Affiliation:
1Department of Internal Medicine, Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital, Chongqing 400030, China
†These authors contributed equally to this work.
Affiliation:
1Department of Internal Medicine, Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital, Chongqing 400030, China
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0003-4172-012X
Affiliation:
2Department of Critical Care Medicine, Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital, Chongqing 400030, China
Affiliation:
3Department of Anesthesia and Surgery, Shengli Oilfield Central Hospital, Dongying 257034, Shandong, China
Affiliation:
4Key Laboratory of Endocrine Glucose & Lipids Metabolism and Brain Aging, Ministry of Education, Department of Endocrinology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, Shandong, China
5Shandong Institute of Endocrine and Metabolic Diseases, Jinan 250021, Shandong, China
6Shandong Engineering Laboratory of Prevention and Control for Endocrine and Metabolic Diseases, Jinan 250021, Shandong, China
ORCID: https://orcid.org/0000-0003-1671-2646
Affiliation:
7Department of Endocrinology and Metabolism, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, Shandong, China
8Department of Endocrinology and Metabolism, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan 250021, Shandong, China
9Shandong Provincial Key Laboratory of Endocrinology and Lipid Metabolism, Jinan 250021, Shandong, China
ORCID: https://orcid.org/0000-0001-6743-8881
Affiliation:
1Department of Internal Medicine, Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital, Chongqing 400030, China
Affiliation:
4Key Laboratory of Endocrine Glucose & Lipids Metabolism and Brain Aging, Ministry of Education, Department of Endocrinology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, Shandong, China
5Shandong Institute of Endocrine and Metabolic Diseases, Jinan 250021, Shandong, China
6Shandong Engineering Laboratory of Prevention and Control for Endocrine and Metabolic Diseases, Jinan 250021, Shandong, China
Email: fxu@sdfmu.edu.cn
ORCID: https://orcid.org/0000-0002-8102-3903
Affiliation:
1Department of Internal Medicine, Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital, Chongqing 400030, China
Email: ling.luo.cqch@hotmail.com
Explor Med. 2023;4:1068–1078 DOI: https://doi.org/10.37349/emed.2023.00195
Received: June 19, 2023 Accepted: September 20, 2023 Published: December 29, 2023
Academic Editor: Lindsay A. Farrer, Boston University School of Medicine, USA
Aim: Respiratory failure is common after esophagectomy for esophageal cancer (EC). This study aimed to identify the risk factors associated with postoperative respiratory failure following esophagectomy for EC.
Methods: A single-center observational study from China was conducted on 262 patients with EC who underwent thoracoscopic esophagectomy between April 2014 and June 2016. The patients were divided into two groups: group I (respiratory failure) and group II (without respiratory failure). Demographic and perioperative variables, tumor-related factors, surgical factors, Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, and clinical course were compared between the groups. Univariable and multivariable logistic regression analyses were performed to assess the risk factors of postoperative respiratory failure after esophagectomy.
Results: Among the 262 patients, 24 (9.2%) developed respiratory failure. Univariable analysis revealed several risk factors, including age, smoking, comorbidities, partial pressure of oxygen (PO2), partial pressure of carbon dioxide (PCO2), forced vital capacity (FVC), FVC percentage (FVC%), urine volume during surgery, and APACHE II score. Multivariable analysis showed that age, comorbidities of diabetes mellitus (DM), FVC%, urine volume during surgery, and APACHE II score were independent predictors of respiratory failure. Specifically, elderly patients (> 65 years) with comorbidities of DM, lower FVC%, higher urine volume during surgery, and elevated APACHE II score were found to be more susceptible to respiratory failure, resulting in prolonged hospitalization and increased healthcare burden. These findings emphasize the importance of considering these factors in the management and care of patients at risk of respiratory failure.
Conclusions: As a common complication following esophagectomy for EC. Respiratory failure is significantly associated with age, comorbidities of DM, FVC%, urine volume during surgery, and APACHE II score in the dataset. The findings will contribute to the evaluation of the risk of respiratory failure and guide early intervention strategies in clinical decision-making.
Esophageal cancer (EC) is a prevalent malignant tumor of the digestive system, characterized by poor prognosis and low survival rates [1, 2]. Esophagectomy is the preferred treatment for resectable EC [3, 4]. However, this surgical procedure is associated with a relatively high morbidity and mortality rate. Pulmonary complications, especially respiratory failure, contribute significantly to extended hospital stays and unfavorable patient outcomes [5–10]. These pulmonary complications occur in 10–20% of patients undergoing esophagectomy [11–13]. Respiratory failure can further lead to poor outcomes, necessitating prolonged intensive care, and incurring higher hospitalization expenses [14–16]. The development of respiratory failure is associated with pneumonia, atelectasis, septic shock, and chest or rib injuries, all of which impair the breathing process [17–19]. Patients with chronic respiratory conditions such as chronic obstructive pulmonary disease (COPD) are at a higher risk of postoperative respiratory failure [20]. Furthermore, the period of one-lung ventilation during esophagectomy plays a crucial role, as the collapse and subsequent re-expansion of the nondependent lung can cause lung injury through an ischemia-reperfusion mechanism [21–23].
Therefore, identifying the risk factors for postoperative respiratory failure after esophagectomy and developing targeted prevention strategies for high-risk populations is of utmost clinical importance. This study aims to explore the risk factors associated with the occurrence of postoperative respiratory failure after esophagectomy for EC.
All patients who underwent thoracoscopic esophagectomy with lymphadenectomy for primary EC between April 2014 and June 2016 at Chongqing University Cancer Hospital were collected. As it is shown in Figure 1, patients with incomplete preoperative, intraoperative, and postoperative data were excluded. To avoid preoperative chemotherapy (CHT) or radiotherapy (RDT) playing a role in the surgical procedure, such as adhesion with the surrounding tissues after CHT/RDT, and the amount of intraoperative bleeding, patients who received CHT or RDT before surgery were excluded. The patients were divided into two groups based on the development of respiratory failure: group I (respiratory failure, N = 24) and group II (without respiratory failure, N = 79, randomly selected from 238 patients). The diagnosis of respiratory failure followed national diagnostic criteria [24]. Following esophagectomy, all patients were initially admitted to the intensive care unit (ICU). This study was approved by the ethics committee of Chongqing University Cancer Hospital (ethical approval number: CZLS2020040-A).
The following variables, including age, gender, Karnofsky Performance Scale (KPS) score, body mass index (BMI), smoking index, and comorbidities [such as COPD, coronary artery disease (CAD), congestive heart failure, and diabetes mellitus (DM)], were analyzed. Additionally, preoperative measurements of hemoglobin (Hb), albumin (Alb), arterial blood gas analysis, lung function, cancer histology, clinical staging (within 2 weeks before surgery), and preoperative hospital stay were also added and included for further investigation. Intraoperative variables, such as operation duration, blood loss, urine volume, and infusion amount, were also considered. Postoperative data, including antibiotic usage, mechanical ventilation, central venous catheterization, urethral catheterization, and Acute Physiology and Chronic Health Evaluation II (APACHE II) scoring system (within 24 h of ICU admission), were collected. Finally, the duration of ventilator use, central venous catheterization, urethral catheterization, and antibiotic usage, as well as postoperative stay, ICU stay, and total hospital stay, were documented.
Descriptive statistics were used to summarize continuous and categorical variables. Continuous variables were presented as mean ± SD (SD: standard deviation), and the normality of the continuous variables was determined by the Shapiro‐Wilk normality test. Categorical variables were expressed as frequencies and percentages. Comparisons involving continuous variables were performed using t-tests or the Wilcoxon rank sum test, depending on the data distribution. Categorical variables were analyzed using the chi-square test or Fisher’s exact test, as appropriate. Variables with a P-value < 0.05 in the univariable analysis [including age, smoking history, COPD, circulatory system diseases, DM, partial pressure of oxygen (PO2), partial pressure of carbon dioxide (PCO2), forced vital capacity (FVC), FVC percentage (FVC%), intraoperative urine volume, and APACHE II score] were included in the multivariable analysis. Multivariable logistic regression analysis with a stepwise regression method was employed to identify the risk factors associated with postoperative respiratory failure after esophagectomy. A P-value < 0.05 was considered statistically significant. Statistical analyses were conducted using SPSS version 26 (IBM Corp, Armonk, NY) and R version 3.5.0 (http://www.R-project.org; R Foundation for Statistical Computing, Vienna, Austria).
The demographic and preoperative parameters of the patients are present in Table 1. A total of 103 patients with a mean age of 61.8 years ± 6.1 years were analyzed. Notably, the average age in group I (65.1 years ± 5.6 years) was significantly higher than in group II (61.4 years ± 6.4 years) (P = 0.018). In group I, 83.3% of patients had a smoking history, with 80.0% being heavy smokers (smoking index ≥ 20 pack-years). In contrast, group II had a lower percentage of patients with a history of smoking (58.23%) and being heavy smokers (52.17%). Consequently, smokers, particularly heavy smokers, had a higher percentage of respiratory failure (P = 0.018). Additionally, the average number of comorbidities was significantly higher in group I (1.08 ± 0.62) compared to group II (0.42 ± 0.17) (P < 0.001). The most common comorbidities were COPD, circulatory system diseases, and DM, all of which were more prevalent in group I (P < 0.001, P = 0.001, and P = 0.029, respectively). In arterial blood gas analysis, group I had a lower average preoperative PO2 (75.80 mmHg ± 9.16 mmHg) and a higher PCO2 (38.56 mmHg ± 4.22 mmHg) compared to group II (PO2: 89.74 mmHg ± 10.25 mmHg; PCO2: 34.70 mmHg ± 3.46 mmHg) (both P < 0.001). Lastly, lung function between the two groups was compared. FVC and FVC% were significantly lower in group I (FVC: 2.68 L ± 0.60 L; FVC%: 85.58% ± 10.64%) than in group II (FVC: 3.22 L ± 0.67 L; FVC%: 94.5% ± 13.43%). There were no differences in gender, BMI, KPS scores, tumor-related factors (including tumor location, histology, and clinical stage), Hb (≤ 110 g/L), Alb (≤ 35 g/L), forced expiratory volume in 1 s (FEV1), the percentage of predicted FEV1 (FEV1%), FEV1/FVC, the maximum ventilation volume (MVV), and the preoperative stay between groups I and II.
Patients’ demographics and preoperative variables
| Variables | Group I (N = 24) | Group II (N = 79) | P-value* |
|---|---|---|---|
| Age (years) | 65.1 ± 5.6 | 61.4 ± 7.4 | 0.018 |
| ≥ 65:< 65 | 15:9 (62.5%:37.5%) | 27:52 (34.18%:65.82%) | 0.005 |
| Gender (males:females) | 20:4 (83.33%:16.67%) | 64:15 (81.01%:18.99%) | 0.788 |
| BMI (kg/m2) | 21.83 ± 3.37 | 21.82 ± 2.35 | 0.923 |
| KPS (≥ 80:< 80) | 16:8 (71.4%:28.6%) | 62:17 (78.48%:21.52%) | 0.183 |
| Smoking history (yes:no) | 20:4 (83.3%:16.7%) | 46:33 (58.23%:41.77%) | 0.017 |
| Smoking index (< 20:≥ 20 pack-years) | 4:16 (20.0%:80.0%) | 22:24 (47.83%:52.17%) | 0.018 |
| Comorbidities | |||
| Respiratory | 10 (41.67%) | 7 (8.86%) | < 0.001 |
| COPD | 8 (33.33%) | 4 (5.06%) | < 0.001 |
| Circulatory | 7 (29.17%) | 7 (8.86%) | 0.001 |
| DM | 6 (25.00%) | 9 (11.39%) | 0.029 |
| Others | 4 (16.67%) | 11 (13.92%) | 0.662 |
| Number of comorbidities | 1.08 ± 0.62 | 0.42 ± 0.17 | < 0.001 |
| Tumor location | 0.528 | ||
| Upper third | 4 (16.67%) | 7 (8.86%) | |
| Middle third | 16 (66.67%) | 63 (79.75%) | |
| Lower third | 2 (8.33%) | 4 (5.06%) | |
| Gastro-esophageal junction | 2 (8.33%) | 5 (6.33%) | |
| Histology | 0.802 | ||
| Squamous cell cancer | 22 (91.67%) | 73 (92.41%) | |
| Adenocarcinoma | 2 (8.33%) | 5 (6.33%) | |
| Other type | 0 (0.00%) | 1 (1.26%) | |
| Clinical stage | 0.058 | ||
| I | 5 (20.83%) | 5 (6.33%) | |
| II | 10 (41.67%) | 36 (45.57%) | |
| III | 9 (37.50%) | 37 (46.84%) | |
| IV | 0 (0.00%) | 1 (1.26%) | |
| Blood test | |||
| Hb (≤ 110 g/L) | 11 (45.83%) | 42 (53.16%) | 0.457 |
| Alb (≤ 35 g/L) | 13 (54.17%) | 37 (46.84%) | 0.697 |
| PO2 (mmHg) | 75.80 ± 9.16 | 87.74 ± 10.25 | < 0.001 |
| PCO2 (mmHg) | 38.56 ± 4.22 | 34.70 ± 3.46 | < 0.001 |
| Lung function | |||
| FVC | 2.68 ± 0.60 | 3.22 ± 0.67 | 0.043 |
| FVC% | 85.58 ± 10.64 | 94.50 ± 13.43 | 0.014 |
| FEV1 | 2.13 ± 0.43 | 2.18 ± 0.51 | 0.698 |
| FEV1% | 85.67 ± 9.76 | 86.67 ± 15.79 | 0.793 |
| MVV | 69.38 ± 22.22 | 69.58 ± 20.59 | 0.973 |
| MVV% | 64.08 ± 16.52 | 62.63 ± 16.79 | 0.937 |
| Preoperative stay (days) | 9.63 ± 4.52 | 8.53 ± 3.32 | 0.120 |
* Compared with the analysis of variance for interval data or chi-square test for categorical data. Blank cells mean not applicable. MVV%: MVV percentage
The comparison of surgical factors between the two groups is present in Table 2. Group I had higher operative blood loss, but no significant association with the occurrence of respiratory failure was observed (group I: 302.08 mL ± 419.23 mL; group II: 193.75 mL ± 79.83 mL; P = 0.220). Further analysis showed a significant difference in the percentage of cases with operative blood loss greater than 350 mL between the groups (group I: 7 cases, 29.16%; group II: 1 case, 1.27%; P = 0.004). Group I also had a larger urine volume during surgery, and the difference was significant (group I: 718.75 mL ± 355.95 mL; group II: 477.08 mL ± 279.74 mL; P = 0.012). The APACHE II scores (group I: 9.54 ± 4.02; group II: 6.75 ± 2.31) and predicted mortality (group I: 10.02% ± 2.86%; group II: 4.47% ± 2.35%) assessed within 24 h after ICU admission from surgery were higher in group I than in group II (both P < 0.05). There were no differences in the duration of surgery between groups.
Comparison of surgical factors between the groups
| Variables | Group I (N = 24) | Group II (N = 79) | P-value* |
|---|---|---|---|
| Duration of surgery (hours) | 4.55 ± 1.62 | 4.30 ± 1.58 | 0.566 |
| ≥ 5 h | 11 (45.8%) | 27 (34.18%) | 0.231 |
| Operative blood loss (mL) | 302.08 ± 419.23 | 193.75 ± 79.83 | 0.220 |
| ≥ 350 mL:< 350 mL | 7:17 (29.16%:70.83%) | 1:78 (1.27%:98.73%) | 0.004 |
| Urine volume during surgery (mL) | 718.75 ± 355.95 | 477.08 ± 279.74 | 0.012 |
| ≥ 600 mL:< 600 mL | 15:9 (62.50%:37.50%) | 23:56 (29.11%:70.89%) | 0.002 |
| Infusion amount | 2478.33 ± 1045.12 | 2110.42 ± 675.34 | 0.265 |
| APACHE II score (first 24 h in ICU) | 9.54 ± 4.02 | 6.75 ± 2.31 | 0.005 |
| ≥ 9:< 9 | 15:9 (62.50%:37.50%) | 13:66 (16.46%:83.54%) | 0.001 |
| Mortality predicted using the APACHE II score (%) | 10.02 ± 2.86 | 4.47 ± 2.35 | 0.002 |
* Compared with the analysis of variance for interval data or chi-square test for categorical data
The comparison of clinical courses between the groups is present in Table 3. The duration of respirator use (group I: 4.17 days ± 1.97 days; group II: 0.13 days ± 0.11 days), central venous catheter use (group I: 19.33 days ± 10.71 days; group II: 14.65 days ± 6.90 days), urethral catheter use (group I: 9.38 days ± 4.61 days; group II: 4.15 days ± 2.54 days), and antibiotic use (group I: 15.13 days ± 8.29 days; group II: 9.30 days ± 4.31 days) were significantly longer in group I than in group II. Consequently, the mean ICU stay (group I: 8.29 days ± 3.30 days; group II: 2.70 days ± 1.75 days), postoperative hospital stay (group I: 24.63 days ± 15.50 days; group II: 16.97 days ± 8.76 days), and total hospital stay (group I: 34.25 days ± 17.03 days; group II: 25.5 days ± 9.29 days) were longer in group I (all P values < 0.001).
Comparison of clinical course between the groups
| Variables | Group I (N = 24) | Group II (N = 79) | P-value* |
|---|---|---|---|
| Duration of respirator use (days) | 4.17 ± 1.97 | 0.13 ± 0.11 | < 0.001 |
| Duration of central venous catheter use (days) | 19.33 ± 10.71 | 14.65 ± 6.90 | 0.003 |
| Duration of urethra catheter use (days) | 9.38 ± 4.61 | 4.15 ± 2.54 | < 0.001 |
| Duration of antibiotic use (days) | 15.13 ± 8.29 | 9.30 ± 4.31 | < 0.001 |
| Post operation stay (days) | 24.63 ± 15.50 | 16.97 ± 8.76 | < 0.001 |
| ICU stay (days) | 8.29 ± 3.30 | 2.70 ± 1.75 | < 0.001 |
| Hospital stay (days) | 34.25 ± 17.03 | 25.50 ± 9.29 | < 0.001 |
* Compared with the analysis of variance for interval data or chi-square test for categorical data
Besides univariable analysis, multivariable logistic regression analysis revealed that age, DM, FVC%, intraoperative urine volume, and APACHE II score were independently associated with postoperative respiratory failure. Patients who were older than 65 years, had DM, lower FVC%, higher intraoperative urine volume, and higher APACHE II score had an increased risk of respiratory failure (Figure 2).
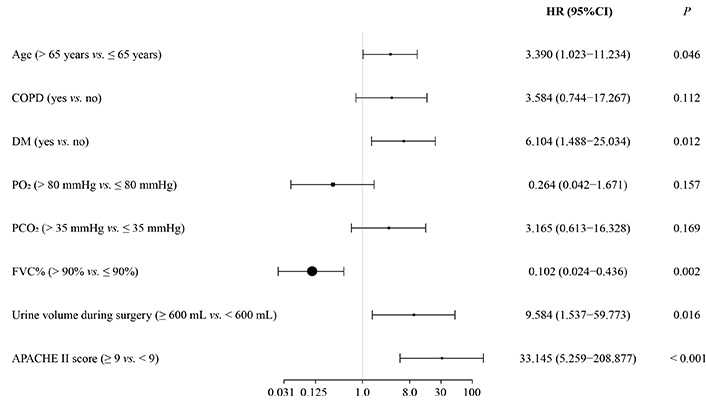
Independent risk factors for postoperative respiratory failure after esophagectomy for EC: multivariable logistic regression analysis. HR: hazard ratio; CI: confidence interval
Although there are many clinical studies on the risk factors for pulmonary complications after esophagectomy for EC, little research on the risk factors for postoperative respiratory failure. This study aims to explore the risk factors associated with the occurrence of postoperative respiratory failure after esophagectomy for EC in the southwest China population. The results showed that age, comorbidities of DM, FVC%, urine volume during surgery, and APACHE II score were independent risk factors for the development of respiratory failure, which was helpful to evaluate the risk factors of respiratory failure and guide early intervention strategies in clinical decision-making.
Pulmonary complications, particularly respiratory failure, have a significant negative impact on the survival of patients undergoing esophageal surgery for EC [9, 12, 13, 19, 25, 26]. According to the survey, the incidence rate of respiratory failure is as high as 30% [27]. Efforts have been made in recent years to reduce the incidence of respiratory failure and postoperative mortality through surgical and medical interventions [8, 15, 23, 28]. While improvements in surgical techniques have likely contributed to better outcomes, it is crucial to thoroughly examine all factors that contribute to postoperative respiratory failure.
The analysis of perioperative data revealed that patients aged 65 years or older, patients with a high smoking index (≥ 20 pack-years), patients with multiple comorbidities, those with lower preoperative arterial PO2 or higher PCO2 in blood gas analysis, and patients with lower FVC% had a significantly higher risk of postoperative respiratory failure. Among the surgical factors, higher intraoperative urine volume and a higher APACHE II score were associated with a greater incidence of respiratory failure. Additionally, there were no statistically significant differences in tumor location, pathology, or clinical stage between the two groups. Multivariable logistic regression analysis identified age, comorbidities of DM, FVC%, urine volume during surgery, and APACHE II score as independent risk factors for the development of respiratory failure.
Several factors have been reported to influence pulmonary complications after esophagectomy, such as age, pulmonary function, performance status, surgical approach, preoperative nutrition, smoking status, and neoadjuvant therapy [12, 29–32]. Ferguson et al. [33] proposed a scoring system based on age, working status, FEV1%, and lung carbon monoxide diffusion capacity (DLCO%). This scoring system accurately predicted pulmonary complications with a 70.8% accuracy. Other studies have also confirmed the independent roles of age, smoking, and impaired lung function in postoperative pulmonary outcomes [33–36]. However, there are few studies on the risk factors for postoperative respiratory failure after esophagectomy for EC. This study identified age, DM, FVC%, urine volume during surgery, and APACHE II score as independent risk factors for the development of respiratory failure.
With the advancements in technology, age is no longer considered a contraindication to surgery, leading to an increasing number of elderly patients with EC undergoing surgical procedures. As elderly patients experience a gradual decline in physical function and decreased respiratory compensation, the management of postoperative care becomes more challenging, often resulting in postoperative respiratory failure. This study confirms that age is indeed a risk factor for the development of postoperative respiratory failure, which aligns with previous research [37–39].
On the basis of the evaluation of preoperative lung function and comorbidities, it is of great significance to further evaluate the patient’s tolerance to surgery and postoperative complications. Previous studies have demonstrated that pulmonary function impairment was a significant indicator of postoperative respiratory failure in patients with EC [27, 40]. Based on the study of Yoshida et al. [36], COPD or DM was considered the main cause of postoperative respiratory failure in patients with EC. Other researchers have highlighted that respiratory tract infection and severe perioperative complications in patients with EC may increase their susceptibility to respiratory failure. Furthermore, patients with poor preoperative pulmonary function, severe perioperative complications, or multiple types of postoperative complications have much higher death risk [40, 41]. The finding of this study suggested that EC patients with preoperative pulmonary function impairment was at a higher risk of experiencing respiratory failure following surgical treatment. Therefore, pulmonary function impairment can be considered a potential risk factor for respiratory failure. The discoveries are consistent with previous studies.
The APACHE II score, alone or in combination with other indicators, has been utilized as a predictive tool for mortality in patients with severe forms of various pathologies [42]. An APACHE II score greater than 10 has been associated with higher mortality rates and serious complications [41]. In this study, when the APACHE II score exceeded 9, there was a significant difference between the two groups, indicating its potential as an indicator of postoperative prognosis. A significant difference in intraoperative urine volume between the two groups was observed, which may be related to intraoperative blood loss and fluid rehydration. However, further investigation is needed to validate this relationship.
Analysis of the clinical course revealed that the respiratory failure group experienced prolonged durations of respirator, central venous catheter, urethral catheter, and antibiotic use, as well as longer stays in the ICU and postoperative hospital stays. These findings are consistent with previous studies and underscore the impact of respiratory failure on hospitalization length and healthcare burden [5, 43, 44].
Nonetheless, this study has certain limitations. Firstly, it was a retrospective, single-center study, and some patients with incomplete data were excluded, resulting in a relatively small sample size. Secondly, similar to other retrospective studies, there may be unbalanced baseline characteristics, which might lead to bias between groups. Moreover, bias might also be inputed due to unmeasured confounders or missing data. Thirdly, the big difference in the number of patients between group I (respiratory failure) and group II (without respiratory failure) might also impair the power of the statistical models. To reduce the sample size difference, this study randomly selected one-third of the sample size from 238 patients as group II (without respiratory failure). Fourthly, the number of events (respiratory failure) was not big in the out dataset. Finally, the study lacked data on intervention measures for identified risk factors. Therefore, future large-scale, multicenter, and prospective studies are essential to further explore these findings.
In conclusion, this study identified age, DM, FVC%, intraoperative urine volume, and APACHE II score as independent risk factors for postoperative respiratory failure. These findings have the potentials to evaluate the risk of respiratory failure and further guide the early interventions in clinical practice.
Alb: albumin
APACHE II: Acute Physiology and Chronic Health Evaluation II
BMI: body mass index
CHT: chemotherapy
COPD: chronic obstructive pulmonary disease
DM: diabetes mellitus
EC: esophageal cancer
FEV1%: percentage of predicted forced expiratory volume in 1 s
FEV1: forced expiratory volume in 1 s
FVC%: forced vital capacity percentage
FVC: forced vital capacity
Hb: hemoglobin
ICU: intensive care unit
KPS: Karnofsky Performance Scale
MVV: maximum ventilation volume
PCO2: partial pressure of carbon dioxide
PO2: partial pressure of oxygen
RDT: radiotherapy
ZF and Hao Y: Conceptualization, Data curation, Formal analysis, Writing—original draft, Writing—review & editing. YL, DX, BS, Huguang Y, and CX: Data curation, Formal analysis. FX: Writing—review & editing. LL: Conceptualization, Writing—review & editing. All authors reviewed and approved the final manuscript.
The authors declare that they have no conflicts of interest.
The ethics committee of Chongqing University Cancer Hospital granted ethical approval for this study (ethical approval number: CZLS2020040-A), which complied with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
All datasets for this study are included in the manuscript.
This work was supported by Chongqing Science and Health Joint Medical Research Project No. [2023MSXM039]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3673
Download: 33
Times Cited: 0
