Affiliation:
1Centro de Investigación y Desarrollo en Criotecnología de Alimentos (CIDCA), CONICET-CICPBA-Facultad de Ciencias Exactas-Universidad Nacional de La Plata (UNLP), La Plata 1900, Buenos Aires, Argentina
ORCID: https://orcid.org/0000-0001-7889-7493
Affiliation:
1Centro de Investigación y Desarrollo en Criotecnología de Alimentos (CIDCA), CONICET-CICPBA-Facultad de Ciencias Exactas-Universidad Nacional de La Plata (UNLP), La Plata 1900, Buenos Aires, Argentina
ORCID: https://orcid.org/0009-0002-4027-8173
Affiliation:
2Spectral Service AG, Emil-Hoffmann-Straße 33, 50996 Cologne, Germany
ORCID: https://orcid.org/0000-0003-4822-5295
Affiliation:
1Centro de Investigación y Desarrollo en Criotecnología de Alimentos (CIDCA), CONICET-CICPBA-Facultad de Ciencias Exactas-Universidad Nacional de La Plata (UNLP), La Plata 1900, Buenos Aires, Argentina
ORCID: https://orcid.org/0000-0003-1439-7064
Affiliation:
1Centro de Investigación y Desarrollo en Criotecnología de Alimentos (CIDCA), CONICET-CICPBA-Facultad de Ciencias Exactas-Universidad Nacional de La Plata (UNLP), La Plata 1900, Buenos Aires, Argentina
3Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata (UNLP), La Plata 1900, Buenos Aires, Argentina
Email: vixtaina@agro.unlp.edu.ar; vanesaix@hotmail.com
ORCID: https://orcid.org/0000-0002-7066-1918
Explor Foods Foodomics. 2024;2:107–124 DOI: https://doi.org/10.37349/eff.2024.00029
Received: October 31, 2023 Accepted: January 05, 2024 Published: April 10, 2024
Academic Editor: Andrea Gomez-Zavaglia, Center for Research and Development in Food Cryotechnology (CIDCA-CONICET), Argentina
The article belongs to the special issue Delivery of Hydrophobic Compounds in Food Systems
Aim: The present study investigates the influence of various homogenization techniques, namely high-pressure valve homogenization and microfluidization, and different forms of modified sunflower lecithin, including deoiled (DL) and hydrolyzed (HL) variants, on the development of monolayer and bilayer nanoemulsions of chia oil.
Methods: Oil-in-water (O/W) nanoemulsions with 5% chia seed oil were prepared using simple (0.5% DL or HL) or double-layer [0.5% DL or HL and 0.3% chitosan (Ch)] stabilization. This involved a two-step homogenization process, utilizing either microfluidization or high-pressure valve homogenization. Chia oil nanoemulsions were characterized by their zeta potential, particle size, and rheological properties. Besides, their physical stability and omega-3 content during refrigerated storage were evaluated.
Results: Overall, the studied modified sunflower lecithin (DL and HL) demonstrated effective capability in stabilizing chia nanoemulsions and facilitating the formation of the double-layered structure following Ch deposition. Concerning the homogenization method, it has been demonstrated that under the same homogenization conditions, microfluidization resulted in significantly smaller droplet sizes and higher apparent viscosities compared to high-pressure valve homogenization. This discrepancy can be attributed to the design of the homogenization chambers, as microfluidization generates a narrow distribution of shear forces, while high-pressure valve homogenization yields a much broader distribution. In contrast to chia monolayer nanoemulsions, the nanoemulsions stabilized by modified sunflower lecithin-Ch demonstrated a noteworthy improvement in their overall stability. This enhancement can be ascribed to their increased apparent viscosity and the highly charged interfaces of the droplets. Furthermore, throughout the entire refrigerated storage period, the omega-3 content in all nanoemulsions remained unchanged.
Conclusions: In this study, mono and bilayer chia oil nanoemulsions were successfully obtained using modified sunflower lecithin and high-energy techniques. Microfluidization outperformed high-pressure valve homogenization, resulting in smaller droplets and increased viscosity. These findings are relevant for designing stable chia oil nanoemulsions with natural components, offering substantial health benefits.
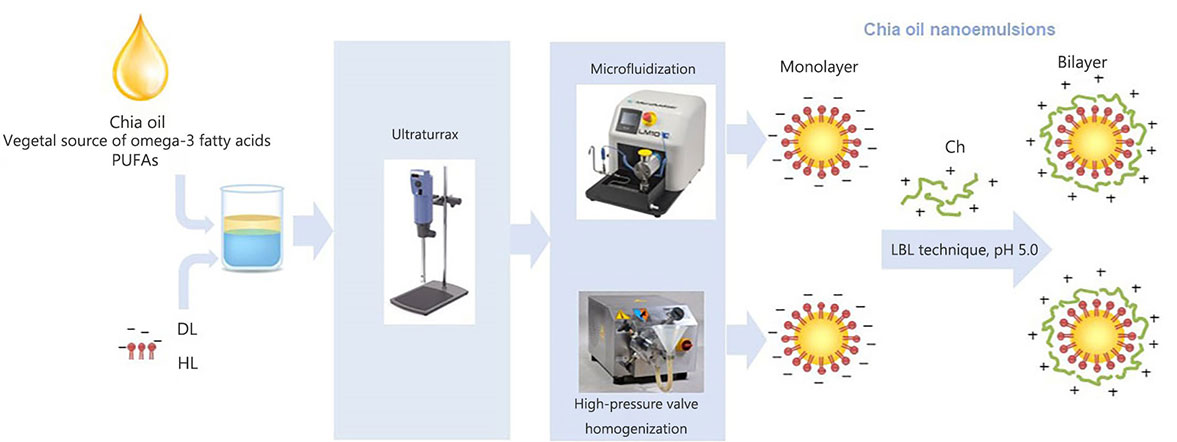
Scheme of the obtention process of chia O/W nanoemulsions using different homogenization technologies and the layer-by-layer (LBL) technique. PUFAs: polyunsaturated fatty acid
Chia seed oil has become an attractive ingredient for the development of functional foods and cosmetic applications due to its high content of polyunsaturated fatty acids (PUFAs) [1]. It is known that omega-3 PUFAs contribute to promoting brain development [2], reducing the incidence of cardiovascular diseases [3], controlling inflammation [4], suppressing tumor carcinogenesis [5], and managing obesity [6]. However, despite the proven health benefits, the intake of these PUFAs does not meet the recommended levels in most countries worldwide. Western diets are characterized by containing an unhealthy omega-6:omega-3 ratio of 20:1 instead of 5:1 to 10:1 recommended by international health authorities [7]. This fact has further intensified the research regarding omega-3 PUFAs-rich oils incorporation into foods. The principal sources of long-chain omega-3 PUFAs in the human diet are marine lipids. However, global demands for PUFAs from vegetal sources have considerably increased in recent years because of the growth of the population with vegetarian and vegan diets and the rising concerns about contamination of fish oil with organic pollutants [8]. In this context, chia seed oil is the vegetable source with the highest content known of α-linolenic acid (ALA, omega-3, approximately 65%), and it presents a low omega-6:omega-3 fatty acids ratio (0.31–0.24), which is favorable because of its benefit to the cardiovascular system [1]. The bioactive potential of chia seed oil extends to reducing the risk of chronic diseases due to its antioxidant, anti-inflammatory, hypoglycemic, and hypolipemic effects when regularly consumed [9]. Regular consumption of chia oil has been shown to enhance antioxidant levels, improve liver plasma status, reduce plasma lipid peroxidation, and protect against oxidative stress, particularly in obese individuals [10]. Also, the anti-inflammatory properties of chia oil alone or in combination with other vegetable oils were demonstrated by Gazem and Chandrashekariah [11].
However, this oil is susceptible to oxidative degradation and requires protection for its potential use in foods. In this regard, emulsion-based delivery systems have proven their effectiveness in preventing the oxidative deterioration of these sensitive ingredients, thereby preserving their quality when incorporated into various food products [12].
Emulsions with varying compositions and structures, and consequently different physicochemical and functional properties, can be created using diverse techniques, homogenization methods, and emulsifying agents. Notably, nanoemulsions have emerged as promising vehicles for encapsulating bioactive food constituents due to their extended global stability and bioavailability increase of highly lipophilic substances [13]. In these systems, the smaller sizes of droplets (20–200 nm) give them more stability against gravitational separation and droplet aggregation than conventional emulsions, thereby extending the shelf life of foods [14].
Food-grade nanoemulsions can be produced using either low-energy methods, such as phase inversion and spontaneous emulsification, or high-energy methods, including high-pressure valve homogenization, microfluidization, and ultrasonication. High-energy processes offer several advantages, such as not requiring additional chemicals, shorter preparation times, and smaller amounts of emulsifying agents to achieve nanoscale droplet sizes in contrast to low-energy methods [15]. Furthermore, the variety of surfactants appropriate to form nanoemulsions through low-energy approaches is limited, and many of them are of synthetic origin, which imposes restrictions on their suitability for applications within the food industry. Thus, high-energy methods are much more frequently used in the food industry. High-pressure valve homogenizers (HPHs), in which separated dispersed and continuous phases, or a pre-emulsion, are forced through a narrow valve under high pressure, have become an inseparable operation in the dairy industry to reduce fat droplet size and prevent phase separation [16]. However, microfluidization has earned a prominent role in food processing with its advantages, such as quick processing times, low-temperature impact, and minimal nutritional loss. This method involves passing a liquid feed through a carefully interaction chamber where it is divided into two or more micro streams, which in turn collide with each other, generating a unique combination of high pressure, high velocity, vibrations, pressure drop, shear rate, and hydrodynamic cavitation [17].
Nanoemulsification may facilitate the oxidative degradation of dispersed oil droplets, mainly due to their larger specific surface area, which increases the possibility for lipid-aqueous phase pro-oxidant interactions. In addition, high levels of pressure applied during the homogenization process may cause lipid deterioration. In this sense, multiple interfacial layers coating the oil droplets in emulsions formed using the layer-by-layer (LBL) technique have previously been proven to inhibit lipid oxidation [18]. It has been reported that emulsions containing oil droplets surrounded by multiple interfacial membranes have better stability to environmental stresses than conventional emulsions consisting of oil droplets surrounded by a single interfacial membrane. In this sense, the LBL deposition method offers an alternative to improve the stability of the emulsion, although it is necessary to choose an appropriate combination of emulsifier and biopolymers [19].
Emulsion composition plays a crucial role in the formation and stability of emulsions. Emulsifiers act as essential components because these agents help prevent the coalescence of oil droplets and maintain the uniform dispersion of active ingredients. There is a growing interest in replacing synthetic emulsifying and stabilizing agents with natural alternatives, one of which is sunflower lecithin [19]. This lecithin is a phospholipid (PL)-rich mixture derived from the degumming process of crude sunflower oil, and it has been successfully utilized to stabilize a wide range of emulsions in the food and pharmaceutical industries [20]. It is worth noting that the surface-active characteristics of the PLs in sunflower lecithin are responsible for its physicochemical and functional properties. Several chemical processes can be utilized to modify the properties of sunflower lecithin. These processes include deoiling, ethanol fractionation, and enzymatic hydrolysis. By employing these methods, the balance between hydrophilic and lipophilic compounds [the hydrophilic-lipophilic balance (HLB)] can be altered, resulting in different functional properties of the modified lecithin [21, 22]. Taking into account the anionic character of lecithin in an acidic medium, it is interesting to consider a cationic polymer, such as chitosan (Ch), to form the second layer around the lecithin stabilized oil droplets applying the electrostatic deposition technique. Ch is a natural, non-toxic, and biodegradable polysaccharide derived from chitin [23]. Thus, lecithin and Ch, widely used in conventional emulsions, can be used at adequate levels to developing nanoemulsions applying high-pressure homogenization technologies. According to Wani et al. [24], it is essential to formulate nanoemulsions using only those ingredients known to be safe when used at required levels. On the other hand, Müller et al. [25] classified nanoparticles based on their size and biodegradability into four classes, considering biodegradable nanoparticles to be in the low-risk class.
Different emulsion-based delivery systems have been applied for chia oil entrapment, including conventional, multilayer, and multiple methods [17, 26–32]. However, the number of studies involving chia oil nanoemulsion formation using high-energy methods is still limited. Notably, there is a scarcity of research on the production of chia bilayer nanoemulsions in the existing literature.
Therefore, the objectives of this study were to investigate the formation of mono and bilayer nanoemulsions containing chia oil and to compare two homogenization methods: high-pressure valve homogenization and microfluidization. Additionally, the emulsifying capabilities of deoiled (DL) and hydrolyzed (HL) sunflower lecithin were examined. Finally, the physical and oxidative stability of chia mono and bilayer nanoemulsions during refrigerated storage were assessed.
Solazteca SDA (Buenos Aires, Argentina) provided cold-pressed chia oil. Sigma Chemical Company (San Luis, MO, USA) supplied Ch (cat# 9012-76-4) with a medium molecular weight of approximately 250 kDa and 75–85% deacetylation. DL and HL sunflower lecithins were provided by Lasenor Emul S.L. (Olesa de Montserrat, Spain). All reagents used were of analytical grade.
A 23 full factorial design, replicated twice, was used to study the effects of the homogenization method (high-pressure valve homogenization or microfluidization), modified sunflower lecithin type (DL or HL), and emulsion type (monolayer or bilayer) on each variable studied. The experimental design and the sample codes are shown in Table 1.
Physicochemical characteristics of chia seed oil
| Fatty acids | Relative percent |
|---|---|
| C16:0 | 8.3 ± 1.5 |
| C18:0 | 3.5 ± 0.4 |
| C18:1 | 5.4 ± 0.3 |
| C18:2 | 18.6 ± 0.5 |
| C18:3 | 64.1 ± 0.8 |
| Peroxide value (meq/kg) | 1.6 ± 0.2 |
| Free fatty acids (g oleic acid/100 g oil) | 0.91 ± 0.05 |
The fatty acid composition of chia seed oil was analyzed by gas chromatography (GC) according to International Union of Pure and Applied Chemistry (IUPAC) 2.302. Fatty acid methyl esters (FAMEs) were prepared using boron trifluoride-methanol (cat# 373-57-9, Alfa Chemistry, Holbrook, NY, USA) reagent following the IUPAC 2.301 method [33]. The peroxide value and the free fatty acid content were also determined according to American Oil Chemists Society (AOCS) recommended practices [34, 35].
Phosphorus-31 nuclear magnetic resonance (31P NMR) spectroscopy was employed to determine the PL composition of both modified sunflower lecithin (DL and HL), according to Diehl [36]. Briefly, 100 mg of each sample was diluted in 1 mL of deuterated chloroform, 1 mL of methanol, and 1 mL of 0.2 mol/L Ch-ethylenediaminetetraacetic acid (Cs-EDTA). The mixture was shaken for 15 min, afterwards, the organic layer was separated and analyzed using a Bruker Avance spectrometer (Bruker Avance 600 MHz automatic spectrometer, UK) and triphenyl phosphate as the internal standard.
Initially, about 500 mL of stock solutions of DL and HL were prepared by dispersing each in 35.46 mmol/L acetic acid (cas# 64-19-7, Cicarelli, Santa Fe, Argentina)/64.54 mmol/L sodium acetate (cas# 127-09-3, Merck, Darmstadt, Germany) buffer solution (pH 5.0). Similarly, 80 mL of a stock biopolymer solution was obtained by dissolving Ch in the same buffer solution and stirring for around 8 h. Afterward, the emulsifying and biopolymer stock solutions were refrigerated at 4°C ± 0.5°C overnight to ensure complete hydration.
The optimal amounts of DL and HL, as well as the suitable pH conditions for facilitating the electrostatic deposition of Ch onto the modified lecithin-stabilized droplets during the formation of double-layered nanoemulsions, while minimizing the residual excess of the emulsifying agent in the continuous phase, were determined based on the results of preliminary experiments (data not shown).The shows the scheme of mono and bilayer chia oil-in-water (O/W) nanoemulsions preparation is presented in Figure 1. In this way, about 250 mL of each O/W nanoemulsion containing 5% w/w of chia seed oil and stabilized with a simple (0.5% w/w of DL or HL) and double layer (0.5% w/w of DL or HL, and 0.3% w/w of Ch) were produced by applying a two-step homogenization process and the LBL deposition technique in the latter case. In the first step, a pre-homogenization was performed using a laboratory scale disperser (Ultraturrax T-25, IKA-Labortechnik, GmbH & Co., Staufen, Germany) for 3 min at 13,000 rpm. The coarse emulsion obtained by ultraturrax was subsequently treated by either a microfluidizer (LM10, Microfluidics, Westwood, CA, USA) or a HPH (Panda 2 K, GEA, NiroSoavi, Parma, Italy) at 1,000 bar for three passes (Figure 1). During the homogenization process, the temperature of the nanoemulsions was maintained below 20°C by employing an ice-water bath. Food-grade preservatives potassium sorbate (1,000 ppm, cas# 24634-61-5, Cicarelli, Argentina) and nisine (12 ppm, cas# 1414-45-5, El Maestro Quesero, Argentina) were added to the nanoemulsions obtained. Finally, all systems were stored for 30 days at 4°C ± 0.5°C in darkness.
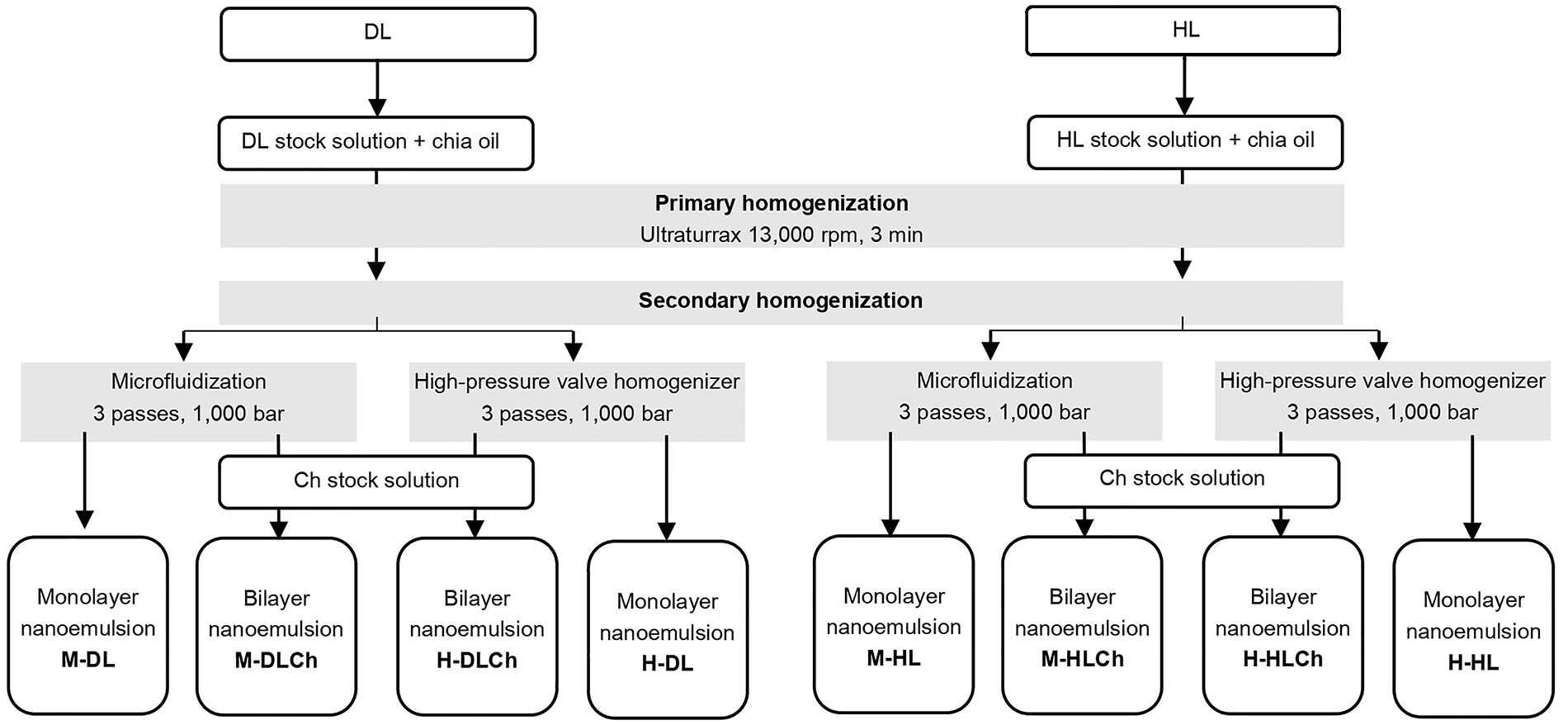
Obtaining O/W nanoemulsions with chia seed oil using DL or HL sunflower lecithin (monolayer) and Ch (bilayer). M-DL: microfluidization-DL lecithin; M-DLCh: microfluidization-DL lecithin-Ch; H-DLCh: high-pressure valve homogenization-DL lecithin-Ch; H-DL: high-pressure valve homogenization-DL lecithin; M-HL: microfluidization-HL lecithin; M-HLCh: microfluidization-HL lecithin-Ch; H-HLCh: high-pressure valve homogenization-HL lecithin-Ch; H-HL: high-pressure valve homogenization-HL lecithin
The zeta-potential was measured using a Zeta-Potential Analyzer (Brookhaven 90Plus/Bi-MAS, New York, NY, USA) immediately after emulsion preparation, according to Julio et al. [17]. For each determination, 0.05 g of the sample was diluted in 100 mL milli-Q water, adjusting the pH to 5.0. Measurements were carried out in triplicate at room temperature using a zeta-potential range of −50 mV to +50 mV.
The particle size distribution (PSD) of the nanoemulsions was measured using the Mastersizer 2000 (Malvern Instruments, Ltd., Worcestershire, UK) based on the laser diffraction technique. About 1 mL of emulsion was diluted in recirculating water at 1,700 rpm for each measurement. A refraction index of 1.45 was used for the particle and 1.33 for the dispersant, corresponding to chia oil and water, respectively. Measurements were performed in duplicate. The PSD, surface-weighted diameter (D [3, 2]) and span were recorded.
Rheological measurements were conducted at 25°C ± 0.5°C using a stress-controlled oscillatory rheometer (AR-2000, TA instrument, New Castle, DE, USA) equipped with a cone-plate sensor system. Apparent viscosity (η) was recorded while varying the shear rate (γ) from 1 s–1 to 500 s–1 for 3 min, holding it at 500 s–1 for 1 min, and then decreasing it from 500 s–1 to 1 s–1 for 3 min. These measurements were performed in triplicate.
The physical stability of nanoemulsions was assessed through the multiple light scattering technique using a Turbiscan MA analyzer (Formulation, France). Fresh undiluted nanoemulsions were put into measurement tubes (approximately 6.5 cm) and scanned periodically to obtain their backscattering (BS) profiles [percentage of backscattered of light (%BS) vs. tube length] over 30 days. Measurements were carried out in duplicate.
Chia mono and bilayer nanoemulsions were analyzed by confocal laser scanning microscopy using a confocal microscope (cat# FV1000, Olympus, Shinjuku, Tokyo, Japan). Samples (about 1 mL) were stained by directly adding Nile Red (cat# 7385-67-3, Sigma Aldrich, St. Louis, MO) and allowing them to rest in the dark. Excitation and emission wavelengths of 488 nm and 518 nm, respectively, were employed. Micrographs were captured at 20× magnifications. The analysis of the images was carried out using the OlyVIA software (3.7 version, Olympus, Japan).
To extract chia oil from the nanoemulsions, 3 mL of each system was mixed with 15 mL of an isopropanol/isooctane (1:3 v/v) mixture. The mixture was then centrifuged at 5,000 g for 2 min. Following centrifugation, the organic phase was collected, and the solvent was subsequently removed using a rotary evaporator (Rotavapor R 110, Buchi) under a pressure range of 95–100 bar at 50°C. Finally, any remaining solvent and surrounding oxygen were eliminated using a stream of nitrogen (N2). After oil extraction, α-linolenic acid content was determined by proton nuclear magnetic resonance (1H NMR) according to Julio et al. [8]. Briefly, 100 mg of each nanoemulsion was dissolved in 1 mL of the mixture deuterated chloroform (DCl3)/methanol-d4 (MeOD)/Cs-EDTA-deuterium oxide (D2O) 0.2 mol/L (1:1:1), ultrasonicated for 30 min, and shaken for at approximately 1 h. The mixture was centrifuged, and the organic layer was analyzed using a nuclear magnetic resonance (NMR) spectrometer Avance III 500 MHz (Bruker, Karlsruhe, Germany), magnetic flux density 11.7 with Tesla BFOPLUS SmartProbe (5 mm PABBO BB) at 25°C. The calibration was performed on the tetramethylsilane (TMS) signal. Data were analyzed using the software Bruker TopSpin 3.2, standard operation procedure SAA-GMR045-03. 1H NMR spectroscopic measurements were performed immediately after their preparation and after 30 days of storage in triplicate.
The experimental results were subjected to multifactorial analysis of variance (ANOVA, P ≤ 0.05) to investigate both the major effects and interactions. The Statgraphics Centurion XV.II program for Windows software (StatPoint Technologies, Warrenton, USA) was used to conduct the analysis. Multiple comparisons between means were performed using the Tukey test (P ≤ 0.05; 95% level of confidence).
The shows the properties of the chia seed oil employed in this study are shown in Table 1. Its fatty acid composition closely resembles those documented in prior studies for chia seed oil [1, 2]. Furthermore, the low peroxide value and free fatty acids content indicate a high quality of the initial chia oil used.
Sunflower lecithin is an effective surface tension-reducing agent thanks to its amphiphilic structure of lipophilic fatty acyl and hydrophilic head groups. Modifications like deoiling and hydrolysis can alter its HLB, impacting emulsifying properties. The HLB number measures an emulsifying agent’s affinity for oil or water, typically ranging from 1 (fully oil-soluble) to 20 (water-soluble). A higher HLB number signifies higher hydrophilicity, promoting water solubility and superior stabilization of O/W emulsions [20].
The composition of modified sunflower lecithin for DL and HL is detailed in Table 2, revealing significant differences between the two types. In this context, the phosphatidylcholine (PC) content in DL was approximately double that of HL. Additionally, DL exhibited higher levels of phosphatidylethanolamine (PE), phosphatidic acid (PA), and phosphatidylinositol (PI) compared to HL. Conversely, HL exhibited a relatively elevated concentration of hydrophilic PLs, accounting for approximately 33% of its composition. These hydrophilic components primarily comprised lysophospholipids [1-lysophosphatidylcholine (1-LPC), 2-LPC, lysophosphatidylethanolamine (LPE), lysophosphatidic acid (LPA)], while hydrophobic compounds were relatively scarce. Consequently, HL possesses a higher HBL than DL, potentially making it a better emulsifying agent for forming O/W emulsions.
PL composition of DL and HL sunflower lecithins by 31P NMR
| PL type | Percentage of total PL | PL/L (g/100 g) | ||
|---|---|---|---|---|
| DL | HL | DL | HL | |
| PC | 36 | 17.98 | 22.98 | 12.5 |
| 1-LPC | nd | 1.58 | nd | 0.74 |
| 2-LPC | 1.2 | 17.52 | 0.53 | 8.15 |
| PI | 31.6 | 27.36 | 21.89 | 20.63 |
| LPI | nd | nd | nd | nd |
| PS-Na | 0.5 | nd | 0.33 | nd |
| PE | 15.4 | 10.56 | 9.27 | 6.91 |
| LPE | 1.2 | 8.3 | 0.48 | 3.52 |
| N-aPE | 1.5 | nd | 1.22 | nd |
| PG | 1.8 | nd | 1.14 | nd |
| DPG | 0.7 | nd | 0.41 | nd |
| PA | 9.1 | 3.8 | 5.15 | 2.35 |
| LPA | 0.4 | 5.68 | 0.13 | 2.2 |
| Other PLs | 0.5 | 7.23 | 0.32 | 5.03 |
| Total | 100 | 100 | 63.85 | 62.03 |
Coefficient of variation < 4%. L: lecithin average values of two replicates; nd: no signal assignment; LPI: lysophosphatidylinositol; PS-Na: phosphatidylserine-Na; N-aPE: n-acyl-PE; PG: phosphatidylglycerol; DPG: diphosphatidylglycerol
The zeta-potential serves as an electrokinetic potential that quantifies the extent of electric charge on the surface of emulsion droplets, which is a vital determinant of their stability [37]. This parameter was assessed for various chia oil nanoemulsions at pH 5.0, confirming the complete electrostatic deposition of Ch onto the oil droplets stabilized by modified sunflower lecithin using the LBL technique. The surface charge of droplets in the different chia nanoemulsions is presented in Table 3. Both the addition of Ch and the homogenization method had a significant impact (P ≤ 0.05) on the surface charge of chia oil droplets. As expected, before the Ch deposition, one-layer nanoemulsions exhibited a negative surface charge, which can be attributed to the anionic PLs from DL or HL at pH 5.0. Conversely, after the addition of Ch, the oil droplets of double layer nanoemulsions exhibited a positive charge which may be due to the amino groups (-NH3+; pKa < 6.3) present in molecules of this cationic biopolymer suggesting thus the electrostatic deposition of Ch onto the entire surface of the droplets of monolayer systems [2]. Furthermore, the droplets coated by double-layer exhibited a relatively high positive charge (+18.37 mV to +34.26 mV), creating a robust electrostatic repulsion between them, effectively preventing aggregation. In contrast, the droplets in single-layer systems carried a relatively low negative charge at pH 5.0 (–5.95 mV to –12.14 mV), which could be insufficient to avoid droplet aggregation.
Particle size, zeta-potential, and rheological parameters for chia O/W mono and bilayer-nanoemulsions formulated with different modified sunflower lecithin (DL or HL), by two homogenization processes (high-pressure valve homogenization or microfluidics)
| Sample code | D [3, 2] (µm) | Span values | Zeta-potential (mV) | Power law parameters | |
|---|---|---|---|---|---|
| n | K × 103 (Pa.s) | ||||
| H-DL | 0.315 ± 0.05d | 1.317 ± 0.01a | –11.45 ± 1.35a | 0.998 ± 0.001b | 1.360 ± 0.001a |
| H-DLCh | 0.229 ± 0.02c | 1.318 ± 0.03a | +34.26 ± 1.47d | 0.956 ± 0.001ab | 4.685 ± 0.001bc |
| H-HL | 0.173 ± 0.02b | 8.440 ± 0.04d | –12.14 ± 3.26a | 0.998 ± 0.001b | 1.233 ± 0.001a |
| H-HLCh | 0.118 ± 0.01a | 2.389 ± 0.17c | +33.77 ± 2.69d | 0.949 ± 0.016a | 3.752 ± 0.002b |
| M-DL | 0.113 ± 0.04a | 11.945 ± 0.04e | –5.95 ± 2.82b | 0.999 ± 0.002b | 1.331 ± 0.110a |
| M-DLCh | 0.121 ± 0.02a | 1.835 ± 0.10b | +18.37 ± 2.88c | 0.947 ± 0.001a | 5.423 ± 0.571cd |
| M-HL | 0.120 ± 0.02a | 11.770 ± 0.01e | –6.64 ± 0.90b | 0.999 ± 0.001b | 1.295 ± 0.001a |
| M-HLCh | 0.110 ± 0.01a | 1.114 ± 0.13a | +19.31 ± 1.03c | 0.927 ± 0.002a | 6.374 ± 0.341d |
Average values ± standard deviations of two replications. See Table 1 for sample code. n: flow behavior index; K: flow consistency index; a, b, c, d, e, ab, bc, cd: different letters in the same column indicate significant differences (P ≤ 0.05) between systems according to the Tukey test
Additionally, the choice of homogenization method also had a significant impact (P ≤ 0.05) on the zeta-potential of the oil droplets. Notably, the absolute value of the zeta-potential in emulsions produced through high-pressure valve homogenization was higher (P ≤ 0.05) than those generated through microfluidization. This divergence may be attributed to the distinct conformational arrangement of the modified lecithin and Ch at the droplet interfaces, resulting in different geometries of the devices. Nevertheless, despite the droplets produced by microfluidization having a lower charge than those from high-pressure valve homogenization, it would be sufficient to provide stability against creaming and flocculation processes.
In this study, the formulations and homogenization conditions applied produced emulsions with nanoscale droplet sizes since the D [3, 2] values ranged from 110–229 nm, as presented in Table 3.
The initial mean diameter was influenced (P ≤ 0.05) by the three factors investigated to varying degrees, with the order of impact as follows: homogenization method > emulsion type > lecithin type. When the emulsifier content and homogenization pressure were kept constant, microfluidization produced significantly smaller droplet sizes (P ≤ 0.05) than high-pressure valve homogenization (Table 3). Lee and Norton [15] also observed similar results, reporting that the microfluidizer produced smaller droplets and a narrower distribution of particle sizes compared to the HPH at 100 MPa and 150 MPa. This contrast is due to the limited range of shear forces generated by the microfluidizer compared to the broader spectrum of forces produced by the HPH in food-grade emulsions. Furthermore, these findings align with prior research by Ciron et al. [38] examining the impact of conventional homogenization and microfluidization on particle size in milk and other dairy-based emulsions. Their study revealed that microfluidization is conducive to producing smaller milk droplets than conventional homogenization. It was noted that as pressure increased, emulsion droplet sizes gradually decreased, and microfluidizer-generated emulsions exhibited significantly smaller droplet sizes in comparison to those produced via HPH under similar homogenization conditions. Other studies have also shown that microfluidization leads to emulsions with smaller droplets, attributed to the ease of emulsifier adsorption on the surface during microfluidization [39]. This fact can be attributed to the distinctive geometry of the microfluidizer, where oil droplets in the interaction chamber experience substantial shearing forces, approximately 15 times higher than those during valve homogenization. The extended capillary tube in the chamber ensures effective emulsifier adsorption [39].
When examining the influence of emulsion type on particle size, it was observed that the mean particle diameter of nanoemulsions produced via high-pressure valve homogenization significantly decreased (P ≤ 0.05) with the addition of Ch to form the interfacial double layer. This phenomenon can be attributed to the electrostatic deposition of this biopolymer on anionic droplets coated with modified sunflower lecithin. As a result, the oil droplets became highly positively charged, potentially impeding recoalescence during the homogenization process through electrostatic repulsion forces. These findings align with those of Wang et al. [40], who investigated bilayer emulsions containing rice bran protein hydrolysate and Ch. They reported that the application of adequate Ch can result in the formation of a complete covering film on the surface of monolayer droplets. This technique has the potential to effectively reduce particle size by preventing emulsion aggregation and flocculation, thanks to the positively charged nature of Ch.
The type of sunflower lecithin also exerted a significant influence on droplet size, albeit to a lesser extent. Specifically, when high-pressure valve homogenization was employed, the use of HL for forming nanoemulsions resulted in smaller droplet sizes (P ≤ 0.05) compared to those obtained with DL. Previous research by Cabezas et al. [41] has demonstrated that two types of HL sunflower lecithin produced droplet mean diameters significantly smaller than those formed with DL sunflower lecithin, especially at levels of 2% w/w. This trend can be attributed, as previously discussed, to the HLB value associated with emulsifiers. The higher concentration of hydrophilic PLs, such as lysophospholipids, in HL increases the empirical HLB value, thereby enhancing its performance as an O/W emulsifying agent.
Moreover, the specific arrangement of different PLs at the oil-water interface significantly influences emulsion formation and stability. PC typically forms stable monolayers or bilayers, while LPC and LPE form hexagonal widespread clusters that facilitate O/W emulsion stabilization. Conversely, PE gives rise to a reversed hexagonal phase, which is more challenging to arrange at the interface [42]. Therefore, the higher content of PE in DL could explain its reduced efficacy as an emulsifying agent compared to HL. In line with D [3, 2] results, the span values were significantly affected (P ≤ 0.05) by the three examined factors (Table 3). Also, all factor interactions were statistically significant, with the type of emulsion exerting the most substantial influence. Bilayer systems exhibited lower span values than monolayer systems, notably evident when employing a HPH in system preparation. Consequently, these findings imply potential recoalescence events during the homogenization of monolayer systems, potentially leading to broader particle distribution curves and the emergence of larger droplet populations.
The PSD curves corresponding to the freshly prepared oil nanoemulsions are shown in Figure 2. As can be seen, systems formed by microfluidization resulted in the PSD curves shifting toward smaller particle sizes compared to high-pressure valve homogenization. This behavior was more evident in systems stabilized with DL (Figure 2A) than in HL (Figure 2B). Besides, nanoemulsions obtained by microfluidization presented unimodal (double-layer nanoemulsions) and trimodal (one-layer nanoemulsions) PSD curves, while those formed by high-pressure valve homogenization were bimodal (one-layer nanoemulsions) and trimodal (double-layer nanoemulsions). Moreover, upon the introduction of Ch and the development of an interfacial double layer, a distinct reduction in the number of peaks associated with larger droplets in the distribution curves becomes evident. This observation also suggests a decrease in coalescence events during the homogenization process.
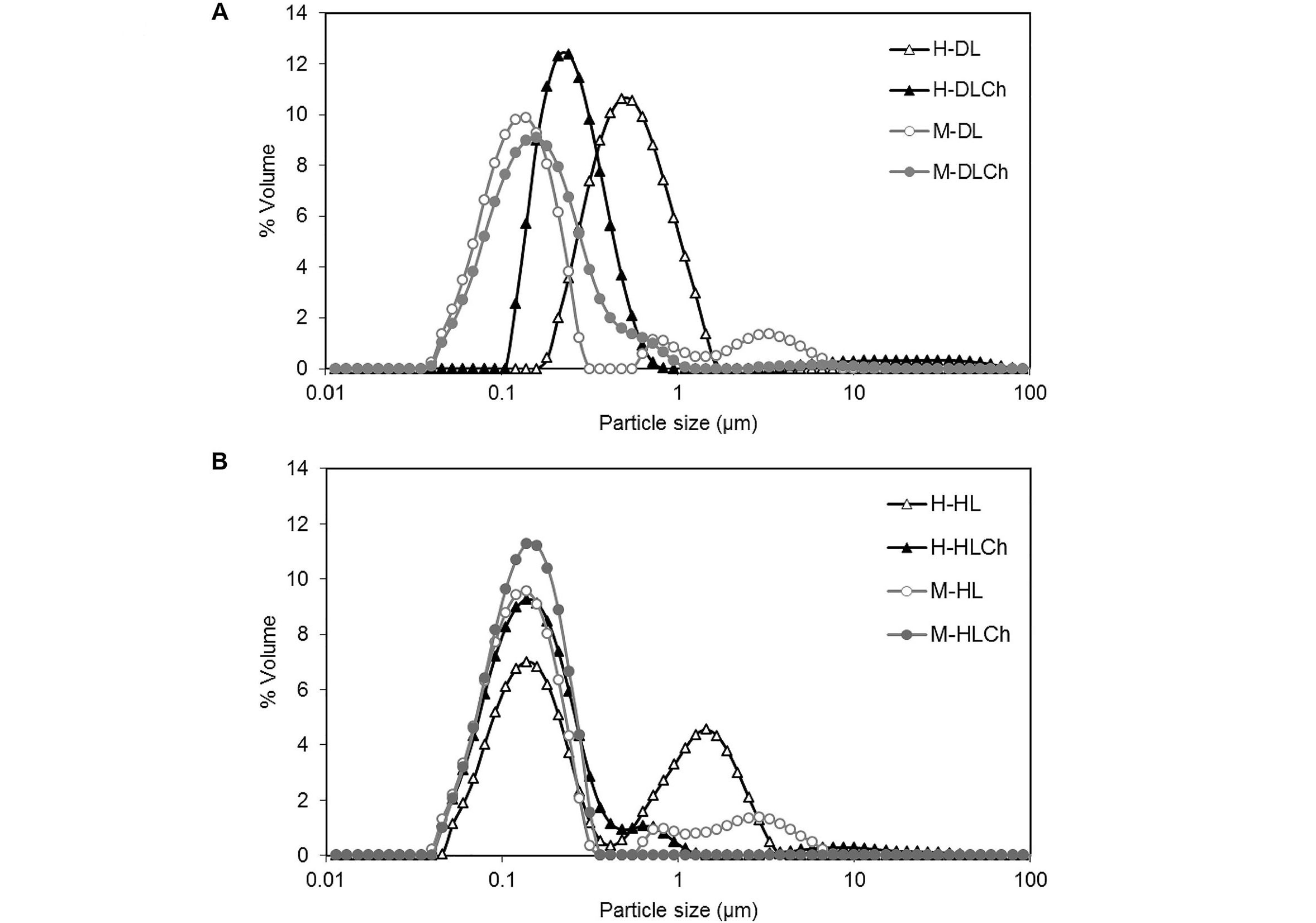
PSDs (% volume) of chia O/W mono and bilayer-nanoemulsions formulated with different modified sunflower lecithin by two homogenization processes (high-pressure valve homogenization or microfluidics). A: DL; B. HL. Average values of two replications. The coefficient of variation was lower than 1%. See Figure 1 for the sample code
The flow behavior of the chia nanoemulsions was assessed at a controlled temperature of 25°C ± 0.5°C. The n and K parameters were obtained from the rheological data fitted to the power-law model (Table 3). According to this, all one-layer emulsions behaved as Newtonian fluids (n = 1), while bilayer ones resulted in pseudoplastic fluids since n < 1. When considering the K coefficient, it was primarily influenced by the type of emulsions, with a relatively minor impact from the homogenization method used. Specifically, emulsions with a double-layer structure, prepared using microfluidization technology, exhibited significantly higher values (P ≤ 0.05) for this parameter. Additionally, for a meaningful comparison of the viscosity among the various nanoemulsions, the apparent viscosity at a shear rate of 100 s–1 (η100) is presented in Figure 3. This parameter holds significance in food science and processing as it offers essential insights into the behavior of a food product during mastication, processing, and quality control [43]. It significantly influences sensory attributes and the efficiency of manufacturing processes. The η100 values of the nanoemulsions, in general, were relatively low, indicating that the droplets in the emulsions did not flocculate, and the dispersion of the entire emulsion system was good. Similar to the K coefficient, the η100 values were significantly influenced (P ≤ 0.05) by the addition Ch and the homogenization method. The Ch addition to form the double layer in nanoemulsions substantially increased η100 in both the systems with DL and HL. This increase can be attributed to the structure formed by a three-dimensional network of interconnected Ch chains [44]. Furthermore, the apparent viscosity of double-layered nanoemulsions obtained by microfluidization exhibited higher η100 values (P ≤ 0.05). These results can be linked to the smaller droplet size in these systems, with increased droplet concentration and interfacial surface area, consequently enhancing the strength of the Ch network.
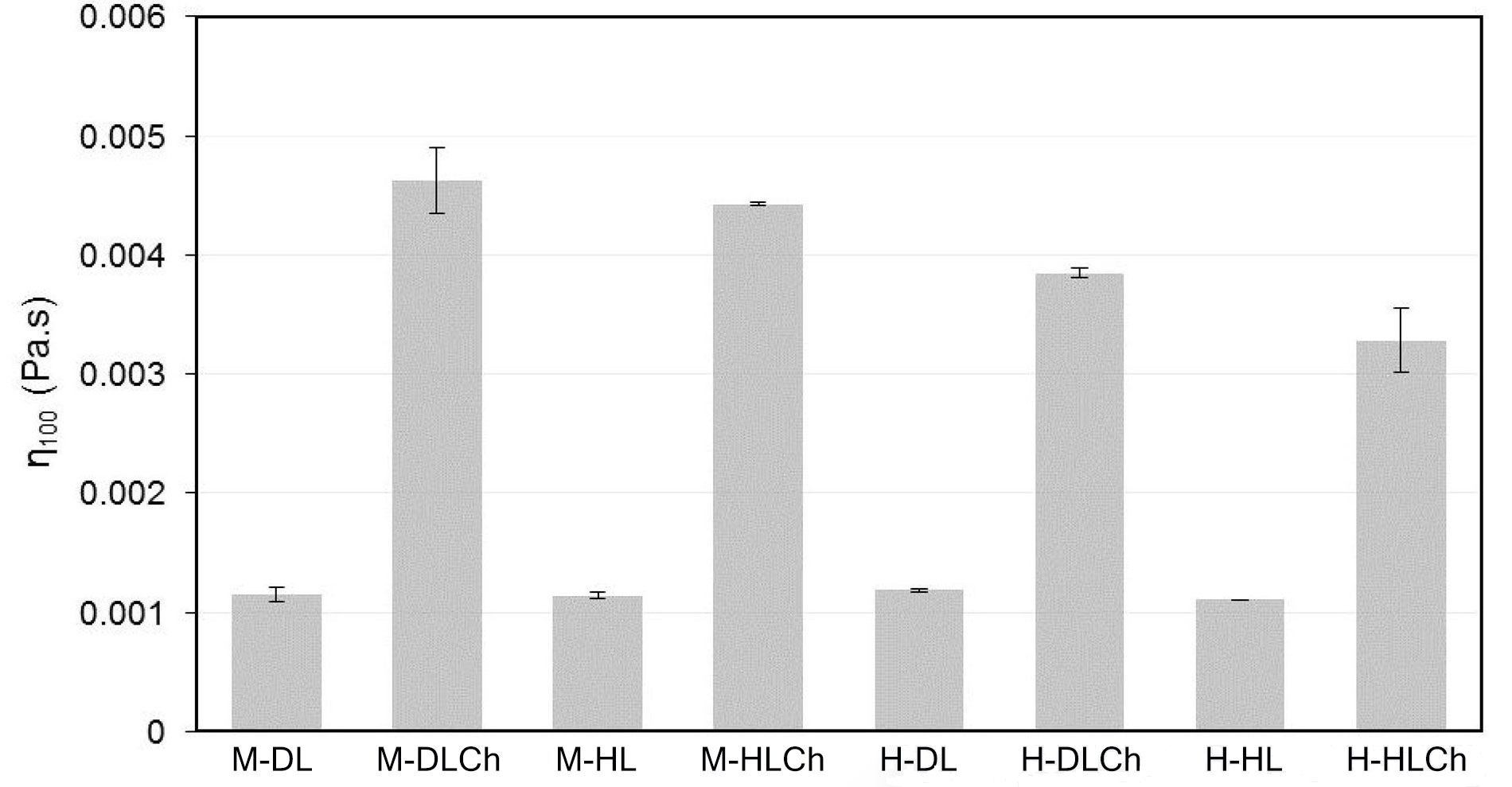
η100 for chia O/W mono and bilayer-nanoemulsions formulated with different modified sunflower lecithin (DL or HL), by two homogenization processes (high-pressure valve homogenization or microfluidics). Average values of two replications. Vertical bars indicate standard deviation. See Figure 1 for sample code
The physical stability of nanoemulsions was determined by analyzing their BS profiles over 30 days. These profiles are depicted as the %BS in relation to the tube length. Upon immediate preparation, the mono-layer systems exhibited an initial %BS (average of initial BS values along the entire sample tube, denoted as BS0) of approximately 76–78%, while the bilayer ones had an initial %BS of 82–84% (Figure 4).
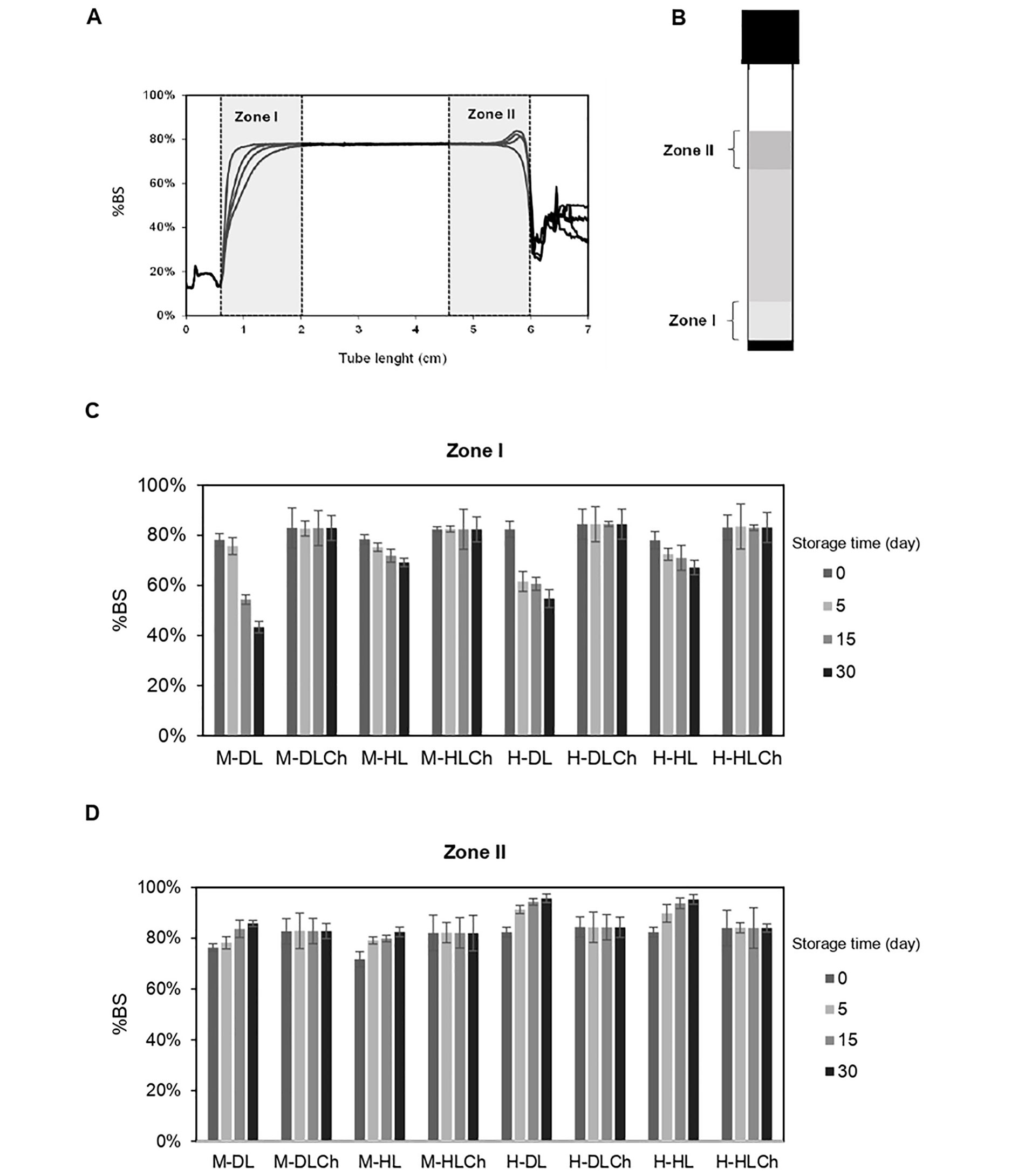
Stability of chia oil nanoemulsions. A. Illustrative BS profile of the monolayer chia nanoemulsion over time (each curve corresponds to a single time measurement); B. turbiscan sample tube with the zones I and II reference; C. average %BS at zones I; D. zone II of the sample tube corresponding to mono and bilayer chia nanoemulsion as a function of the storage time (0 day, 5 days, 15 days, and 30 days). The coefficient of variation was lower than 1%. See Figure 1 for the sample code
Immediately after the emulsion preparation, it can be considered a homogeneous PSD and thus associate their BS0 with their mean droplet diameter. According to this, when the droplet size is smaller than the incident wavelength (λ = 0.8 μm), it is expected that the %BS increases [45]. Thus, it would explain the lower values of BS0 found in the former, which presented larger droplets.
In order to spot signs of destabilization processes such as creaming or flocculation, the analysis of their BS profiles in two distinct zones within the sample tube, zone I (bottom of the sample tube) and zone II (top of the sample tube) was conducted (Figure 4). The bilayer nanoemulsions demonstrated remarkable physical stability, as evidenced by their unchanged BS profiles. Throughout the entire storage period, there were negligible variations in the average %BS values at the bottom and top of the sample tube. The high physical stability of these systems may be accounted for by different reasons. One of them could be the high surface charge of their oil droplets which would cause enough electrostatic repulsion between droplets and thus prevent the nanoemulsion coalescence. Another plausible explanation could be the smaller droplet size and higher apparent viscosity of bilayer nanoemulsions compared to monolayer systems. This combination may reduce droplet mobility and, as a result, hinder their upward movement, as predicted by Stokes’s law. In accordance with findings from previous studies, the Ch incorporation to create the double interfacial layer enveloping the oil droplets resulted in an elevation of the viscosity of the continuous phase. This viscosity increase contributed to the enhancement of creaming stability by retarding the diffusion of droplets and flocs [46].
Conversely, in the case of monolayer systems, a continuous creaming process was observed, starting as early as day 5 and continuing over time, resulting in the formation of a cream phase. The non-uniform distribution of oil droplets along the sample tube was confirmed by a decrease in the %BS in zone I and a simultaneous increase of this parameter in zone II, signifying destabilization due to creaming (Figure 4). Consequently, a gradual clarification process in the lower section of the sample tube (zone I) and the formation of a cream layer in zone II were noted. This phenomenon is attributed to the migration of oil droplets towards the upper zone, driven by the lower oil density in comparison to the aqueous phase. These observations may be linked to the lower apparent viscosity and zeta-potential recorded for the monolayer systems compared to their bilayer counterparts.
The confocal micrographs of the chia nanoemulsions produced using the HPH is shown in Figure 5. In agreement with the D [3, 2] results, the micrographs illustrated larger particle sizes in the monolayer nanoemulsions (Figure 5A and B) compared to the bilayer ones (Figure 5C and D), also observing variations in the size of the droplets depending on the type of lecithin used.
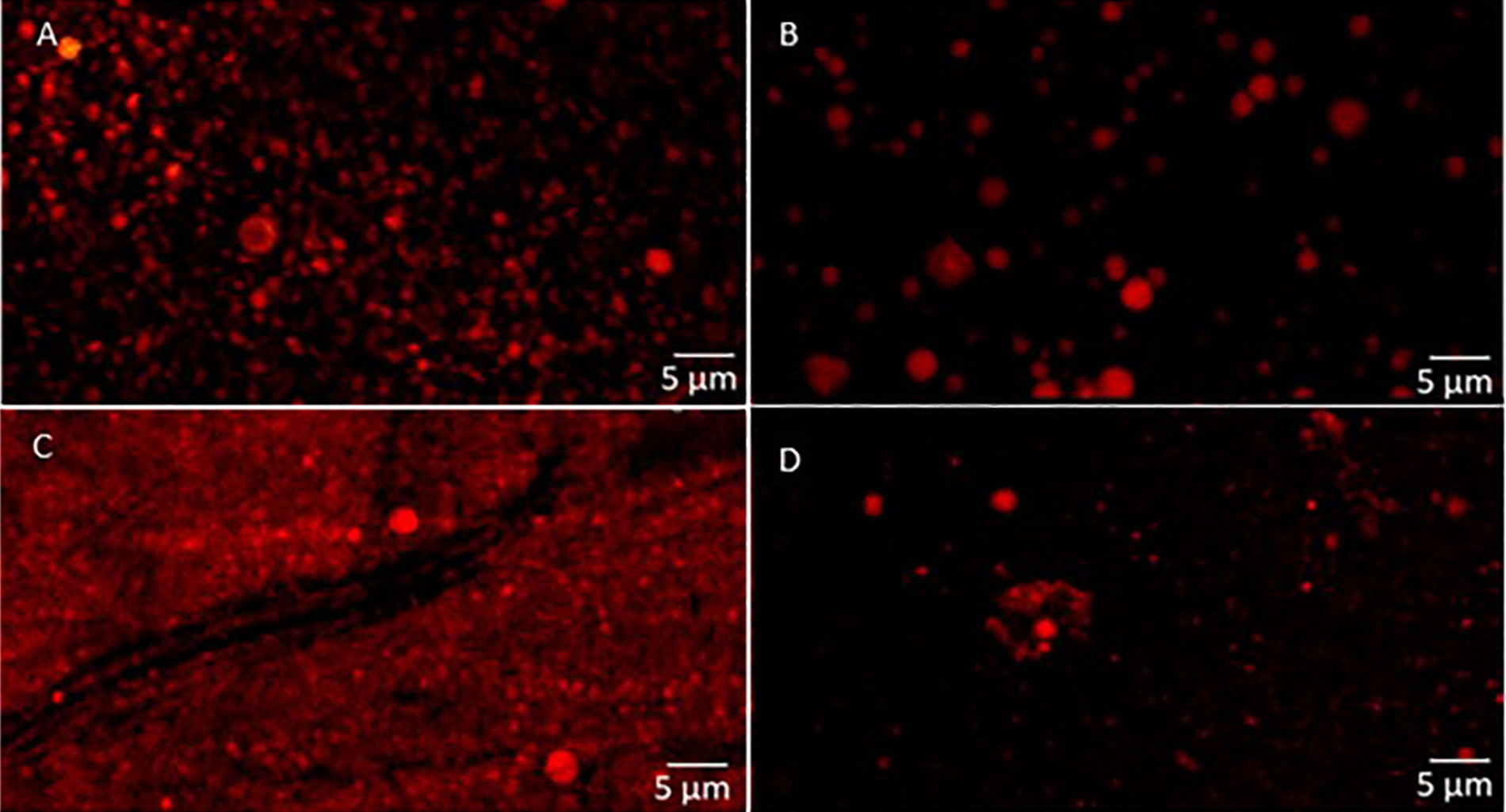
Confocal laser scanning micrographs of chia oil nanoemulsions obtained by HPH. A. H-DL, monolayer nanoemulsions with DL sunflower lecithin; B. H-HL, monolayer nanoemulsions with HL sunflower lecithin; C. H-DLCh, bilayer nanoemulsions with DL sunflower lecithin and Ch; D. H-HLCh, bilayer nanoemulsions with HL sunflower lecithin and Ch
Furthermore, it could be seen from Figure 5C and D that chia oil droplets appear to be uniformly distributed and trapped within the Ch network matrix in bilayer systems. In line with what was mentioned before, this arrangement may play a crucial role in the physical stability of these systems by reducing the upper movement of oil droplets, preventing the creaming.
The micrographs corresponding to the systems produced by microfluidization were similar to the bilayer systems with HL lecithin, and no differences were found between them (not shown).
Since one of the main objectives of this research was to deliver the omega-3 fatty acids from chia oil, their content immediately after nanoemulsions preparation and after 30 days of refrigerated storage was determined by 1H NMR (Figure 6).
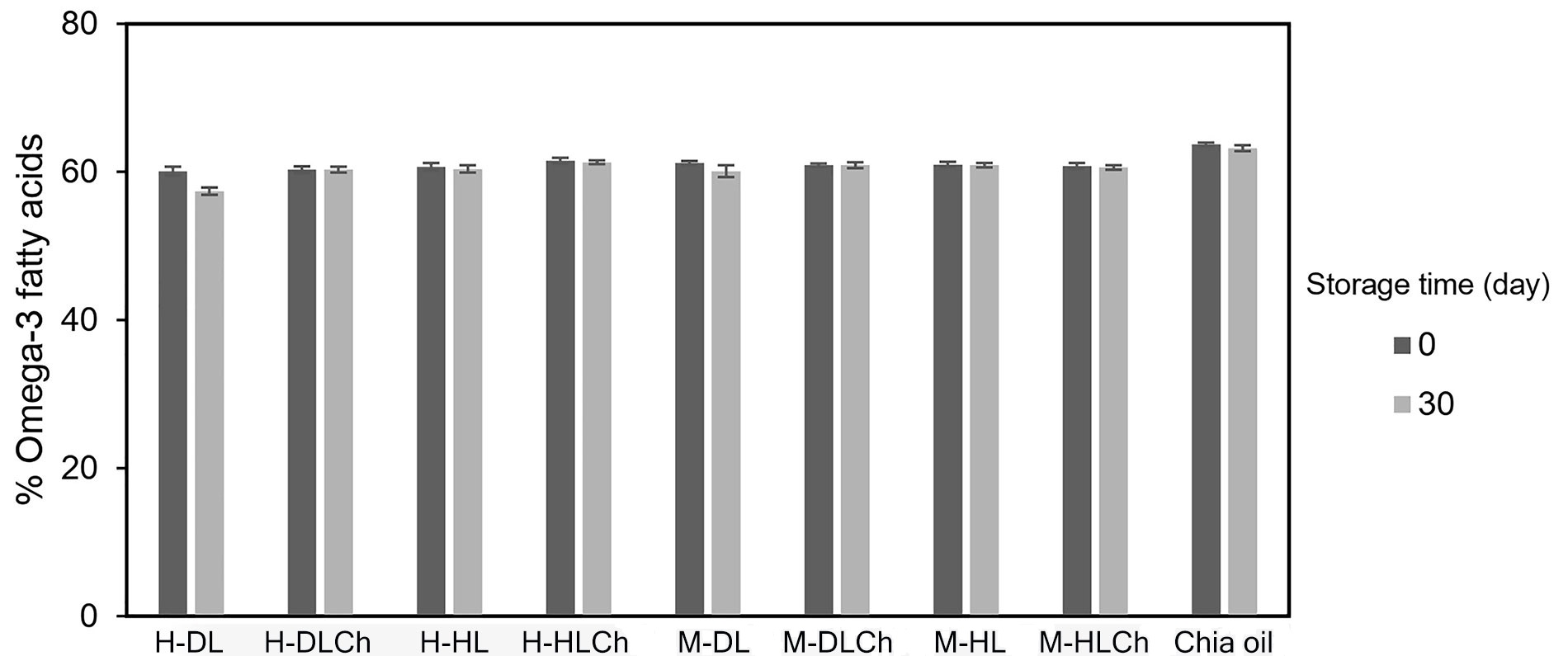
Omega-3 fatty acid content of chia mono and bilayer nanoemulsions at initial time and after 30 days of refrigerated storage. Average values of two replications. Vertical bars indicate standard deviation. See Figure 1 for sample code
As can be seen, the initial omega-3 content of the different nanoemulsions indicated that the emulsification process applied produced a slight loss of these bioactive compounds since it was similar to the bulk chia oil used as a control sample. In addition, it was observed that all nanoemulsions studied prevented the omega-3 degradation since their content remained constant for the entire storage period.
In this study, both mono and bilayer chia oil nanoemulsions were successfully produced using two different types of modified sunflower lecithin and high-energy methods. Overall, both variants of modified sunflower lecithin proved to be effective in the production of mono and bilayer chia nanoemulsions. The type of lecithin only influenced the droplet size of the nanoemulsions produced through high-pressure valve homogenization, with systems containing HL resulting in smaller droplets than DL.
Furthermore, the microfluidization process demonstrated superior performance compared to high-pressure valve homogenization in the production of chia oil nanoemulsions. Under identical homogenization conditions, the droplet sizes achieved through microfluidization were significantly smaller than those obtained via high-pressure valve homogenization, and emulsions exhibited higher apparent viscosities.
Additionally, the LBL technique employed to create a double interfacial layer in chia oil nanoemulsion-based delivery systems proved to be an efficient strategy in delaying the degradation of bioactive lipidic components and ensuring the production of highly physically stable systems. The addition of Ch altered the charge of the droplets from negative to positive and increased their absolute value, resulting in strong electrostatic repulsion among them. This modification also enhanced the apparent viscosity of the systems, further contributing to the physical stability of the nanoemulsions.
These results hold significance in the design and production of chia oil nanoemulsions, particularly bilayer systems using naturally sourced components to protect and deliver omega-3 PUFA into foods and nutraceuticals, with potential additional health benefits. Nevertheless, it is necessary to complement these studies with clinical research to guarantee the safety of these nanoemulsions when incorporated into food matrices intended for human consumption.
%BS: percentage of backscattered of light
1H NMR: proton nuclear magnetic resonance
1-LPC: 1-lysophosphatidylcholine
BS: backscattering
Ch: chitosan
D [3, 2]: surface-weighted diameter
DL: deoiled
HL: hydrolyzed
HLB: hydrophilic-lipophilic balance
HPHs: high-pressure valve homogenizers
K: flow consistency index
LBL: layer-by-layer
LPE: lysophosphatidylethanolamine
n: flow behavior index
O/W: oil-in-water
PC: phosphatidylcholine
PE: phosphatidylethanolamine
PSD: particle size distribution
PUFAs: polyunsaturated fatty acids
The authors wish to thank the donation of chia seed oil sunflower lecithins (Lasenor Emul S.L., Spain). The authors are also grateful to Flavio Accinelli (Bio Esanco S.A., Argentina), Mariela Fernández, and her working group (CETMIC, Argentina) for their technical assistance, particularly in measuring emulsion stability and zeta potential, respectively.
LMJ: Conceptualization, Investigation, Data curation, Methodology, Formal analysis, Writing—original draft, Visualization. CNC: Conceptualization, Investigation, Methodology. BWKD: Investigation, Methodology. MCT: Conceptualization, Methodology, Funding acquisition, Project administration, Supervision. VYI: Conceptualization, Investigation, Methodology, Writing—review & editing, Supervision, Visualization, Project administration. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Data from the present manuscript will be made available upon request.
This work was supported by Universidad Nacional de La Plata (UNLP) [11/X907]; Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) [PIP 2007]; Agencia Nacional de Promoción Científica y Tecnológica [PICT 2020-1274, PICT 2019-01775]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Angela Daniela Carboni ... María Cecilia Puppo
Lucas O. Benitez ... Juan M. Castagnini
Deniz Günal-Köroğlu ... Esra Capanoglu
Juliana Ripari Garrido ... María Victoria Salinas
Lucía Cassani, Andrea Gomez-Zavaglia
