization without a major effect on the overall location in the tumor. In the study by Kirpotin et al. [33], both targeted and non-targeted liposomes achieved high levels (7–8% of injected dose/g of tumor tissue) of accumulation in HER2-overexpressing breast cancer tissue, with targeted liposomes being located in tumor cells and unlabeled molecules being present mainly in the extracellular stroma and macrophages. A common problem in drug distribution is the delivery of high molecular weight molecules (e.g., proteins, RNA, DNA, etc.) to target cells. These structures are susceptible to enzymatic degradation and cannot passively cross the cell membrane to reach the active site in target cells. Their entry into cells requires the initiation of endocytosis, which can be facilitated by appropriate ligands on the NCs surface and the induction of active drug transport. Receptor-mediated endocytosis can enhance cellular uptake and additionally direct the delivery of NCs to specific subcellular sites [34].
The effective design of actively targeted carriers (NCs) for the delivery of intracellular therapies, such as nucleic acids or intracellular antibodies, requires consideration of several key aspects. Traditional markers, such as CD19 or HER2, are clinically important for the use of monoclonal antibody therapy, but may not ensure efficient internalization of NCs and escape from the endosome. This means that when designing such carriers, attention must be paid not only to the expression level of tumor markers, but also to the ability of these molecules to rapidly internalize and enable the release of therapeutic agents after their delivery into the cell. The complexity of targeted therapy is also problematic, which is increased by the heterogeneity of the tumor and the presence of different components of the tumor microenvironment, such as tumor-associated macrophages, fibroblasts, or other stromal cells. This heterogeneity means that the traditional approach of actively targeting a single receptor present on the surface of tumor cells is not always sufficient, as it does not take into account the variability in marker expression within the tumor. Moreover, such an approach may lead to the selection of cell clones resistant to treatment, resulting in disease relapse and development of resistance. This is why there is a need to develop more versatile carriers—NPs that target a wider range of molecular targets. This may include the use of carriers targeting different receptors, which will allow for greater flexibility in overcoming tumor heterogeneity and reducing the risk of developing therapy-resistant clones. Alternatively, combining several different NCs with different targeting molecules can significantly improve the efficacy of therapy, increasing the chances of hitting a wide range of tumor cells and their microenvironment. Such an approach could therefore help reduce the risk of relapse and resistance to treatment, improving the overall therapeutic outcome. However, such conjugation of targeting ligands to actively targeted carriers (NCs) involves a more complex manufacturing process compared to passive NCs, which poses a significant challenge in the context of their clinical implementation. Additional steps of chemical synthesis, purification, and quality control increase costs, extend production time, and complicate quality monitoring, which may hinder the widespread use of this technology, especially in the context of large-scale access.
The figure below (Figure 2) schematically shows the mechanism of passive and active drug transport.
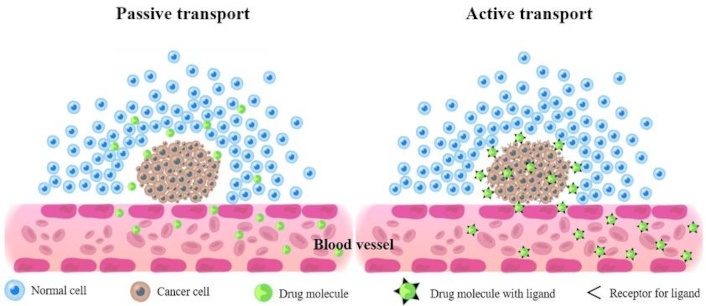
The mechanism of passive and active drug transport across cell membrane. Passive transport moves molecules down their concentration gradient, from high to low concentration, without requiring energy. Active transport, on the other hand, moves molecules against their concentration gradient, requiring energy, typically in the form of ATP
Endocytosis is an energy-intensive process in which cells internalize substances from their environment using vesicles from the plasma membrane [35]. Endocytosis pathways are classified into phagocytosis and pinocytosis, depending on the proteins involved. Pinocytosis is a process that occurs in almost every eukaryotic cell and is responsible for the transport of NPs into the interior of cancer cells. This process can be divided into clathrin-dependent endocytosis (the most well-known in terms of its use in anticancer therapy), clathrin-independent endocytosis involving caveolae, and macropinocytosis [36]. Phagocytosis mainly affects phagocytes and is an undesirable phenomenon in the case of intracellular drug transport, because NPs are degraded in this way. Studies have shown that NC modification with polyethylene glycol protects NP from phagocytosis by both (M1, M2) macrophage phenotypes, while coating NC with CD47 molecule reduces the phagocytic function of M1 macrophages, which are characterized by higher activity [37].
Clathrin-mediated endocytosis (CME) is a classic route of cargo introduction into mammalian cells. CME is involved in nutrient uptake, regulation of signaling pathways, and membrane recycling. Classical receptors involved in this process are transferrin receptor (TfR), low-density lipoprotein receptor (LDLR), epidermal growth factor receptor (EGFR), and folate receptor (FR), which are responsible for internalization of Tf, LDL, epidermal growth factor, and folic acid, respectively [38]. Some of these receptors are overexpressed in cancer cells, which is why by modifying the NC surface with specific ligands, it is possible to increase the affinity of these molecules for cancer cells. For example, TfR expression on the surface of cancer cells is about 100 times higher than on normal cells, which makes this receptor one of the most attractive targets for cancer therapy [39].
However, NP endocytosis is not synonymous with drug delivery and its effect on the cancer cell. The mechanism of transport of molecules by endocytosis encounters certain physiological barriers resulting from their interactions with target cells. After the endocytosis process, NPs are directed to various subcellular locations, some of which may negatively affect their fate. For example, NPs internalized via the clathrin-dependent pathway are preferentially directed to lysosomes, where they are degraded. In order for the drug contained in NPs to act in the cytosol or cell nucleus, it must escape from the endosome before it is degraded. This process is called endosomal escape. Initiation of this process is a major challenge for contemporary researchers. Many strategies have been tested to facilitate this process, including pH buffering substances using the “proton sponge effect”, fusogenic pH-sensitive NPs, peptides, and porous proteins [40]. Particular attention has been paid to membrane fusion peptides found in viral envelopes. For example, the influenza virus has an amphiphilic anionic peptide (HA2) on its surface, which has properties facilitating escape from endosomes. Additionally, HA2 has a helical structure dependent on the ambient pH, which favors fusion with the endosomal membrane and at the same time facilitates the release of its contents into the cytoplasm [41]. Over the years, attempts have been made to design comparable, synthetic peptides mimicking the fusogenic functions of virus-derived peptides. The best-known synthetic peptide is GALA, which, similarly to viral peptides, is pH-sensitive and has amphiphilic properties. Studies have shown significantly higher efficiency of silencing lung cancer cell genes with siRNA when NPs containing gamma-aminobutyric acid (GABA) are used, which resulted from the increased endosomal escape process [42]. Another problem is the recycling process of the endosome containing NPs. Studies conducted by Sahay et al. [43] have shown that the vast majority of internalized liposomal NPs (70%) underwent exocytosis by escaping from late endosomes/lysosomes. Understanding the mechanisms of endocytosis proves the need for further work to ensure the highest possible, selective uptake of NCs by cancer cells, but also draws attention to the need to develop methods that allow for a higher degree of endosomal escape, which would increase the effectiveness of therapy.
Lipid-based NPs are the oldest and broadest group of NCs that have been used in drug and gene delivery. The milestone that decided to start research on liposomal NC was the approval in 1995 of the drug Doxil, a liposomal preparation of DOX for second-line therapy of Kaposi’s sarcoma. Doxil therapy was associated with a lower percentage of adverse effects, such as hair loss or peripheral neuropathy, and most importantly, with lower cardiotoxicity while maintaining high treatment efficacy [44]. The positive conclusion of research on the first type of NP encouraged researchers to continue working in this direction. Over the past 30 years, intensive research has led to the approval of 23 products in the US and Europe, 63% of which were for cancer therapy [45].
Five generations of lipid NCs can be distinguished: liposomes, solid lipid NPs (SLNs), nanostructured lipid carriers (NLCs), lipid drug conjugates (LDCs), and hybrid polymer-lipid NPs (PLNs).
Liposomes are composed of a lipid bilayer enclosing a hydrophilic core inside the NC. Due to this structure, they are ideal carriers because hydrophilic drugs can usually be placed in the core, while hydrophobic drugs are trapped in the lipid bilayer. Individual liposomal NCs differ in size, lamellarity (number of lipid layers), and surface charge. They can be classified as single- or multilayer, while single-layer liposomes can be further divided into small [small unilamellar vesicles (SUVs); 70 nm] and large [large unilamellar vesicles (LUVs); 100–250 nm]. Both SUVs and LUVs consist of a single lipid bilayer and a large aqueous core; hence, they are mainly suitable for transporting hydrophilic drugs, whereas multilayer liposomes, composed of several lipid bilayers and a limited aqueous space, are used for transporting hydrophobic drugs [46]. Furthermore, based on the charge of the lipid components, cationic and anionic liposomes are distinguished. Cationic liposomes have been used for transporting nucleic acids containing negatively charged phosphate groups; therefore, they can serve as vectors for miRNA, DNA, and siRNA therapies [47]. In turn, anionic liposomes have been shown to be effective in transporting plasmid DNA [48]. An example of a liposomal drug carrier is the previously mentioned form of Doxil, in which the drug is located in the aqueous phase of the core as a crystalline salt (DOX)2SO4, which is transported into the interior of the liposome due to the transmembrane gradient of ammonium ions, which also determines the release of the drug in the tumor [49].
Liposomes are not ideal carriers; their main disadvantages are the relatively expensive preparation process, a relatively low degree of drug load capacity and stability, most importantly, rapid decomposition in the human body before the drug reaches therapeutic concentration [50]. Due to these limitations, research has been conducted to introduce new, improved generations of lipid NCs.
SLNs consist of fully crystallized lipid components, characterized by a highly ordered crystalline structure in combination with emulsifiers. Their advantages include: high stability, excellent drug protection, and their controlled release, which drew the attention of researchers as early as 1991 [51]. However, they have two main disadvantages, which are poor, long-term drug retention and low drug loading capacity. This is due to the storage-related change in the structure of the lipid matrix to a more ordered one with simultaneous gradual excretion of encapsulated drugs [52]. Due to these limitations, solutions were sought to improve the stability of this form of NC. By incorporating liquid lipids into their structure, NLCs have gained an advantage over SLNs. The mixture of solid and liquid lipids takes the form of a partially crystallized lipid structure, which provides better stability and greater drug loading capacity with minimal leakage during storage [53]. Initially, they were considered for the transport of lipophilic drugs, but over time, it turned out that they can also effectively transport hydrophilic drug molecules. Studies comparing the efficacy of co-delivery of vincristine (VCR) and temozolomide (TMZ) using engineered lipid molecules, SLNs and NLCs showed significantly higher anticancer efficacy against glioma cells for drugs transported using NLCs compared to SLNs, both in vitro and in vivo [54]. LDCs are particularly well suited for the delivery of water-soluble drugs, due to the ability of the LDC lipid matrix to conjugate with hydrophilic drug molecules (via covalent bonding or salt formation with fatty acids). They are considered the best method to increase the bioavailability of drugs with limited absorption during oral administration due to the increased ability of the NC to avoid enzymatic degradation or damage due to changing pH values of the gastrointestinal environment [55]. A study on the efficacy of LDC NPs for the delivery of 5-fluorouracil (5-FU) to brain tumor cells confirmed the higher cytotoxicity of the NC-conjugated form of the drug against glioma cells. It was shown that LDC provides higher drug concentrations in the systemic circulation (blood), while pure 5-FU was largely available in peripheral tissues. The half-life of the conjugated form of the drug was approximately 12 times longer, and the maximum concentration in brain tissue was twice as high as in the traditional form of the drug, which clearly proved the greater efficacy of glioma therapy using LDC [56]. Hybrid PLNs consist of at least two types of materials integrated into one drug delivery system, combining the advantages of each of the individual components. Classic PLNs have a core-shell structure (polymer core surrounded by a lipid layer) and are derived from both liposomes and polymer NPs [57]. Due to this structure, they are characterized by high stability and high biocompatibility. Due to the dual structure, this method is particularly interesting in terms of the possibility of combined drug delivery, which is particularly useful in oncology. An example is a loaded lipid-polymer hybrid NP containing DOX and edelfosine (EDL), which was designed to increase the efficacy of osteosarcoma treatment. It was shown that the targeted NPs were characterized by increased cellular internalization and subcellular distribution in cancer cells. Additionally, it was proven that the combination of drugs in a 1:1 ratio achieves the most synergistic effect in inducing cancer cell death in in vitro studies. In vivo results confirmed high efficacy in destroying cancer cells without detectable side effects. These results indicate that hybrid lipid-polymer NCs, due to their properties such as better physiological stability, the ability to carry a large drug load, drug co-delivery, and prolonged blood circulation, are promising in terms of increasing the efficacy of oncological treatment [58].
The figure below (Figure 3) shows the structural diagrams of selected forms of lipid-based NPs.
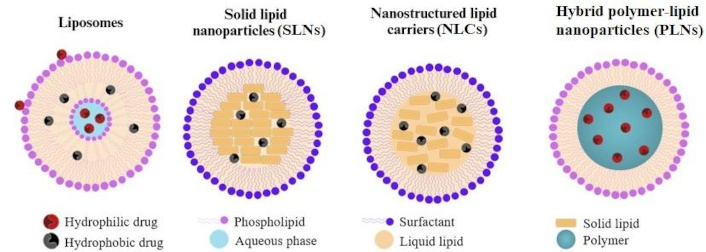
Schematic diagram of the structure of selected forms of lipid-based nanoparticles (LNPs). LNPs are diverse in structure but typically consist of a lipid core and a shell, often containing phospholipids, cholesterol, and PEGylated lipids. They can be categorized into liposomes, solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), and hybrid polymer-lipid nanoparticles (PLNs)
In addition to lipid-polymer hybrid NPs, lipid-metal hybrid NPs have also been designed, such as lipid-coated silver hybrid NPs (lipid-AgNPs), lipid-aluminum NPs, and liposome gold NPs (LiposAuNPs) [59, 60]. Metal-containing NPs are widely used for probing and labeling, but in vivo studies on their use for drug transport have shown some limitations due to low cellular uptake and poor stability. These assumptions have been confirmed, among others, in studies on AuNPs coated with lysophosphocholine. The results showed that lysophosphocholines-AuNPs formed aggregates with a core-shell structure, characterized by high stability, without significant structural changes, even when the temperature was increased to 50°C. Additionally, AuNPs showed the ability to kill cancer cells using photothermal therapy. In vivo studies in a mouse tumor xenograft model showed complete ablation of the tumor mass using near infra-red (NIR) laser illumination (750 nm), thus prolonging disease-free survival [61].
Block copolymer micelles are formed by the self-assembly of amphiphilic or oppositely charged copolymers in an aqueous environment. Hydrophilic and hydrophobic materials interact to form the corona and core of the micelle, respectively. They are particularly advantageous for poorly soluble, small molecules with high potency and significant toxicity, due to the possibility of encapsulating them inside the micelle. The appropriate size of the micelles determines the long circulation time. The micelles are large enough to avoid renal excretion (> 50 kDa), but small enough (< 200 kDa) to bypass filtration through the gaps between the endothelial cells in the spleen. In addition, it is believed that the presence of a non-ionic water-soluble coating and the appropriate size (10–100 nm) of polymer micelles limits their uptake by the mononuclear phagocyte system and allows passive penetration into the tumor tissue and retention of micelles in the tumor microenvironment. Currently, efforts are being made to increase the physical stability of micelles, which would increase the resistance to dissociation of this carrier and allow for higher drug accumulation within the tumor [62]. Micelles are a particularly good solution for the transport of substances with limited bioavailability. An example is quercetin (QUE)—a therapeutic substance with potential anticancer activity. The low bioavailability of QUE is a consequence of its lipophilic nature and low solubility in water, gastric juice, as well as poor permeability through the barrier in the form of the gastrointestinal epithelium, which results in low QUE absorption when administered orally. The use of micelles, in addition to increasing the bioavailability of drugs, can also be used to reduce the side effects resulting from the use of highly cytotoxic drugs, especially in oncological therapy. For example, DOX is one of the most potent anticancer drugs, but its use is limited due to strong cardiotoxicity resulting from the generation of free radicals and the presence of highly cardiotoxic metabolites, such as doxorubicinol. The toxic effect of this drug could be limited as a result of the use of strong antioxidants, such as QUE, which has the ability to scavenge free radicals and bind transition metal ions. In the study conducted by Chang et al. [63], lecithin-stabilized polymeric micelles (LsbPMs) were prepared for the transport of QUE and the effect of combining QUE with DOX in micelles was examined. Cytotoxicity assay showed that QUE-loaded LsbPM had significant antitumor activity against human breast cancer cells in vitro and in a mouse colon cancer model. In a pharmacokinetic study, compared with free QUE, intravenous and oral administration of QUE-loaded LsbPM significantly increased the relative bioavailability to 158% and 360%, respectively, and the absolute bioavailability to 5.13%. The action of QUE-loaded LsbPM combined with DOX could effectively inhibit the growth of colon cancer cells and reduce the toxicity to the heart tissue in a mouse model, which proved the effect of QUE on enhancing the antitumor activity and reducing the cardiotoxicity [63].
Albumins are ubiquitous, biodegradable, biocompatible, non-toxic, and non-immunogenic protein NCs. Albumin NPs can be easily modified by introducing functional groups on their surface or by enriching them with specific ligands directing the drug to a specific molecular target. Additionally, cancer cells have an increased ability to internalize albumin molecules, which results from the function of this protein as a basic source of energy and building blocks in rapidly dividing tumor cells [64]. Albumin retention in the tumor microenvironment is also facilitated by the increased expression of the glycoprotein SPARC (secreted protein, acidic and rich in cysteine), characteristic of solid tumors, an acidic secretory protein rich in cysteine, also known as osteonectin [65]. The first drug approved by the FDA in a form bound to albumin was Abraxane—a polymeric NP based on HSA (human serum albumin) and paclitaxel. Paclitaxel is a cytotoxic drug belonging to the taxane group, which inhibits mitotic divisions by binding to microtubules, thus disrupting the formation of the mitotic spindle. It is a drug with high efficacy, but its serious limitation is its strong hydrophobicity and the resulting need for emulsifiers to dissolve the drug in water. The most commonly used emulsifier was polyoxyethylated castor oil, which effectively increased the solubility of paclitaxel, but at the same time increased the risk of adverse effects associated with excessive histamine secretion as a result of hypersensitivity reactions, including anaphylaxis. The production of paclitaxel in combination with albumin allows avoiding the use of CrEL as a stabilizer, while increasing the safety of the therapy [66]. Clinical studies have shown that albumin-bound paclitaxel demonstrated higher response rates and better tolerability than solvent-based formulations in patients with metastatic breast cancer and advanced non-small cell lung cancer. Additionally, Abraxane has been shown to achieve 33% higher tumor uptake than traditional paclitaxel, which is explained by increased drug accumulation due to albumin uptake by SPARC. Abraxane is currently approved by the FDA for monotherapy for the treatment of metastatic breast cancer in adult patients who have failed first-line therapy for metastatic disease and who are not candidates for standard anthracycline therapy in combination with gemcitabine. It is also indicated for the first-line treatment of metastatic pancreatic adenocarcinoma in adults and in combination with carboplatin for the first-line treatment of non-small cell lung cancer in adult patients who are not candidates for radical surgery and/or radiotherapy [67].
Polymer NPs include two types of NCs—nanocapsules and nanospheres, which differ in terms of morphology. Nanocapsules are referred to as reservoir systems and consist of an oily core in which the drug is dissolved and a polymer shell responsible for controlling the release profile of the drug from the core. Nanospheres, considered a matrix system, have a structure of a continuous polymer network. The drug can be retained inside the polymer network or absorbed on its surface [68]. The polymers that form NPs can be linear, branched, or spherical, and their size is strictly controlled. The main synthetic polymers include polylactic acid (PLA), polyglycolic acid (PGA), poly(lactic-co-glycolic acid) (PLGA), polyglutamic acid, and N-(2-hydroxypropyl)-methacrylamide (HPMA) [69]. PLGA is one of the most commonly used polymers for the construction of NPs, partly due to its minimal systemic toxicity. This compound is hydrolyzed in the body to monomers in the form of lactic acid and glycolic acid, which are then metabolized in the Krebs cycle and removed as carbon dioxide and water [70]. PLGA NPs are commonly used to encapsulate various types of anticancer drugs, which allows for their more effective delivery in vivo. The most common forms of PLGA-based NPs are microspheres or microparticles [71]. They are used in oncology to transport many drugs, including VCR, cisplatin, etoposide, paclitaxel, DOX, curcumin, triptorelin, dexamethasone, rapamycin, and many others [72–79].
The main barrier to oncological treatment is the development of multidrug resistance (MDR). It appears after a longer period of chemotherapy and causes a decrease in the effectiveness of the treatment, which may consequently lead to the failure of the therapy. One of the most common mechanisms of resistance development is the P-glycoprotein-based pumping of the drug from the cancer cell. P-glycoprotein, encoded by the MDR-1 gene, exports a wide range of chemotherapeutic drugs, reducing their accumulation in tumor cells and thus leading to low treatment efficacy. For example, VCR sulfate is a widely used anticancer agent, but many cancer cells are resistant to its action due to the drug efflux mechanism via the P-glycoprotein complex-related pump. P-glycoprotein probably identifies only those drugs that are bound to the plasma membrane. VCR-loaded PLGA NPs, after internalization, usually end up in endolysosomal complexes, which allows them to escape from the drug efflux mechanism [80]. Additionally, the use of P-glycoprotein inhibitors such as verapamil (VRP), cyclosporine A, or quinidine could help overcome the resistance of cancer cells to treatment. In vivo studies compared the efficacy of PLGA NPs containing VCR and VRP (VCR-VRP-PLGANPs) with free VCR, free VCR/VRP combinations, and single-drug NP combinations against MDR breast cancer cells. A statistically significant difference in the rate of tumor growth inhibition was observed between the NPs and the other study groups. The slowest tumor growth was observed with VCR-VRP-PLGANPs. As expected, VRP increased the antitumor potency of VCR, and encapsulation of both substances into PLGA NPs further enhanced the effect of this drug combination, probably through increased accumulation of NPs in the tumor microenvironment and rapid release in the weakly acidic environment inside the tumor cells [81].
Dendrimers are spherical molecules characterized by a symmetrical, highly branched three-dimensional structure. Their central part is a core, composed of a single atom or group of atoms, from which symmetrical branches called dendrons extend. At the ends of dendrimers, covalent or non-covalent attachment of biologically active molecules, drugs, genes, or contrast agents is possible. The structure of dendrons is largely responsible for the physical and chemical properties of dendrimers [82]. There are complete dendrimers, in the case of molecules with amine (–NH2) or hydroxyl (–OH) end groups, and half-dendrimers, terminated with carboxyl groups (–COOH). The increase in the number of repeating branching units determines the generation of the dendrimer and is responsible for the formation of a circular structure. Therefore, the higher the generation of the dendrimer, the higher the density of the molecule packing, which is why it is possible to synthesize dendrimers only up to 10 generations. The high degree of control over the possible structure of the dendrimer makes these molecules attractive for delivering drugs to tissues. Therapeutic compounds can be enclosed in their cavities, bound to the surface via hydrophobic or electrostatic interactions, or covalently linked to end groups. There are different types of dendrimers, including phosphate dendrimers (P-dendrimers), polyamines, carbosilicates (CBS), polypeptides, polyesters, and the best known polyamidoamines (PAMAMs) [83].
Dendrimers derived from PAMAM are the most commonly used group of this type of NPs. They are hydrophilic, biocompatible, and non-immunogenic systems, which favors their use as drug carriers or nucleic acids [84]. In an in vivo study, the effect of conjugation of DOX with carboxyl end groups of PAMAM dendrimers on the efficacy of intrapulmonary therapy in a mouse model of melanoma lung metastases was assessed. The results showed a significant increase in the therapeutic efficacy of DOX, as indicated by a smaller number of nodules observed in the lungs and increased survival rates compared to the free form of DOX. Additionally, the intrapulmonary route of administration and conjugation with PAMAM allowed for a longer drug accumulation in the lungs compared to intravenous administration, while significantly reducing the distribution of DOX to the heart tissue [85].
CNTs are cylindrical structures with a wide variety of structures. CNTs include two classes of nanomaterials, including single-walled CNTs (SWCNTs), composed of a single graphene layer rolled into a cylindrical tube, and multi-walled CNTs (MWCNTs), containing many concentric graphene cylinders. The diameter of MWCNTs, due to their multilayer structure, is usually larger than SWCNTs (approx. 1 nm in diameter) and ranges from 5 to 20 nm. CNTs, especially MWCNTs, have attracted attention due to their promising properties, such as large absorption surface area, high material strength, high charging capacity, optical and electrical properties, as well as high stability, biocompatibility, and the ability to release therapeutic agents [86]. In addition, they are considered to be “intelligent” materials, closely related to the mechanism of microtubule action. MWCNTs can penetrate many biological barriers, including the interior of cells, where they exhibit unique biomimetic properties with cytoskeletal elements, and primarily with microtubules, thus demonstrating a common anticancer effect with drugs, consisting of inhibiting the cell cycle in the mitotic phase. In vivo studies have shown the benefits of using MWCNTs as a drug carrier, due to the additional inhibition of the cancer cell cycle in the M phase. For example, a combination of 5-FU with a carrier in the form of MWCNTs (5-FU-MWCNT) was tested. 5-FU is a widely used anticancer drug, the mechanism of action of which is based on inhibiting the cell cycle in the S phase. A study conducted on a mouse model of melanoma showed the highest efficacy of 5-FU-MWCNT in reducing tumor mass compared to 5-FU monotherapy and MWCNT alone. These results confirmed the possibility of increasing the effectiveness of the treatment by using two complementary cytotoxic mechanisms generated by both therapies, disrupting two different stages of the cell cycle [87]. Carbon tubes are not ideal materials, however, due to their structure, they also have certain disadvantages, such as lack of biodegradability, tendency to accumulate and resulting toxicity. The toxic effect is mainly due to the similarity in structure to asbestos fibers. The side effects reported in studies include long-term inflammation, but also the development of neoplastic changes, mainly in the form of pleural mesothelioma (when inhaling MWCNT) [88]. Despite the great benefits of using MWCNT, toxic effects on specific cell types, such as alveolar epithelial cells or normal bronchial epithelial cells, have also been demonstrated [89]. However, the results of studies on the potential toxicity of MWCNT are inconsistent, hence, further work is required in this direction.
NPs can be used not only as drug carriers, but also as contrast agents, enabling imaging at the molecular level. More precise visualization of pathologically changed tissues is possible due to the small size of NCs, optical properties, and the ability to accumulate in the tumor area. Additionally, the use of specific ligands allows detection of pathological changes at the cellular level, allowing for earlier detection of changes, which in turn increases the probability of complete recovery of the patient [90].
The concept of theragnosis is becoming increasingly common in medicine. Theragnosis (from the words “therapy” and “diagnosis”) is a modern concept that combines diagnostics and therapy in one process. Multifunctional NPs can be combined simultaneously with diagnostic markers and therapeutic agents, thus allowing for detection, assessment of advancement, and simultaneous treatment. One of the most attractive materials in the context of theragnosis is AuNPs, which results from their properties of absorbing and scattering light [the phenomenon of surface plasmon resonance (SPR)]; additionally, they are characterized by biocompatibility and limited toxicity [91]. AuNP-based theragnosis has some limitations, but it is a promising perspective for the use of NPs for better diagnostics and treatment of cancer.
After demonstrating the effectiveness of anticancer drugs, various schemes of combined chemotherapy began to be constructed to increase therapeutic efficacy. In recent years, the focus has been on the mechanism of co-delivery of these drugs, combining molecules with different physicochemical and pharmacological properties using NDCDS nanotechnology. Thanks to this, it is possible to minimize the side effects of therapy and the toxicity of individual molecules. This delays tumor adaptation, and thanks to this, also limits the number of mutations occurring within it. This is a therapeutic option based on combination therapy, from which it differs, among other things, in the pharmaceutical compatibility of the drugs used—they can be mixed in one infusion preparation and administered together—conventional chemotherapy rarely provides such a possibility. Thanks to this, sequential and precise action of anticancer drugs with different target points is possible. The most commonly combined are DOX and paclitaxel molecules, which are administered in free form in a molar ratio of 5:1, while in the NDCDS formula the highest effectiveness was demonstrated by their combination in a ratio of 2:1. Zhang et al. [92] in their studies proved that the use of nanotechnology improves the effectiveness of inhibiting the growth of cancer cells compared to the use of separate supply of the same drugs in the same concentrations, but separately. High effectiveness of the above-mentioned has been demonstrated especially in the treatment of non-small cell lung cancer, and in the combination of cisplatin with DOX, paclitaxel, or rifampicin in the therapy of ovarian cancer and melanoma. An example of NDCDS is also the previously described polymer-lipid molecules combining drugs with both hydrophilic and hydrophobic character. NDCDS systems are subject to continuous research combining molecules of different sizes, including monoclonal antibodies, for example, co-delivery of trastuzumab with DOX, cisplatin, or paclitaxel in the case of breast cancer with overexpression of HER2/neu. A challenge in NDCDS research, apart from pharmaceutical compatibility, is the type of biomaterial from which the carrier for individual drugs is made. Mainly liposome and polymer materials are used, but also inorganic materials based on AuNPs [92]. DNA damage induced by cis-platinum (Cis-Pt) mediated chemotherapy was significantly impaired by the highly expressed programmed death ligand-1 in tumor cells [93]. The immune checkpoint blockade therapies that target the programmed cell death ligand-1 have been used as revolutionary cancer treatments in the clinic [94]. NPs with efficient tumor-targeting capacity, tumor-responsive prosperity, and versatility for combination therapy were identified as new avenues for programmed death ligand-1 targeting cancer immunotherapies [95].
The use of NPs in medicine has significantly contributed to the improvement of anticancer therapy. Thanks to the variety of NC forms, such as liposomes, polymers, albumin NCs, and dendrimers, it is possible to precisely match the therapy to the individual needs of the patient, which leads to more effective and safe methods of diagnosis and treatment. The increase in knowledge and technological progress in this field gives hope for a revolution in the treatment of cancer and other diseases, opening up new possibilities for various areas of medicine, particularly in molecular diagnostics or gene therapy. NPs offer numerous benefits, especially in the context of the method of drug delivery, enabling targeted and controlled release of active substances, which allows for longer maintenance of therapeutic concentration in the tumor microenvironment while minimizing side effects and increasing the effectiveness of therapy. Nevertheless, despite their numerous advantages, conventional drug delivery systems still have their place in medicine. Oral tablets or capsules remain a convenient form of treatment, especially for patients who require therapy at home. In addition, the use of NPs for some drugs can be expensive and does not always provide sufficient benefits compared to traditional methods, which is why the classical form of drug delivery in such cases remains the primary method of treatment. Therefore, although nanotechnology is the future of medicine, there is still a need to balance innovative solutions with traditional methods to provide patients with optimal treatment, tailored to their specific needs and capabilities. Nanotechnology in medicine has undergone impressive modifications, pushing the drug to a new level with significant healthcare outcomes. There is still a need to study the significant capabilities of nanotechnology in healthcare.
5-FU: 5-fluorouracil
AuNPs: gold nanoparticles
CME: clathrin-mediated endocytosis
CNTs: carbon nanotubes
DOX: doxorubicin
Doxil: liposomal doxorubicin
EPR: enhanced permeability and retention
iNPG: injectable nanoparticle generator
LDCs: lipid drug conjugates
LDL: low-density lipoprotein
LsbPM: lecithin-stabilized polymeric micelles
LUVs: large unilamellar vesicles
MDR: multidrug resistance
MWCNTs: multi-walled carbon nanotubes
NCs: nanocarriers
NDCDS: nano drug co-delivery system
NLCs: nanostructured lipid carriers
NPs: nanoparticles
PAMAMs: polyamidoamines
PLGA: poly(lactic-co-glycolic acid)
PLNs: polymer-lipid nanoparticles
QUE: quercetin
SLNs: solid lipid nanoparticles
SPARC: secreted protein, acidic and rich in cysteine
SUVs: small unilamellar vesicles
SWCNTs: single-walled carbon nanotubes
TfR: transferrin receptor
VCR: vincristine
VRP: verapamil
DBA, AK, and KK: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. DA: Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
