





Aim:
To evaluate angiotensin II (Ang II) and Ang-(1-7) levels and the cytokine profile in patients hospitalized with mild coronavirus disease 2019 (COVID-19) and contrast them with patients with identical clinical conditions but treated with high doses of vitamin D (vitD).
Methods:
From the 218 patients recruited (ClinicalTrials.gov NCT04411446), 16 participated in this sub-study and were randomized to a single oral dose of 500,000 IU vitD (n = 10) or placebo (n = 6). Plasmatic Ang II and Ang-(1-7) levels were determined by radioimmunoassay and interleukins (ILs) 1, 6, 8, and 10 and tumor necrosis factor alpha (TNF-α) by enzyme-linked immunosorbent assay before and after treatment. Parallel, serum 25-hydroxyvitamin D3 (25-OH vitD) concentrations as vitD status was measured by a chemiluminescence immunoassay.
Results:
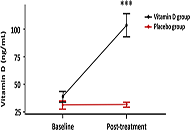
A trend towards an increase in Ang-(1-7) and a decrease in Ang II levels were observed in placebo- and vitD-treated COVID-19 patients compared to baseline values. There was no difference in Ang II and Ang-(1-7) levels between placebo- and vitD-treated COVID-19 patients. Similar results were obtained with ILs profile. COVID-19 patients showed an increase in the protective component of the RAS which was not improved by vitD treatment.
Conclusions:
VitD did not improve RAS disbalance in COVID-19. Notwithstanding, the authors visualize that acute treatment with high doses of vitD may show a trend to a decline in inflammatory ILs and an increase in protective markers. Finally, the authors would like to highlight the limitations of this preliminary study, namely the small number of patients and the use of a large single bolus dose of vitD rather than lower daily doses for extended periods with prolonged follow-up times. All these factors need special consideration in the designs of new vitD supplementation trials. All these factors need special consideration in the designs of new vitD supplementation trials (ClinicalTrials.gov identifier: NCT04411446).
Aim:
To evaluate angiotensin II (Ang II) and Ang-(1-7) levels and the cytokine profile in patients hospitalized with mild coronavirus disease 2019 (COVID-19) and contrast them with patients with identical clinical conditions but treated with high doses of vitamin D (vitD).
Methods:
From the 218 patients recruited (ClinicalTrials.gov NCT04411446), 16 participated in this sub-study and were randomized to a single oral dose of 500,000 IU vitD (n = 10) or placebo (n = 6). Plasmatic Ang II and Ang-(1-7) levels were determined by radioimmunoassay and interleukins (ILs) 1, 6, 8, and 10 and tumor necrosis factor alpha (TNF-α) by enzyme-linked immunosorbent assay before and after treatment. Parallel, serum 25-hydroxyvitamin D3 (25-OH vitD) concentrations as vitD status was measured by a chemiluminescence immunoassay.
Results:
A trend towards an increase in Ang-(1-7) and a decrease in Ang II levels were observed in placebo- and vitD-treated COVID-19 patients compared to baseline values. There was no difference in Ang II and Ang-(1-7) levels between placebo- and vitD-treated COVID-19 patients. Similar results were obtained with ILs profile. COVID-19 patients showed an increase in the protective component of the RAS which was not improved by vitD treatment.
Conclusions:
VitD did not improve RAS disbalance in COVID-19. Notwithstanding, the authors visualize that acute treatment with high doses of vitD may show a trend to a decline in inflammatory ILs and an increase in protective markers. Finally, the authors would like to highlight the limitations of this preliminary study, namely the small number of patients and the use of a large single bolus dose of vitD rather than lower daily doses for extended periods with prolonged follow-up times. All these factors need special consideration in the designs of new vitD supplementation trials. All these factors need special consideration in the designs of new vitD supplementation trials (ClinicalTrials.gov identifier: NCT04411446).
DOI: https://doi.org/10.37349/emed.2023.00137
This article belongs to the special issue Lung Fibrosis—Models and Mechanisms

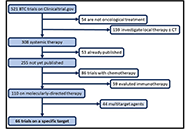
Patients with unresectable biliary tract carcinomas (BTCs) have a poor prognosis with a median overall survival of fewer than 12 months following systemic chemotherapy. In recent years, the identification of distinct molecular alterations with corresponding targeted therapies is modifying this therapeutic algorithm. The aim of this review is to present an overview of targeted therapy for BTCs, describing published available data and potential future challenges in ongoing trials. From clinicaltrials.gov online database all ongoing trials for BTCs (any stage) was examinated in July 2021, and data regarding study design, disease characteristics and type of treatments were registered. Oncogenic-driven therapy (targeted therapy) was investigated in 67 trials. According to research, 15 ongoing trials (22.4%) are investigating fibroblast growth factor (FGF) receptor (FGFR)-inhibitors in BTCs. Three (18.7%) are open-label randomized multicenter phase 3 trials, 8 (50%) are single-arm phase two trials, and 4 (25%) are phase one studies. Twelve (17.9%) clinical trials dealt with isocitrate dehydrogenase (IDH) 1/2 targeting therapy either in combination with cisplatin (Cis) and gemcitabine (Gem) as first-line treatment for BTCs or in monotherapy in patients with IDH1 mutant advanced malignancies, including cholangiocarcinoma (CCA). Nine (13.4%) clinical trials tested human epidermal growth factor receptor (HER) 2 targeting therapy. Four (44.4%) studies are phase I trials, two (22.2%) are phase I/II trials, and three (33.3%) phase II trials. Rare molecular alterations in BTCs, such as anaplastic lymphoma kinase (ALK), c-ros oncogene1 receptor tyrosine kinase (ROS1), and v-RAF murine sarcoma viral oncogene homologue B1 (BRAF), are also under investigation in a few trials. Forty-four clinical trials (17.2%) are investigating not oncogenic-driven multitarget therapy like multireceptor tyrosin kinase inhibitors and antiangiogenetic agents. In conclusion, this review shows that BTCs management is experiencing important innovations, especially in biomarker-based patient selection and in the new emerging therapeutic approach. Many ongoing trials could answer questions regarding the role of molecular inhibitors leading to new therapeutic frontiers for molecular subcategories of BTCs.
Patients with unresectable biliary tract carcinomas (BTCs) have a poor prognosis with a median overall survival of fewer than 12 months following systemic chemotherapy. In recent years, the identification of distinct molecular alterations with corresponding targeted therapies is modifying this therapeutic algorithm. The aim of this review is to present an overview of targeted therapy for BTCs, describing published available data and potential future challenges in ongoing trials. From clinicaltrials.gov online database all ongoing trials for BTCs (any stage) was examinated in July 2021, and data regarding study design, disease characteristics and type of treatments were registered. Oncogenic-driven therapy (targeted therapy) was investigated in 67 trials. According to research, 15 ongoing trials (22.4%) are investigating fibroblast growth factor (FGF) receptor (FGFR)-inhibitors in BTCs. Three (18.7%) are open-label randomized multicenter phase 3 trials, 8 (50%) are single-arm phase two trials, and 4 (25%) are phase one studies. Twelve (17.9%) clinical trials dealt with isocitrate dehydrogenase (IDH) 1/2 targeting therapy either in combination with cisplatin (Cis) and gemcitabine (Gem) as first-line treatment for BTCs or in monotherapy in patients with IDH1 mutant advanced malignancies, including cholangiocarcinoma (CCA). Nine (13.4%) clinical trials tested human epidermal growth factor receptor (HER) 2 targeting therapy. Four (44.4%) studies are phase I trials, two (22.2%) are phase I/II trials, and three (33.3%) phase II trials. Rare molecular alterations in BTCs, such as anaplastic lymphoma kinase (ALK), c-ros oncogene1 receptor tyrosine kinase (ROS1), and v-RAF murine sarcoma viral oncogene homologue B1 (BRAF), are also under investigation in a few trials. Forty-four clinical trials (17.2%) are investigating not oncogenic-driven multitarget therapy like multireceptor tyrosin kinase inhibitors and antiangiogenetic agents. In conclusion, this review shows that BTCs management is experiencing important innovations, especially in biomarker-based patient selection and in the new emerging therapeutic approach. Many ongoing trials could answer questions regarding the role of molecular inhibitors leading to new therapeutic frontiers for molecular subcategories of BTCs.
DOI: https://doi.org/10.37349/etat.2021.00056
This article belongs to the special issue Precision Medicine for Cholangiocarcinoma

Lynch syndrome is a hereditary cancer predisposition syndrome caused by germline alterations in mismatch repair (MMR) genes leading to increased risk of colon cancer as well as other cancer types. Non-small cell lung cancer (NSCLC) is not among typical Lynch syndrome-associated tumors: pembrolizumab, an immune checkpoint inhibitor, is actually approved for the treatment of NSCLC patients and represents a promising treatment option for patients with advanced metastatic MMR-deficient cancer, regardless of tumor origin. This case report describes the clinical presentation and management of a 74-year-old female with a history of rectal adenocarcinoma and ovarian cancer, who has a documented frameshift pathogenic variant in the exon 8 of MSH6 gene and an intronic variant in the BRCA2 gene (classified as a variant of uncertain significance), affected by NSCLC with brain metastases. Despite these premises, the patient was treated with pembrolizumab and she did not benefit from this kind of treatment.
Lynch syndrome is a hereditary cancer predisposition syndrome caused by germline alterations in mismatch repair (MMR) genes leading to increased risk of colon cancer as well as other cancer types. Non-small cell lung cancer (NSCLC) is not among typical Lynch syndrome-associated tumors: pembrolizumab, an immune checkpoint inhibitor, is actually approved for the treatment of NSCLC patients and represents a promising treatment option for patients with advanced metastatic MMR-deficient cancer, regardless of tumor origin. This case report describes the clinical presentation and management of a 74-year-old female with a history of rectal adenocarcinoma and ovarian cancer, who has a documented frameshift pathogenic variant in the exon 8 of MSH6 gene and an intronic variant in the BRCA2 gene (classified as a variant of uncertain significance), affected by NSCLC with brain metastases. Despite these premises, the patient was treated with pembrolizumab and she did not benefit from this kind of treatment.
DOI: https://doi.org/10.37349/etat.2021.00044

Aim:
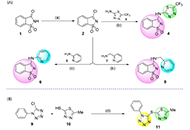
This study discloses the synthesis and the antimicrobial and anticancer activities of four molecules of structural basis saccharin-thiadiazolyl (4), saccharin-pyridyl (6, 8), and tetrazole-thiadiazolyl (11).
Methods:
Antimicrobial properties of the molecules were evaluated by the well-diffusion method, against Gram-positive bacteria [Staphylococcus aureus American Type Culture Collection (ATCC) 25923, Staphylococcus epidermidis ATCC 12228, Mycobacterium smegmatis ATCC 607], Gram-negative bacteria (Pseudomonas aeruginosa ATCC 27853) and yeast (Saccharomyces cerevisiae ATCC 2601 and Candida albicans ATCC 10231) strains. The anticancer activity of the compounds was assessed through i) proliferation assays for HCT116, MCF-7, and A375 human cell lines [cells were treated with serial dilutions of compounds and the effect on cell propagation was evaluated by sulforhodamine B (SRB) assay]; ii) antiproliferative and cytotoxic assays for glioma-type cell lines A172 (glioblastoma), U87 (brain-likely glioblastoma), and H4 (neuroglioma; cells were treated with diverse concentrations and the cell viability was assessed using a modified Alamar blue® assay).
Results:
Compound 11 exhibited significant inhibitory activity against S. aureus and S. epidermidis, with the further molecules demonstrating some inhibitory potential against all the tested Gram-positive, Gram-negative, and yeast strains. Similarly, derivative 11 showed an interesting antiproliferative activity against human colon adenocarcinoma (HCT116), human breast adenocarcinoma (MCF-7), and melanoma (A375) cells, with 50% growth inhibition (GI50) values varying from 3.55 µmol/L to 11.5 µmol/L, in the same order of magnitude of those shown by etoposide. Treatment of brain-like glioblastoma cells (U87) with 11, at the concentration of 100 µg/mL, induced a decrease in cell viability by 50% after 48 h and 72 h. Besides, results attained for A172 cells have shown that compound 11 only induces a significant decrease in cell viability upon treatment at 100 µg/mL for 72 h. A divergent observation was recorded for H4 cells, where the treatment with derivative 11 had promoted a significant decrease in cell viability (< 40–60%), even at concentrations as low as 0.39 µg/mL, after 24 h.
Conclusions:
This investigation reveals the potential of distinct azole-based conjugates, in particular the tetrazole-thiadiazolyl (11) derivative, as scaffolds worth further investigations, in the frame of antimicrobial and antineoplastic chemotherapy.
Aim:
This study discloses the synthesis and the antimicrobial and anticancer activities of four molecules of structural basis saccharin-thiadiazolyl (4), saccharin-pyridyl (6, 8), and tetrazole-thiadiazolyl (11).
Methods:
Antimicrobial properties of the molecules were evaluated by the well-diffusion method, against Gram-positive bacteria [Staphylococcus aureus American Type Culture Collection (ATCC) 25923, Staphylococcus epidermidis ATCC 12228, Mycobacterium smegmatis ATCC 607], Gram-negative bacteria (Pseudomonas aeruginosa ATCC 27853) and yeast (Saccharomyces cerevisiae ATCC 2601 and Candida albicans ATCC 10231) strains. The anticancer activity of the compounds was assessed through i) proliferation assays for HCT116, MCF-7, and A375 human cell lines [cells were treated with serial dilutions of compounds and the effect on cell propagation was evaluated by sulforhodamine B (SRB) assay]; ii) antiproliferative and cytotoxic assays for glioma-type cell lines A172 (glioblastoma), U87 (brain-likely glioblastoma), and H4 (neuroglioma; cells were treated with diverse concentrations and the cell viability was assessed using a modified Alamar blue® assay).
Results:
Compound 11 exhibited significant inhibitory activity against S. aureus and S. epidermidis, with the further molecules demonstrating some inhibitory potential against all the tested Gram-positive, Gram-negative, and yeast strains. Similarly, derivative 11 showed an interesting antiproliferative activity against human colon adenocarcinoma (HCT116), human breast adenocarcinoma (MCF-7), and melanoma (A375) cells, with 50% growth inhibition (GI50) values varying from 3.55 µmol/L to 11.5 µmol/L, in the same order of magnitude of those shown by etoposide. Treatment of brain-like glioblastoma cells (U87) with 11, at the concentration of 100 µg/mL, induced a decrease in cell viability by 50% after 48 h and 72 h. Besides, results attained for A172 cells have shown that compound 11 only induces a significant decrease in cell viability upon treatment at 100 µg/mL for 72 h. A divergent observation was recorded for H4 cells, where the treatment with derivative 11 had promoted a significant decrease in cell viability (< 40–60%), even at concentrations as low as 0.39 µg/mL, after 24 h.
Conclusions:
This investigation reveals the potential of distinct azole-based conjugates, in particular the tetrazole-thiadiazolyl (11) derivative, as scaffolds worth further investigations, in the frame of antimicrobial and antineoplastic chemotherapy.
DOI: https://doi.org/10.37349/eds.2023.00028

Aim:
This study provides a comparative analysis of oral dysbiosis of patients with periodontal diseases: chronic catarrhal gingivitis and generalized periodontitis, associated with various systemic pathologies, using a combination of the enzymatic method and interval scale. Studying the differences in the oral microbiota of patients with periodontal diseases and systemic pathologies can help comprehend the underlying mechanisms and create successful treatments.
Methods:
An enzymatic method was used to diagnose and monitor the degree of oral dysbiosis of patients with different systemic pathologies and periodontal diseases. We applied particular inclusion and exclusion criteria to include patients in a study. The level of microbial presence in the oral cavity can be measured by analyzing urease enzyme activity.
Results:
The research established that oral dysbiosis is observed in all groups of patients with periodontal diseases and systemic pathology: chronic colitis, chronic pancreatitis, and primary hypothyroidism. The article discusses an express method of diagnosing the microbiota of the oral cavity in combination with an interval scale. This combination makes it possible to classify patients according to the level of oral dysbiosis and prescribe further recommendations for treatment.
Conclusions:
The association of periodontitis and linked comorbidities is a complex interplay involving common risk factors, pathophysiology, and bidirectional causal relationships. The imbalance of microorganisms in the oral cavities of patients with systemic and periodontal diseases highlights the need for a personalized medical treatment approach. Correcting dysbiosis of the oral cavity should complement antimicrobial treatment for periodontal diseases and the normalization of metabolic processes in the periodontium. It has been confirmed that there is a correlation between patients’ microbial colonization of the oral cavity and the values obtained by the enzymatic method, suggesting that this approach can serve as a rapid assessment of the oral cavity’s microbiocenosis.
Aim:
This study provides a comparative analysis of oral dysbiosis of patients with periodontal diseases: chronic catarrhal gingivitis and generalized periodontitis, associated with various systemic pathologies, using a combination of the enzymatic method and interval scale. Studying the differences in the oral microbiota of patients with periodontal diseases and systemic pathologies can help comprehend the underlying mechanisms and create successful treatments.
Methods:
An enzymatic method was used to diagnose and monitor the degree of oral dysbiosis of patients with different systemic pathologies and periodontal diseases. We applied particular inclusion and exclusion criteria to include patients in a study. The level of microbial presence in the oral cavity can be measured by analyzing urease enzyme activity.
Results:
The research established that oral dysbiosis is observed in all groups of patients with periodontal diseases and systemic pathology: chronic colitis, chronic pancreatitis, and primary hypothyroidism. The article discusses an express method of diagnosing the microbiota of the oral cavity in combination with an interval scale. This combination makes it possible to classify patients according to the level of oral dysbiosis and prescribe further recommendations for treatment.
Conclusions:
The association of periodontitis and linked comorbidities is a complex interplay involving common risk factors, pathophysiology, and bidirectional causal relationships. The imbalance of microorganisms in the oral cavities of patients with systemic and periodontal diseases highlights the need for a personalized medical treatment approach. Correcting dysbiosis of the oral cavity should complement antimicrobial treatment for periodontal diseases and the normalization of metabolic processes in the periodontium. It has been confirmed that there is a correlation between patients’ microbial colonization of the oral cavity and the values obtained by the enzymatic method, suggesting that this approach can serve as a rapid assessment of the oral cavity’s microbiocenosis.
DOI: https://doi.org/10.37349/emed.2024.00241
This article belongs to the special issue Oral Health Interconnections and Multidisciplinary Approaches

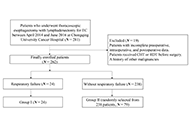
Aim:
Respiratory failure is common after esophagectomy for esophageal cancer (EC). This study aimed to identify the risk factors associated with postoperative respiratory failure following esophagectomy for EC.
Methods:
A single-center observational study from China was conducted on 262 patients with EC who underwent thoracoscopic esophagectomy between April 2014 and June 2016. The patients were divided into two groups: group I (respiratory failure) and group II (without respiratory failure). Demographic and perioperative variables, tumor-related factors, surgical factors, Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, and clinical course were compared between the groups. Univariable and multivariable logistic regression analyses were performed to assess the risk factors of postoperative respiratory failure after esophagectomy.
Results:
Among the 262 patients, 24 (9.2%) developed respiratory failure. Univariable analysis revealed several risk factors, including age, smoking, comorbidities, partial pressure of oxygen (PO2), partial pressure of carbon dioxide (PCO2), forced vital capacity (FVC), FVC percentage (FVC%), urine volume during surgery, and APACHE II score. Multivariable analysis showed that age, comorbidities of diabetes mellitus (DM), FVC%, urine volume during surgery, and APACHE II score were independent predictors of respiratory failure. Specifically, elderly patients (> 65 years) with comorbidities of DM, lower FVC%, higher urine volume during surgery, and elevated APACHE II score were found to be more susceptible to respiratory failure, resulting in prolonged hospitalization and increased healthcare burden. These findings emphasize the importance of considering these factors in the management and care of patients at risk of respiratory failure.
Conclusions:
As a common complication following esophagectomy for EC. Respiratory failure is significantly associated with age, comorbidities of DM, FVC%, urine volume during surgery, and APACHE II score in the dataset. The findings will contribute to the evaluation of the risk of respiratory failure and guide early intervention strategies in clinical decision-making.
Aim:
Respiratory failure is common after esophagectomy for esophageal cancer (EC). This study aimed to identify the risk factors associated with postoperative respiratory failure following esophagectomy for EC.
Methods:
A single-center observational study from China was conducted on 262 patients with EC who underwent thoracoscopic esophagectomy between April 2014 and June 2016. The patients were divided into two groups: group I (respiratory failure) and group II (without respiratory failure). Demographic and perioperative variables, tumor-related factors, surgical factors, Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, and clinical course were compared between the groups. Univariable and multivariable logistic regression analyses were performed to assess the risk factors of postoperative respiratory failure after esophagectomy.
Results:
Among the 262 patients, 24 (9.2%) developed respiratory failure. Univariable analysis revealed several risk factors, including age, smoking, comorbidities, partial pressure of oxygen (PO2), partial pressure of carbon dioxide (PCO2), forced vital capacity (FVC), FVC percentage (FVC%), urine volume during surgery, and APACHE II score. Multivariable analysis showed that age, comorbidities of diabetes mellitus (DM), FVC%, urine volume during surgery, and APACHE II score were independent predictors of respiratory failure. Specifically, elderly patients (> 65 years) with comorbidities of DM, lower FVC%, higher urine volume during surgery, and elevated APACHE II score were found to be more susceptible to respiratory failure, resulting in prolonged hospitalization and increased healthcare burden. These findings emphasize the importance of considering these factors in the management and care of patients at risk of respiratory failure.
Conclusions:
As a common complication following esophagectomy for EC. Respiratory failure is significantly associated with age, comorbidities of DM, FVC%, urine volume during surgery, and APACHE II score in the dataset. The findings will contribute to the evaluation of the risk of respiratory failure and guide early intervention strategies in clinical decision-making.
DOI: https://doi.org/10.37349/emed.2023.00195

Aim:
The study aims to evaluate the incidence of recurrent thromboses in patients with primary antiphospholipid syndrome (PAPS) and its association with the presence of different antiphospholipid antibodies (aPLs) and known thrombogenic risk factors.
Methods:
This retrospective study included 52 patients. The median age of the patients was 38.5 years [31.5; 43.5], and the duration of the disease was 9.0 years [3.1; 13.0]. aPLs, including IgG/IgM/IgA antibodies to cardiolipin (aCLs), IgG/IgM/IgA anti-beta2-glycoprotein I (anti-β2-GPI), IgG anti-domain I-β2-GPI (anti-β2-GPIDI) antibodies, IgG/IgM antibodies to the phosphatidylserine/prothrombin complex (aPS/PT), and other thrombosis risk factors were included for analysis.
Results:
Recurrent thrombosis was reported in 34 (65%) out of 52 patients and 18 (35%) did not have it. The main reason for the recurrence of thrombosis was the lack of anticoagulant therapy: in 18 (52.9%) out of 34 patients with recurrent thrombosis. Three patients were taking warfarin at the time of thrombosis recurrence, but target international normalized ratio (INR) levels were not achieved. Other patients with recurrent thrombosis were taking direct oral anticoagulants (DOACs). The risk of recurrent thrombotic events with positive IgG aCL was 10.33 (P = 0.002) and 21 (P = 0.007) times higher were examined in enzyme-linked immunoassay (ELISA) and chemiluminescent assay (CLA), respectively. The risk of thrombosis was 4.58 times higher in patients who were IgA aCL-positive (P = 0.01). Compared with other antibodies, with positive IgG values of anti-β2-GPI and IgG aPS/PT by ELISA, a lower probability of thrombosis recurrence was observed: 7.56 and 7.25, respectively. A high risk of recurrent thrombosis [odds ratio (OR) = 32.0] was observed in IgG anti-β2-GPI (CLA). The combination of IgG aCL with IgG anti-β2-GPI and with IgG anti-β2-GPIDI is more informative with respect to the risks of thrombosis recurrence compared to double positivity for aCL with anti-β2-GPI (OR = 20.71 vs. OR = 10.18). Triple positivity for IgG aCL with IgG anti-β2-GPI and with IgG aPS/PT also shows better results compared to positivity for aCL with anti-β2-GPI (OR = 6.06 vs. OR = 5.79). Among other risk factors, arterial hypertension (AH) and obesity were significant in relation to the recurrence of thrombosis. AH occurred in 22 (42%) of 52 patients with PAPS. AH was associated with recurrent thrombosis in PAPS patients: 18 (53%) out of 34 with recurrent thrombosis had AH versus 4 out of 18 without recurrent thrombosis (P = 0.003).
Conclusions:
Recurrent thrombosis in antiphospholipid syndrome (APS) is largely associated with IgG aCL, IgG anti-β2-GPI, IgG anti-β2-GPIDI, IgG aPS/PT, and IgA aCL positivity. AH was a significant risk factor for recurrent thrombosis.
Aim:
The study aims to evaluate the incidence of recurrent thromboses in patients with primary antiphospholipid syndrome (PAPS) and its association with the presence of different antiphospholipid antibodies (aPLs) and known thrombogenic risk factors.
Methods:
This retrospective study included 52 patients. The median age of the patients was 38.5 years [31.5; 43.5], and the duration of the disease was 9.0 years [3.1; 13.0]. aPLs, including IgG/IgM/IgA antibodies to cardiolipin (aCLs), IgG/IgM/IgA anti-beta2-glycoprotein I (anti-β2-GPI), IgG anti-domain I-β2-GPI (anti-β2-GPIDI) antibodies, IgG/IgM antibodies to the phosphatidylserine/prothrombin complex (aPS/PT), and other thrombosis risk factors were included for analysis.
Results:
Recurrent thrombosis was reported in 34 (65%) out of 52 patients and 18 (35%) did not have it. The main reason for the recurrence of thrombosis was the lack of anticoagulant therapy: in 18 (52.9%) out of 34 patients with recurrent thrombosis. Three patients were taking warfarin at the time of thrombosis recurrence, but target international normalized ratio (INR) levels were not achieved. Other patients with recurrent thrombosis were taking direct oral anticoagulants (DOACs). The risk of recurrent thrombotic events with positive IgG aCL was 10.33 (P = 0.002) and 21 (P = 0.007) times higher were examined in enzyme-linked immunoassay (ELISA) and chemiluminescent assay (CLA), respectively. The risk of thrombosis was 4.58 times higher in patients who were IgA aCL-positive (P = 0.01). Compared with other antibodies, with positive IgG values of anti-β2-GPI and IgG aPS/PT by ELISA, a lower probability of thrombosis recurrence was observed: 7.56 and 7.25, respectively. A high risk of recurrent thrombosis [odds ratio (OR) = 32.0] was observed in IgG anti-β2-GPI (CLA). The combination of IgG aCL with IgG anti-β2-GPI and with IgG anti-β2-GPIDI is more informative with respect to the risks of thrombosis recurrence compared to double positivity for aCL with anti-β2-GPI (OR = 20.71 vs. OR = 10.18). Triple positivity for IgG aCL with IgG anti-β2-GPI and with IgG aPS/PT also shows better results compared to positivity for aCL with anti-β2-GPI (OR = 6.06 vs. OR = 5.79). Among other risk factors, arterial hypertension (AH) and obesity were significant in relation to the recurrence of thrombosis. AH occurred in 22 (42%) of 52 patients with PAPS. AH was associated with recurrent thrombosis in PAPS patients: 18 (53%) out of 34 with recurrent thrombosis had AH versus 4 out of 18 without recurrent thrombosis (P = 0.003).
Conclusions:
Recurrent thrombosis in antiphospholipid syndrome (APS) is largely associated with IgG aCL, IgG anti-β2-GPI, IgG anti-β2-GPIDI, IgG aPS/PT, and IgA aCL positivity. AH was a significant risk factor for recurrent thrombosis.
DOI: https://doi.org/10.37349/ei.2023.00114
This article belongs to the special issue Autoantibodies Associated to Thrombosis and Hemostasis

Aim:
The present study investigates the influence of various homogenization techniques, namely high-pressure valve homogenization and microfluidization, and different forms of modified sunflower lecithin, including deoiled (DL) and hydrolyzed (HL) variants, on the development of monolayer and bilayer nanoemulsions of chia oil.
Methods:
Oil-in-water (O/W) nanoemulsions with 5% chia seed oil were prepared using simple (0.5% DL or HL) or double-layer [0.5% DL or HL and 0.3% chitosan (Ch)] stabilization. This involved a two-step homogenization process, utilizing either microfluidization or high-pressure valve homogenization. Chia oil nanoemulsions were characterized by their zeta potential, particle size, and rheological properties. Besides, their physical stability and omega-3 content during refrigerated storage were evaluated.
Results:
Overall, the studied modified sunflower lecithin (DL and HL) demonstrated effective capability in stabilizing chia nanoemulsions and facilitating the formation of the double-layered structure following Ch deposition. Concerning the homogenization method, it has been demonstrated that under the same homogenization conditions, microfluidization resulted in significantly smaller droplet sizes and higher apparent viscosities compared to high-pressure valve homogenization. This discrepancy can be attributed to the design of the homogenization chambers, as microfluidization generates a narrow distribution of shear forces, while high-pressure valve homogenization yields a much broader distribution. In contrast to chia monolayer nanoemulsions, the nanoemulsions stabilized by modified sunflower lecithin-Ch demonstrated a noteworthy improvement in their overall stability. This enhancement can be ascribed to their increased apparent viscosity and the highly charged interfaces of the droplets. Furthermore, throughout the entire refrigerated storage period, the omega-3 content in all nanoemulsions remained unchanged.
Conclusions:
In this study, mono and bilayer chia oil nanoemulsions were successfully obtained using modified sunflower lecithin and high-energy techniques. Microfluidization outperformed high-pressure valve homogenization, resulting in smaller droplets and increased viscosity. These findings are relevant for designing stable chia oil nanoemulsions with natural components, offering substantial health benefits.
Aim:
The present study investigates the influence of various homogenization techniques, namely high-pressure valve homogenization and microfluidization, and different forms of modified sunflower lecithin, including deoiled (DL) and hydrolyzed (HL) variants, on the development of monolayer and bilayer nanoemulsions of chia oil.
Methods:
Oil-in-water (O/W) nanoemulsions with 5% chia seed oil were prepared using simple (0.5% DL or HL) or double-layer [0.5% DL or HL and 0.3% chitosan (Ch)] stabilization. This involved a two-step homogenization process, utilizing either microfluidization or high-pressure valve homogenization. Chia oil nanoemulsions were characterized by their zeta potential, particle size, and rheological properties. Besides, their physical stability and omega-3 content during refrigerated storage were evaluated.
Results:
Overall, the studied modified sunflower lecithin (DL and HL) demonstrated effective capability in stabilizing chia nanoemulsions and facilitating the formation of the double-layered structure following Ch deposition. Concerning the homogenization method, it has been demonstrated that under the same homogenization conditions, microfluidization resulted in significantly smaller droplet sizes and higher apparent viscosities compared to high-pressure valve homogenization. This discrepancy can be attributed to the design of the homogenization chambers, as microfluidization generates a narrow distribution of shear forces, while high-pressure valve homogenization yields a much broader distribution. In contrast to chia monolayer nanoemulsions, the nanoemulsions stabilized by modified sunflower lecithin-Ch demonstrated a noteworthy improvement in their overall stability. This enhancement can be ascribed to their increased apparent viscosity and the highly charged interfaces of the droplets. Furthermore, throughout the entire refrigerated storage period, the omega-3 content in all nanoemulsions remained unchanged.
Conclusions:
In this study, mono and bilayer chia oil nanoemulsions were successfully obtained using modified sunflower lecithin and high-energy techniques. Microfluidization outperformed high-pressure valve homogenization, resulting in smaller droplets and increased viscosity. These findings are relevant for designing stable chia oil nanoemulsions with natural components, offering substantial health benefits.
DOI: https://doi.org/10.37349/eff.2024.00029
This article belongs to the special issue Delivery of Hydrophobic Compounds in Food Systems

The influenza virus glycoprotein hemagglutinin (HA) participates in critical steps of the attachment of viral particles to the host cell membrane receptor and membrane fusion. Due to its crucial involvement in the initial phases of influenza A infections, HA emerges as a promising target in the search of novel drug-like candidates. Given its pivotal role in the early stages of influenza A infections, intense drug discovery efforts have been undertaken to target HA in the past decades. Drug discovery studies mainly rely on preventing the recognition of sialic acid units by the receptor binding site in the globular head (GH) domain, or the conformational rearrangement required for the fusion of viral and cell membranes. In this work, the aim is to summarize the progress made in HA-targeted development of small molecule fusion inhibitors. To this end, attention will primarily be focused on the analysis of the X-ray crystallographic structures of HA bound to fusion inhibitors. Furthermore, this study also aims to highlight the efforts made in exploiting the structural information in conjunction with molecular modeling techniques to discern the mechanism of action of the fusion inhibitors and to assist the design and interpretation of structure-activity relationships of novel lead compounds will be highlighted. The final section will be dedicated to elucidating novel and promising antiviral strategies proceeding from the transformation of known small molecule antivirals in proteolysis targeting chimera (PROTAC)-based targeted protein degradation. This knowledge will be valuable to assist the exploitation of classical and novel antiviral structure-based strategies, together with a deeper understanding of the mechanism of action and minimization of the impact of drug resistance.
The influenza virus glycoprotein hemagglutinin (HA) participates in critical steps of the attachment of viral particles to the host cell membrane receptor and membrane fusion. Due to its crucial involvement in the initial phases of influenza A infections, HA emerges as a promising target in the search of novel drug-like candidates. Given its pivotal role in the early stages of influenza A infections, intense drug discovery efforts have been undertaken to target HA in the past decades. Drug discovery studies mainly rely on preventing the recognition of sialic acid units by the receptor binding site in the globular head (GH) domain, or the conformational rearrangement required for the fusion of viral and cell membranes. In this work, the aim is to summarize the progress made in HA-targeted development of small molecule fusion inhibitors. To this end, attention will primarily be focused on the analysis of the X-ray crystallographic structures of HA bound to fusion inhibitors. Furthermore, this study also aims to highlight the efforts made in exploiting the structural information in conjunction with molecular modeling techniques to discern the mechanism of action of the fusion inhibitors and to assist the design and interpretation of structure-activity relationships of novel lead compounds will be highlighted. The final section will be dedicated to elucidating novel and promising antiviral strategies proceeding from the transformation of known small molecule antivirals in proteolysis targeting chimera (PROTAC)-based targeted protein degradation. This knowledge will be valuable to assist the exploitation of classical and novel antiviral structure-based strategies, together with a deeper understanding of the mechanism of action and minimization of the impact of drug resistance.
DOI: https://doi.org/10.37349/eds.2024.00037

Aim:
The present study aims to generate chimeric mouse single-chain variable fragment (scFv) and immunoglobulin G1 (IgG1) crystallizable fragment (Fc) antibody against disialoganglioside (GD2) for the treatment of neuroblastoma (NB). The generated scFv-IgG
Methods:
Vector for scFv-IgG
Results:
Using plasmid fusion-human IgG1-Fc2 tag vector (pFUSE-hIgG1-Fc2), a plasmid vector encoding chimeric mouse scFv and hIgG1
Conclusions:
The results indicate that chimeric scFv-hIgG
Aim:
The present study aims to generate chimeric mouse single-chain variable fragment (scFv) and immunoglobulin G1 (IgG1) crystallizable fragment (Fc) antibody against disialoganglioside (GD2) for the treatment of neuroblastoma (NB). The generated scFv-IgG
Methods:
Vector for scFv-IgG
Results:
Using plasmid fusion-human IgG1-Fc2 tag vector (pFUSE-hIgG1-Fc2), a plasmid vector encoding chimeric mouse scFv and hIgG1
Conclusions:
The results indicate that chimeric scFv-hIgG
DOI: https://doi.org/10.37349/etat.2023.00188
This article belongs to the special issue Novel Strategies and Targets for Immunotherapy of Cancer

Third-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) have shown impressive results in EGFR mutant lung cancer (LC) patients in terms of disease control rate with a positive impact on overall survival. Nevertheless, after months of treatment with targeted therapy, progression inevitably occurs. Some patients develop oligoprogression and local treatment is required for optimal disease control while maintaining EGFR-TKIs. This work features a clinical case of a patient harboring an EGFR mutant LC undergoing oligoprogression to EGFR-TKIs, first into the brain and afterward to the primary tumor, requiring local ablative strategies, including primary tumor resection three years after the start of osimertinib. Currently, the patient is still alive and continues with a complete response upon EGFR-TKIs maintenance. Hence, oligoprogression, even in driven oncogenic tumors, represents a distinct biological entity and potential curative disease that deserves particular consideration in multidisciplinary tumor boards. In this case, tumor primary resection after three years of the initial diagnosis represents a paradigm shift in the treatment of EGFR mutant patients.
Third-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) have shown impressive results in EGFR mutant lung cancer (LC) patients in terms of disease control rate with a positive impact on overall survival. Nevertheless, after months of treatment with targeted therapy, progression inevitably occurs. Some patients develop oligoprogression and local treatment is required for optimal disease control while maintaining EGFR-TKIs. This work features a clinical case of a patient harboring an EGFR mutant LC undergoing oligoprogression to EGFR-TKIs, first into the brain and afterward to the primary tumor, requiring local ablative strategies, including primary tumor resection three years after the start of osimertinib. Currently, the patient is still alive and continues with a complete response upon EGFR-TKIs maintenance. Hence, oligoprogression, even in driven oncogenic tumors, represents a distinct biological entity and potential curative disease that deserves particular consideration in multidisciplinary tumor boards. In this case, tumor primary resection after three years of the initial diagnosis represents a paradigm shift in the treatment of EGFR mutant patients.
DOI: https://doi.org/10.37349/etat.2023.00191

The pathophysiological mechanisms underlying the close relationship between nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM) are multiple, complex and only partially known. The purpose of this paper was to review the current knowledge of these mechanisms in a unified manner. Subjects with NAFLD and T2DM have established insulin resistance (IR), which exacerbates the two comorbidities. IR worsens NAFLD by increasing the accumulation of free fatty acids (FFAs) in the liver. This occurs due to an increase in the influx of FFAs from peripheral adipose tissue by the activation of hormone-sensitive lipase. In addition, there is de novo increased lipogenesis, a transcription factor, the sterols regulatory element-binding transcription factor 1c (SREBP-1c), which activates the expression of several genes strongly promotes lipogenesis by the liver and facilitate storage of triglycerides. Lipids accumulation in the liver induces a chronic stress in the endoplasmic reticulum of the hepatocytes. Genome-wide association studies have identified genetic variants associated with NAFLD severity, but unrelated to IR. In particular, the alteration of patatin-like phospholipase domain-containing protein 3 contributes to the susceptibility to NAFLD. Furthermore, the lipotoxicity of ceramides and diacylglycerol, well known in T2DM, triggers a chronic inflammatory process favoring the progression from hepatic steatosis to steatohepatitis. Reactive oxygen species produced by mitochondrial dysfunction trigger both liver inflammation and beta-cells damage, promoting the progression of both NAFLD and T2DM. The close association between NAFLD and T2DM is bidirectional, as T2DM may trigger both NAFLD onset and its progression, but NAFLD itself may contribute to the development of IR and T2DM. Future studies on the mechanisms will have to deepen the knowledge of the interaction between the two pathologies and should allow the identification of new therapeutic targets for the treatment of NAFLD, currently substantially absent.
The pathophysiological mechanisms underlying the close relationship between nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM) are multiple, complex and only partially known. The purpose of this paper was to review the current knowledge of these mechanisms in a unified manner. Subjects with NAFLD and T2DM have established insulin resistance (IR), which exacerbates the two comorbidities. IR worsens NAFLD by increasing the accumulation of free fatty acids (FFAs) in the liver. This occurs due to an increase in the influx of FFAs from peripheral adipose tissue by the activation of hormone-sensitive lipase. In addition, there is de novo increased lipogenesis, a transcription factor, the sterols regulatory element-binding transcription factor 1c (SREBP-1c), which activates the expression of several genes strongly promotes lipogenesis by the liver and facilitate storage of triglycerides. Lipids accumulation in the liver induces a chronic stress in the endoplasmic reticulum of the hepatocytes. Genome-wide association studies have identified genetic variants associated with NAFLD severity, but unrelated to IR. In particular, the alteration of patatin-like phospholipase domain-containing protein 3 contributes to the susceptibility to NAFLD. Furthermore, the lipotoxicity of ceramides and diacylglycerol, well known in T2DM, triggers a chronic inflammatory process favoring the progression from hepatic steatosis to steatohepatitis. Reactive oxygen species produced by mitochondrial dysfunction trigger both liver inflammation and beta-cells damage, promoting the progression of both NAFLD and T2DM. The close association between NAFLD and T2DM is bidirectional, as T2DM may trigger both NAFLD onset and its progression, but NAFLD itself may contribute to the development of IR and T2DM. Future studies on the mechanisms will have to deepen the knowledge of the interaction between the two pathologies and should allow the identification of new therapeutic targets for the treatment of NAFLD, currently substantially absent.
DOI: https://doi.org/10.37349/emed.2020.00019
This article belongs to the special issue Exploring NAFLD/NASH

Aim:
Aloysia citrodora has a long history of traditional use in treating various ailments. This study evaluated the in vivo chemopreventive efficacy and systemic toxicity of an extract of A. citrodora in a transgenic mouse model of HPV16 (human papillomavirus type 16)-induced cancer.
Methods:
The experiment involved six groups (n = 5): group 1 (G1, wild-type (WT), water), group 2 (G2, HPV, water), group 3 (G3, WT, 0.013 g/mL), group 4 (G4, HPV, 0.006 g/mL), group 5 (G5, HPV, 0.008 g/mL), and group 6 (G6, HPV, 0.013 g/mL). Throughout the assay, humane endpoints, body weight, food, and water consumption were recorded weekly. The internal organs and skin of the mice were collected for analysis after they were sacrificed. Toxicological parameters that were studied included hematological and biochemical blood markers, splenic and hepatic histology, and hepatic oxidative stress.
Results:
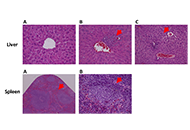
A. citrodora extract seems to reduce the incidence of dysplastic and in situ carcinoma skin lesions induced by HPV16 in this model, suggesting that dietary supplementation with concentrations of 0.008 g/mL and 0.013 g/mL may have beneficial chemopreventive effects.
Conclusions:
The extract did not induce any concentration-dependent toxicological effects on any of the parameters included in the study, indicating a favorable toxicological profile under these experimental conditions.
Aim:
Aloysia citrodora has a long history of traditional use in treating various ailments. This study evaluated the in vivo chemopreventive efficacy and systemic toxicity of an extract of A. citrodora in a transgenic mouse model of HPV16 (human papillomavirus type 16)-induced cancer.
Methods:
The experiment involved six groups (n = 5): group 1 (G1, wild-type (WT), water), group 2 (G2, HPV, water), group 3 (G3, WT, 0.013 g/mL), group 4 (G4, HPV, 0.006 g/mL), group 5 (G5, HPV, 0.008 g/mL), and group 6 (G6, HPV, 0.013 g/mL). Throughout the assay, humane endpoints, body weight, food, and water consumption were recorded weekly. The internal organs and skin of the mice were collected for analysis after they were sacrificed. Toxicological parameters that were studied included hematological and biochemical blood markers, splenic and hepatic histology, and hepatic oxidative stress.
Results:
A. citrodora extract seems to reduce the incidence of dysplastic and in situ carcinoma skin lesions induced by HPV16 in this model, suggesting that dietary supplementation with concentrations of 0.008 g/mL and 0.013 g/mL may have beneficial chemopreventive effects.
Conclusions:
The extract did not induce any concentration-dependent toxicological effects on any of the parameters included in the study, indicating a favorable toxicological profile under these experimental conditions.
DOI: https://doi.org/10.37349/emed.2024.00228

Aim:
The association of echocardiographic findings and subsequent risk of left-sided native valve endocarditis (LS-NVE) is undefined. The aim of this study was to determine if transthoracic echocardiography (TTE) measurements are associated with the subsequent development of LS-NVE in patients without cardiac predisposing conditions.
Methods:
Institutional databases were evaluated for adults diagnosed with LS-NVE from 2008 to 2020. Patients with prosthetic valves, cardiovascular implantable electronic devices, intracardiac devices, injection drug use, and predisposing cardiac conditions were excluded. Only patients who had a TTE performed 6 months to 3 years before the development of LS-NVE were included as cases. Controls were patients within the same Mayo location with a TTE report and were matched in a 1:3 ratio according to age, gender, Charlson comorbidity index, and echocardiography date.
Results:
There were 148 cases and 431 matched controls. As compared to controls, infective endocarditis (IE) cases had a higher prevalence of diabetes mellitus (46.6% vs. 30.4%) and chronic kidney disease (46.6% vs. 28.1%) (P < 0.001). Left ventricular outflow tract velocity (P = 0.017), left ventricular ejection fraction (P = 0.018), and E:e’ ratio (P = 0.050) were associated with LS-NVE.
Conclusions:
Echocardiographic measurements were associated with subsequent LS-NVE development in this pilot study. A larger cohort of LS-NVE patients, however, is needed to validate these findings.
Aim:
The association of echocardiographic findings and subsequent risk of left-sided native valve endocarditis (LS-NVE) is undefined. The aim of this study was to determine if transthoracic echocardiography (TTE) measurements are associated with the subsequent development of LS-NVE in patients without cardiac predisposing conditions.
Methods:
Institutional databases were evaluated for adults diagnosed with LS-NVE from 2008 to 2020. Patients with prosthetic valves, cardiovascular implantable electronic devices, intracardiac devices, injection drug use, and predisposing cardiac conditions were excluded. Only patients who had a TTE performed 6 months to 3 years before the development of LS-NVE were included as cases. Controls were patients within the same Mayo location with a TTE report and were matched in a 1:3 ratio according to age, gender, Charlson comorbidity index, and echocardiography date.
Results:
There were 148 cases and 431 matched controls. As compared to controls, infective endocarditis (IE) cases had a higher prevalence of diabetes mellitus (46.6% vs. 30.4%) and chronic kidney disease (46.6% vs. 28.1%) (P < 0.001). Left ventricular outflow tract velocity (P = 0.017), left ventricular ejection fraction (P = 0.018), and E:e’ ratio (P = 0.050) were associated with LS-NVE.
Conclusions:
Echocardiographic measurements were associated with subsequent LS-NVE development in this pilot study. A larger cohort of LS-NVE patients, however, is needed to validate these findings.
DOI: https://doi.org/10.37349/ec.2024.00034

Aim:
Inositol 1,4,5-trisphosphate receptor (IP3R) is a ubiquitous calcium (Ca2+) channel involved in the regulation of cellular fate and motility. Its modulation by anti-apoptotic protein B-cell lymphoma 2 (Bcl-2) plays an important role in cancer progression. Disrupting this interaction could overcome apoptosis avoidance, one of the hallmarks of cancer, and is, thus, of great interest. Earlier reports have shown the involvement of both the Bcl-2 homology 4 (BH4) and the transmembrane domains (TMDs) of Bcl-2 in regulating IP3R activity, while the Bcl-2 hydrophobic cleft was associated primarily with its anti-apoptotic and IP3R-independent action at the mitochondria (Oncotarget. 2016;7:55704–20. doi: 10.18632/oncotarget.11005). The aim of this study was to investigate how targeting the BH3 hydrophobic cleft of Bcl-2 affects IP3R:Bcl-2 interaction.
Methods:
Organelle membrane-derived (OMD) patch-clamp and circular dichroism (CD) thermal melting experiments were used to elucidate the effects of the ABT-199 (venetoclax) on the IP3R:Bcl-2 interaction. Molecular dynamics (MD) simulations of free and ABT-199 bound Bcl-2 were used to propose a molecular model of such interaction.
Results:
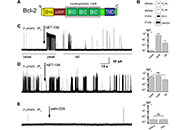
It was shown that occlusion of Bcl-2’s hydrophobic cleft by the drug ABT-199 finely modulates IP3R gating in the low open probability (Po) regime, characteristic of the basal IP3R activity in non-excited cells. Complementary MD simulations allowed to propose a model of this modulation, involving an allosteric interaction with the BH4 domain on the opposite side of Bcl-2.
Conclusions:
Bcl-2 is an important regulator of IP3R activity and, thus of Ca2+ release from internal stores and associated processes, including cellular proliferation and death. The presence of multiple regulatory domains in both proteins suggests a complex interaction. Thus, it was found that the occlusion of the hydrophobic cleft of Bcl-2 by ABT-199 disrupts IP3R activity, leading to Bcl-2 rebinding with smaller affinity and lesser inhibitory effect. MDs simulations of free and ABT-199 bound Bcl-2 propose a molecular model of such disruption, involving an allosteric interaction with the BH4 domain on the opposite side of Bcl-2.
Aim:
Inositol 1,4,5-trisphosphate receptor (IP3R) is a ubiquitous calcium (Ca2+) channel involved in the regulation of cellular fate and motility. Its modulation by anti-apoptotic protein B-cell lymphoma 2 (Bcl-2) plays an important role in cancer progression. Disrupting this interaction could overcome apoptosis avoidance, one of the hallmarks of cancer, and is, thus, of great interest. Earlier reports have shown the involvement of both the Bcl-2 homology 4 (BH4) and the transmembrane domains (TMDs) of Bcl-2 in regulating IP3R activity, while the Bcl-2 hydrophobic cleft was associated primarily with its anti-apoptotic and IP3R-independent action at the mitochondria (Oncotarget. 2016;7:55704–20. doi: 10.18632/oncotarget.11005). The aim of this study was to investigate how targeting the BH3 hydrophobic cleft of Bcl-2 affects IP3R:Bcl-2 interaction.
Methods:
Organelle membrane-derived (OMD) patch-clamp and circular dichroism (CD) thermal melting experiments were used to elucidate the effects of the ABT-199 (venetoclax) on the IP3R:Bcl-2 interaction. Molecular dynamics (MD) simulations of free and ABT-199 bound Bcl-2 were used to propose a molecular model of such interaction.
Results:
It was shown that occlusion of Bcl-2’s hydrophobic cleft by the drug ABT-199 finely modulates IP3R gating in the low open probability (Po) regime, characteristic of the basal IP3R activity in non-excited cells. Complementary MD simulations allowed to propose a model of this modulation, involving an allosteric interaction with the BH4 domain on the opposite side of Bcl-2.
Conclusions:
Bcl-2 is an important regulator of IP3R activity and, thus of Ca2+ release from internal stores and associated processes, including cellular proliferation and death. The presence of multiple regulatory domains in both proteins suggests a complex interaction. Thus, it was found that the occlusion of the hydrophobic cleft of Bcl-2 by ABT-199 disrupts IP3R activity, leading to Bcl-2 rebinding with smaller affinity and lesser inhibitory effect. MDs simulations of free and ABT-199 bound Bcl-2 propose a molecular model of such disruption, involving an allosteric interaction with the BH4 domain on the opposite side of Bcl-2.
DOI: https://doi.org/10.37349/etat.2022.00088
This article belongs to the special issue The Role of Bcl-2 Family Proteins in Cancer Progression and Their Relevance to Cancer Therapy

Aim:
This study evaluates the in vitro and in vivo antiplasmodial, hemolytic, and antioxidant activities of a combined extract of Ageratum conyzoides (A. conyzoides) and Bidens pilosa (B. pilosa), a traditionally used but scientifically unvalidated combination.
Methods:
Plant leaves were extracted via aqueous decoction and cold maceration, combining equal parts to mimic traditional preparation. In vitro antiplasmodial activity against the chloroquine-sensitive Plasmodium falciparum 3D7 (Pf3D7) strain was assessed using the SYBR Green I assay. Cytotoxicity was evaluated via hemolysis test, and antioxidant potential using DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS [2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)], and FRAP (ferric ion reducing antioxidant potential) assays. The most potent combination was tested for acute toxicity and curative antimalarial activity in a rodent model.
Results:
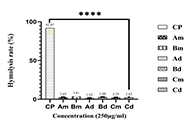
Extract yields ranged from 6.6% (cold maceration extract of B. pilosa) to 29.2% [aqueous decoction extract of combination (Cd)]. Extracts showed moderate to mild in vitro antiplasmodial activity [IC50 (median inhibitory concentration): 24.8–96.6 µg/mL], with the aqueous Cd showing potential synergism [CI (combination index) < 1]. No significant cytotoxicity was observed (< 10% hemolysis). Moderate to good antioxidant activity was found in DPPH [SC50 (median scavenging concentration): 134.65–307.55 µg/mL] and ABTS assays (SC50: 92.23–183.45 µg/mL), with Cd showing the highest activity. FRAP values were low. The Cd extract demonstrated no significant acute toxicity up to 5,000 mg/kg and significant in vivo antimalarial activity, achieving 65% parasite inhibition at 200 mg/kg/day. It also prolonged survival time, with a maximum survival of 28 days at 200 mg/kg/day.
Conclusions:
This preliminary investigation suggests that combined extracts of A. conyzoides and B. pilosa exhibit noteworthy in vitro and in vivo antiplasmodial activity against the tested strains. Further studies are warranted to validate these findings and develop optimized formulations as potential antimalarials.
Aim:
This study evaluates the in vitro and in vivo antiplasmodial, hemolytic, and antioxidant activities of a combined extract of Ageratum conyzoides (A. conyzoides) and Bidens pilosa (B. pilosa), a traditionally used but scientifically unvalidated combination.
Methods:
Plant leaves were extracted via aqueous decoction and cold maceration, combining equal parts to mimic traditional preparation. In vitro antiplasmodial activity against the chloroquine-sensitive Plasmodium falciparum 3D7 (Pf3D7) strain was assessed using the SYBR Green I assay. Cytotoxicity was evaluated via hemolysis test, and antioxidant potential using DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS [2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)], and FRAP (ferric ion reducing antioxidant potential) assays. The most potent combination was tested for acute toxicity and curative antimalarial activity in a rodent model.
Results:
Extract yields ranged from 6.6% (cold maceration extract of B. pilosa) to 29.2% [aqueous decoction extract of combination (Cd)]. Extracts showed moderate to mild in vitro antiplasmodial activity [IC50 (median inhibitory concentration): 24.8–96.6 µg/mL], with the aqueous Cd showing potential synergism [CI (combination index) < 1]. No significant cytotoxicity was observed (< 10% hemolysis). Moderate to good antioxidant activity was found in DPPH [SC50 (median scavenging concentration): 134.65–307.55 µg/mL] and ABTS assays (SC50: 92.23–183.45 µg/mL), with Cd showing the highest activity. FRAP values were low. The Cd extract demonstrated no significant acute toxicity up to 5,000 mg/kg and significant in vivo antimalarial activity, achieving 65% parasite inhibition at 200 mg/kg/day. It also prolonged survival time, with a maximum survival of 28 days at 200 mg/kg/day.
Conclusions:
This preliminary investigation suggests that combined extracts of A. conyzoides and B. pilosa exhibit noteworthy in vitro and in vivo antiplasmodial activity against the tested strains. Further studies are warranted to validate these findings and develop optimized formulations as potential antimalarials.
DOI: https://doi.org/10.37349/eds.2025.1008122

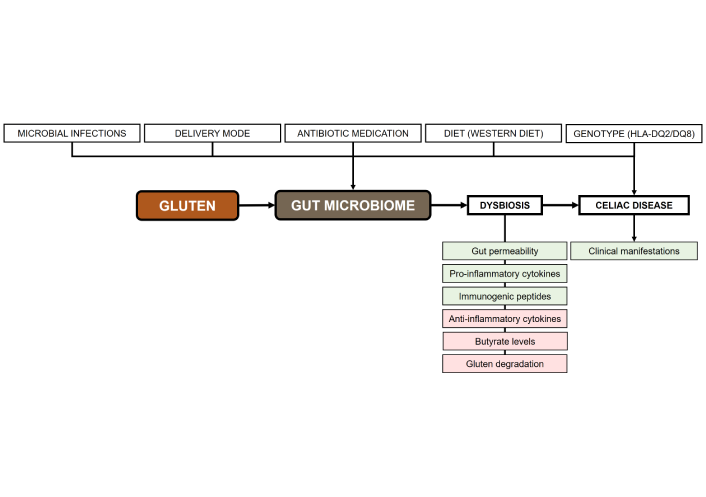
Celiac disease is an immune-mediated disorder with significant metabolic implications. Several factors have been proposed to explain the association between celiac disease in patients following a gluten-free diet and metabolic disorders, including metabolic syndrome. Growing evidence suggests a pivotal role of gut microbiome dysbiosis in the onset of celiac disease and its associated metabolic disturbances. The present narrative review examines (i) the connections between celiac disease and metabolism-related comorbidities, including metabolic syndrome and metabolic dysfunction-associated steatotic liver disease; (ii) the role of the gut microbiome in celiac disease, including the outcomes of gut microbiome dysbiosis in celiac children and adults; and (iii) the potential of microbial therapeutic strategies within the context of personalized medicine for patients with celiac disease and comorbid metabolic conditions. A synthesis of existing studies highlights several protective factors and interventions for future celiac disease prevention research. Adopting plant-based, health-promoting dietary patterns such as the Mediterranean or vegetarian diet within the first two years of life reduces celiac disease risk. These fiber- and phytochemical-rich diets support beneficial gut microbiota growth and short-chain fatty acid production, which maintain intestinal barrier integrity by enhancing mucus and tight junction proteins. Short-chain fatty acids also modulate immunity by inducing Tregs that secrete IL-10, suppressing pro-inflammatory Th1 responses and autoantibody production. Precision probiotics offer diverse therapeutic benefits in celiac disease by reducing inflammation, restoring beneficial microbes, and degrading immunogenic gliadin peptides. Postbiotics complement these effects by reinforcing barrier integrity and counteracting gliadin-induced inflammation. Thus, integrating clinical models with microbial biomarkers promises to improve celiac disease diagnosis and monitoring, enabling better risk stratification, earlier detection, and personalized management of this heterogeneous disease.
Celiac disease is an immune-mediated disorder with significant metabolic implications. Several factors have been proposed to explain the association between celiac disease in patients following a gluten-free diet and metabolic disorders, including metabolic syndrome. Growing evidence suggests a pivotal role of gut microbiome dysbiosis in the onset of celiac disease and its associated metabolic disturbances. The present narrative review examines (i) the connections between celiac disease and metabolism-related comorbidities, including metabolic syndrome and metabolic dysfunction-associated steatotic liver disease; (ii) the role of the gut microbiome in celiac disease, including the outcomes of gut microbiome dysbiosis in celiac children and adults; and (iii) the potential of microbial therapeutic strategies within the context of personalized medicine for patients with celiac disease and comorbid metabolic conditions. A synthesis of existing studies highlights several protective factors and interventions for future celiac disease prevention research. Adopting plant-based, health-promoting dietary patterns such as the Mediterranean or vegetarian diet within the first two years of life reduces celiac disease risk. These fiber- and phytochemical-rich diets support beneficial gut microbiota growth and short-chain fatty acid production, which maintain intestinal barrier integrity by enhancing mucus and tight junction proteins. Short-chain fatty acids also modulate immunity by inducing Tregs that secrete IL-10, suppressing pro-inflammatory Th1 responses and autoantibody production. Precision probiotics offer diverse therapeutic benefits in celiac disease by reducing inflammation, restoring beneficial microbes, and degrading immunogenic gliadin peptides. Postbiotics complement these effects by reinforcing barrier integrity and counteracting gliadin-induced inflammation. Thus, integrating clinical models with microbial biomarkers promises to improve celiac disease diagnosis and monitoring, enabling better risk stratification, earlier detection, and personalized management of this heterogeneous disease.
DOI: https://doi.org/10.37349/edd.2025.100584
This article belongs to the special issue Gut Microbiota towards Personalized Medicine in Metabolic Disease

Aim:
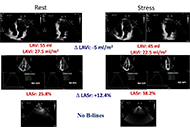
Left atrial volume index (LAVI), left atrial reservoir function through left atrial reservoir strain (LASr), and B-lines in lung ultrasound serve as supplementary indicators of left ventricular filling pressures. This study analyzes the interrelation between LAVI, LASr, and B-lines in both resting and peak vasodilator stress.
Methods:
Dipyridamole stress echocardiography (SE) was conducted on 252 individuals (180 males, 71%, age 65 years ± 10 years) with chronic coronary syndromes. LAVI was quantified using the biplane disk summation method; LASr was obtained using 2-dimensional speckle tracking echocardiography; B-lines were evaluated through a simplified 4-site scan in the third intercostal space during lung ultrasound.
Results:
During SE, a reduction in LAVI (26 ml/m2 ± 14 ml/m2 vs. 24 ml/m2 ± 12 ml/m2, P < 0.001) and an increase in LASr from rest (33% ± 8% vs. 38% ± 10%, P < 0.001) were respectively observed from rest to stress. B-lines were increased significantly during SE, from 19 (7.5%) to 29 (11.5%), P < 0.001. A substantial, inverse linear correlation was identified between LAVI and LASr both at rest (r = –0.301, P < 0.001) and peak stress (r = –0.279, P < 0.001). At group analysis, peak B-lines showed a direct correlation with peak LAVI (r = 0.151, P = 0.017) and an inverse correlation with peak LASr (r = –0.234, P < 0.001). In individual assessments, 9.7% (20/207) of patients displayed stress B-lines with normal LAVI and preserved LASr, while 20% (9/45) exhibited stress B-lines with abnormalities in both LAVI and LASr.
Conclusions:
Vasodilator SE with combined left atrial and volume assessment, related to pulmonary congestion, is feasible with a high success rate. Pulmonary congestion is more frequent with dilated left atrium with reduced atrial contractile reserve (ClinicalTrials.gov identifier: NCT030.49995; NCT050.81115).
Aim:
Left atrial volume index (LAVI), left atrial reservoir function through left atrial reservoir strain (LASr), and B-lines in lung ultrasound serve as supplementary indicators of left ventricular filling pressures. This study analyzes the interrelation between LAVI, LASr, and B-lines in both resting and peak vasodilator stress.
Methods:
Dipyridamole stress echocardiography (SE) was conducted on 252 individuals (180 males, 71%, age 65 years ± 10 years) with chronic coronary syndromes. LAVI was quantified using the biplane disk summation method; LASr was obtained using 2-dimensional speckle tracking echocardiography; B-lines were evaluated through a simplified 4-site scan in the third intercostal space during lung ultrasound.
Results:
During SE, a reduction in LAVI (26 ml/m2 ± 14 ml/m2 vs. 24 ml/m2 ± 12 ml/m2, P < 0.001) and an increase in LASr from rest (33% ± 8% vs. 38% ± 10%, P < 0.001) were respectively observed from rest to stress. B-lines were increased significantly during SE, from 19 (7.5%) to 29 (11.5%), P < 0.001. A substantial, inverse linear correlation was identified between LAVI and LASr both at rest (r = –0.301, P < 0.001) and peak stress (r = –0.279, P < 0.001). At group analysis, peak B-lines showed a direct correlation with peak LAVI (r = 0.151, P = 0.017) and an inverse correlation with peak LASr (r = –0.234, P < 0.001). In individual assessments, 9.7% (20/207) of patients displayed stress B-lines with normal LAVI and preserved LASr, while 20% (9/45) exhibited stress B-lines with abnormalities in both LAVI and LASr.
Conclusions:
Vasodilator SE with combined left atrial and volume assessment, related to pulmonary congestion, is feasible with a high success rate. Pulmonary congestion is more frequent with dilated left atrium with reduced atrial contractile reserve (ClinicalTrials.gov identifier: NCT030.49995; NCT050.81115).
DOI: https://doi.org/10.37349/ec.2024.00018

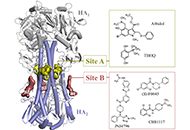
Aim:
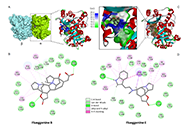
New microtubule-targeting agents are needed to improve cancer treatment. The recent characterization of the anticancer alkaloid securinine as a tubulin-binding agent prompted us to explore the interaction of related monomeric and dimeric analogues with tubulin. The interaction between the α/β-tubulin dimer and alkaloids fluevirines A–F and flueggenines A–I, isolated from the bush Flueggea virosa (Roxb. ex Willd.) Royle, was investigated using molecular docking.
Methods:
Two molecular models were initially compared for the binding of securinine to α/β-tubulin. The pironetin-binding site model (5FNV) was selected for the subsequent docking analysis with all compounds. Empirical energies of interaction (ΔE) were measured and compared.
Results:
Fluevirine A has been identified as a potent tubulin binder. This dimeric alkaloid formed more stable complexes with tubulin than the monomeric counterparts, such as fluevirines B–D. The bis-indole derivative fluevirine E also provided more stable complexes than (nor)securinine. The study was extended to the dimeric alkaloids flueggenines A–I and three compounds were identified as potential tubulin binders: the polycyclic product flueggenine B, the norsecurinine-indole hybrid flueggenine E, and the norsecurinine dimer flueggenine I. This later compound proved to be well adapted to fit into the pironetin site of tubulin, extending its two norsecurinine units between the colchicine-binding area and the pironetin site, in close proximity to the pironetin-reactive cysteine-316 residue. Structure-binding relationships were delineated.
Conclusions:
The study identifies the dimeric alkaloids fluevirine A and flueggenine I as potential α-tubulin binding agents. For the first time, dimeric alkaloids including two C-C connected norsecurinine units are characterized as tubulin ligands. The study contributes to a better understanding of the mechanism of action of Flueggea alkaloids and should help the design of anticancer analogues targeting the pironetin site of α-tubulin.
Aim:
New microtubule-targeting agents are needed to improve cancer treatment. The recent characterization of the anticancer alkaloid securinine as a tubulin-binding agent prompted us to explore the interaction of related monomeric and dimeric analogues with tubulin. The interaction between the α/β-tubulin dimer and alkaloids fluevirines A–F and flueggenines A–I, isolated from the bush Flueggea virosa (Roxb. ex Willd.) Royle, was investigated using molecular docking.
Methods:
Two molecular models were initially compared for the binding of securinine to α/β-tubulin. The pironetin-binding site model (5FNV) was selected for the subsequent docking analysis with all compounds. Empirical energies of interaction (ΔE) were measured and compared.
Results:
Fluevirine A has been identified as a potent tubulin binder. This dimeric alkaloid formed more stable complexes with tubulin than the monomeric counterparts, such as fluevirines B–D. The bis-indole derivative fluevirine E also provided more stable complexes than (nor)securinine. The study was extended to the dimeric alkaloids flueggenines A–I and three compounds were identified as potential tubulin binders: the polycyclic product flueggenine B, the norsecurinine-indole hybrid flueggenine E, and the norsecurinine dimer flueggenine I. This later compound proved to be well adapted to fit into the pironetin site of tubulin, extending its two norsecurinine units between the colchicine-binding area and the pironetin site, in close proximity to the pironetin-reactive cysteine-316 residue. Structure-binding relationships were delineated.
Conclusions:
The study identifies the dimeric alkaloids fluevirine A and flueggenine I as potential α-tubulin binding agents. For the first time, dimeric alkaloids including two C-C connected norsecurinine units are characterized as tubulin ligands. The study contributes to a better understanding of the mechanism of action of Flueggea alkaloids and should help the design of anticancer analogues targeting the pironetin site of α-tubulin.
DOI: https://doi.org/10.37349/eds.2024.00047

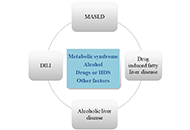
Drug-induced liver injury (DILI) poses a complex and heterogeneous clinical challenge, which often resembles non-drug related acute or chronic liver diseases, such as metabolic dysfunction-associated steatotic liver disease (MASLD). Furthermore, certain drugs can induce hepatic steatosis, which is considered a rare variant of hepatotoxicity. Additionally, the detection and diagnosis of DILI in patients with non-alcoholic liver disease present additional challenges that require attention. The importance of achieving an accurate diagnosis is highlighted by the different therapeutic approaches needed for each of these diseases. Nonetheless, as definitive diagnostic tests and distinct biomarkers often remain elusive, the differential diagnosis must rely on a combination of clinical, biochemical, histological, and immunophenotypic profiling. The diagnosis of hepatotoxicity is predicated upon the temporal nexus between the administration of a potentially hepatotoxic drug and the onset of hepatic injury, concomitantly excluding alternative hepatic pathologies. More frequently, this condition presents an acute course, with a more pronounced elevation of cytolytic and cholestatic parameters as compared to fatty liver disease. Advances in elucidating the underlying mechanisms hold promise for bolstering the diagnosis and management of these conditions. This article aims to thoroughly examine and emphasize the currently available scientific evidence to provide valuable insights into the diagnostic strategies for DILI, metabolic-associated liver disease, and drug-induced steatosis (DIS).
Drug-induced liver injury (DILI) poses a complex and heterogeneous clinical challenge, which often resembles non-drug related acute or chronic liver diseases, such as metabolic dysfunction-associated steatotic liver disease (MASLD). Furthermore, certain drugs can induce hepatic steatosis, which is considered a rare variant of hepatotoxicity. Additionally, the detection and diagnosis of DILI in patients with non-alcoholic liver disease present additional challenges that require attention. The importance of achieving an accurate diagnosis is highlighted by the different therapeutic approaches needed for each of these diseases. Nonetheless, as definitive diagnostic tests and distinct biomarkers often remain elusive, the differential diagnosis must rely on a combination of clinical, biochemical, histological, and immunophenotypic profiling. The diagnosis of hepatotoxicity is predicated upon the temporal nexus between the administration of a potentially hepatotoxic drug and the onset of hepatic injury, concomitantly excluding alternative hepatic pathologies. More frequently, this condition presents an acute course, with a more pronounced elevation of cytolytic and cholestatic parameters as compared to fatty liver disease. Advances in elucidating the underlying mechanisms hold promise for bolstering the diagnosis and management of these conditions. This article aims to thoroughly examine and emphasize the currently available scientific evidence to provide valuable insights into the diagnostic strategies for DILI, metabolic-associated liver disease, and drug-induced steatosis (DIS).
DOI: https://doi.org/10.37349/edd.2023.00034
This article belongs to the special issue Drug-induced Liver Injury: From Bench to Clinical Application
 Previous
Previous