Affiliation:
Department of Thromboinflammation, V.A. Nasonova Research Institute of Rheumatology, 115522 Moscow, Russian Federation
Email: fariza_cheldieva@mail.ru
ORCID: https://orcid.org/0000-0001-5217-4932
Affiliation:
Department of Thromboinflammation, V.A. Nasonova Research Institute of Rheumatology, 115522 Moscow, Russian Federation
ORCID: https://orcid.org/0000-0003-1318-1894
Affiliation:
Department of Thromboinflammation, V.A. Nasonova Research Institute of Rheumatology, 115522 Moscow, Russian Federation
ORCID: https://orcid.org/0000-0002-3246-1157
Affiliation:
Department of Thromboinflammation, V.A. Nasonova Research Institute of Rheumatology, 115522 Moscow, Russian Federation
ORCID: https://orcid.org/0000-0002-4285-0869
Affiliation:
Department of Thromboinflammation, V.A. Nasonova Research Institute of Rheumatology, 115522 Moscow, Russian Federation
ORCID: https://orcid.org/0000-0002-6068-3080
Affiliation:
Department of Thromboinflammation, V.A. Nasonova Research Institute of Rheumatology, 115522 Moscow, Russian Federation
ORCID: https://orcid.org/0000-0002-1598-8360
Affiliation:
Department of Thromboinflammation, V.A. Nasonova Research Institute of Rheumatology, 115522 Moscow, Russian Federation
Email: t_reshetnyak@yahoo.com
ORCID: https://orcid.org/0000-0003-3552-2522
Explor Immunol. 2023;3:475–489 DOI: https://doi.org/10.37349/ei.2023.00114
Received: February 11, 2023 Accepted: April 20, 2023 Published: October 12, 2023
Academic Editor: Jean Amiral, Hyphen BioMed, France
The article belongs to the special issue Autoantibodies Associated to Thrombosis and Hemostasis
Aim: The study aims to evaluate the incidence of recurrent thromboses in patients with primary antiphospholipid syndrome (PAPS) and its association with the presence of different antiphospholipid antibodies (aPLs) and known thrombogenic risk factors.
Methods: This retrospective study included 52 patients. The median age of the patients was 38.5 years [31.5; 43.5], and the duration of the disease was 9.0 years [3.1; 13.0]. aPLs, including IgG/IgM/IgA antibodies to cardiolipin (aCLs), IgG/IgM/IgA anti-beta2-glycoprotein I (anti-β2-GPI), IgG anti-domain I-β2-GPI (anti-β2-GPIDI) antibodies, IgG/IgM antibodies to the phosphatidylserine/prothrombin complex (aPS/PT), and other thrombosis risk factors were included for analysis.
Results: Recurrent thrombosis was reported in 34 (65%) out of 52 patients and 18 (35%) did not have it. The main reason for the recurrence of thrombosis was the lack of anticoagulant therapy: in 18 (52.9%) out of 34 patients with recurrent thrombosis. Three patients were taking warfarin at the time of thrombosis recurrence, but target international normalized ratio (INR) levels were not achieved. Other patients with recurrent thrombosis were taking direct oral anticoagulants (DOACs). The risk of recurrent thrombotic events with positive IgG aCL was 10.33 (P = 0.002) and 21 (P = 0.007) times higher were examined in enzyme-linked immunoassay (ELISA) and chemiluminescent assay (CLA), respectively. The risk of thrombosis was 4.58 times higher in patients who were IgA aCL-positive (P = 0.01). Compared with other antibodies, with positive IgG values of anti-β2-GPI and IgG aPS/PT by ELISA, a lower probability of thrombosis recurrence was observed: 7.56 and 7.25, respectively. A high risk of recurrent thrombosis [odds ratio (OR) = 32.0] was observed in IgG anti-β2-GPI (CLA). The combination of IgG aCL with IgG anti-β2-GPI and with IgG anti-β2-GPIDI is more informative with respect to the risks of thrombosis recurrence compared to double positivity for aCL with anti-β2-GPI (OR = 20.71 vs. OR = 10.18). Triple positivity for IgG aCL with IgG anti-β2-GPI and with IgG aPS/PT also shows better results compared to positivity for aCL with anti-β2-GPI (OR = 6.06 vs. OR = 5.79). Among other risk factors, arterial hypertension (AH) and obesity were significant in relation to the recurrence of thrombosis. AH occurred in 22 (42%) of 52 patients with PAPS. AH was associated with recurrent thrombosis in PAPS patients: 18 (53%) out of 34 with recurrent thrombosis had AH versus 4 out of 18 without recurrent thrombosis (P = 0.003).
Conclusions: Recurrent thrombosis in antiphospholipid syndrome (APS) is largely associated with IgG aCL, IgG anti-β2-GPI, IgG anti-β2-GPIDI, IgG aPS/PT, and IgA aCL positivity. AH was a significant risk factor for recurrent thrombosis.
Antiphospholipid syndrome (APS) is an autoimmune multisystemic disorder, characterized by recurrent thrombosis and pregnancy morbidity [1]. Thrombotic APS is characterized by venous, arterial, and/or small vessel thrombosis in the context of persistently positive antiphospholipid antibodies (aPLs). The serological markers of APS include IgG and/or IgM antibodies to cardiolipin (aCLs) in serum or plasma, which are present in medium or high levels, IgG and/or IgM anti-beta2-glycoprotein I (anti-β2-GPI) and lupus anticoagulant (LA), which are detected two or more times at a study time interval of at least 12 weeks [2].
APLs play a decisive role in the pathogenesis of thrombosis [3]. In addition to the classical aPLs, new autoantibodies and antibody complexes of different Ig subtypes are now recognized as significant contributors to the pathogenesis of APS. The role of other antibodies such as IgA aCL, IgA anti-β2-GPI, anti-domain 1-β2-GPI (anti-β2-GPIDI) antibodies, IgG/IgM antibodies to the phosphatidylserine/prothrombin complex (aPS/PT) in the recurrence of thrombosis continues to be discussed [4].
The classical risk factors for arterial and venous thrombosis described for the general population are important in their relapses in patients with primary APS (PAPS). Stratification of patients depending on classical risk factors of thrombosis in combination with various aPLs would allow the best assessment of concomitant thrombotic risk factors of PAPS patients [5].
Thrombosis is the leading cause of mortality worldwide and can lead to irreversible organ damage, early disability, and premature death of patients. In patients with APS, the problem of recurrent thrombosis remains acute at the present time, despite the anticoagulant therapy.
To evaluate the frequency of recurrent thrombosis in patients with PAPS and their relationship with the presence of various aPL and known risk factors for thrombosis, this retrospective study included 52 patients with PAPS, of whom 30 (58%) were women and 22 (42%) were men. The median age of the patients was 38.5 [31.5; 43.5] years and the duration of the disease was 9.0 [3.1; 13.0] years (Table 1).
The clinical and laboratory characteristics of patients included in the study
| Parameter | Patients with PAPS, n = 52 |
|---|---|
| Average age, median [25; 75 percentiles], years | 38.5 [31.5; 43.5] |
| Duration of the disease, median [25; 75 percentiles], years | 9.0 [3.1; 13.0] |
| History of thrombosis, n (%) | |
| Arterial | 19 (37) |
| Venous | 23 (44) |
| Arterial + venous | 10 (19) |
| Recurrent thrombosis, n (%) | 34 (65) |
| Obstetric pathology*, n (%)/n | 18 (95)/19 |
| IgG аCL, n (%) | |
| by ELISA | 40 (77) |
| by CLA | 44 (85) |
| IgM аCL, n (%) | |
| by ELISA | 11 (21) |
| by CLA | 19 (36) |
| IgG anti-β2-GPI, n (%) | |
| by ELISA | 38 (73) |
| by CLA | 41 (80)/51 |
| IgМ anti-β2-GPI, n (%) | |
| by ELISA | 12 (23) |
| by CLA | 19 (36) |
| LA**, n (%)/n | 6 (75)/8 |
| IgA aCL by CLA, n (%)/N | 26 (53)/49 |
| IgA anti-β2-GPI by CLA, n (%)/N | 26 (53)/49 |
| IgG anti-β2-GPIDI by CLA, n (%)/N | 37 (77)/48 |
| AH, n (%) | 22 (42) |
| Hypercholesterolemia, n (%)/n | 1 (2)/46 |
| Active smoking, n (%) | 5 (10) |
| Type 2 diabetes mellitus, n (%) | 1 (2) |
| Obesity, n (%) | 15 (29) |
| Peri-operative, n (%) | 0 (0) |
| Hormonal contraception, n (%) | 0 (0) |
| Factor G20210A prothrombin (FII) mutation, n (%)/n | 2 (6)/32 |
| Factor V Leiden (FVL), n (%) | 0 (0)/32 |
| Therapy***, n (%) | |
| DOACs | 28 (54) |
| Warfarin | 12 (23) |
| LMWH | 4 (8) |
| Without anticoagulant therapy | 8 (15)**** |
| Low dose aspirin | 18 (35)***** |
| Hydroxychloroquine | 34 (65) |
*: obstetric pathology was calculated in women who had pregnancy in their disease course, in the numerator—“n” and “%” of women with obstetric pathology, in the denominator—“n” of women who had pregnancy in their disease course; numerator is the “n” and “%” of patients with positive aPLs, and denominator is the “n” of patients who had aPLs determination; **: LA study was performed in patients who have not taken anticoagulant therapy; ***: therapy at the time of inclusion in the study; ****: patients were diagnosed with APS for the first time, so they did not receive anticoagulant therapy before being included in the study; *****: five patients received aspirin in combination with anticoagulants, 13 patients received aspirin without anticoagulants. AH: arterial hypertension; CLA: chemiluminescent assay; DOACs: direct oral anticoagulants; ELISA: enzyme-linked immunoassay; LMWH: low-molecular-weight heparin; median [25; 75 percentiles]: median with interquartile range; n: number of patients
The diagnosis of APS was based on the 2006 international classification criteria [2]. PAPS was verified in a patient in the absence of signs of any other disease and in the presence of those with definite APS. Patients with obstetric APS alone were not included in this study.
AH was defined by elevated systolic blood pressure (BP) > 140 mmHg and/or diastolic BP > 90 mmHg on at least two occasions or the use of oral antihypertensive medications.
Serum total cholesterol levels were determined by standard enzymatic methods and interpreted according to the values obtained at the time of inclusion of patients in the study. Hyperlipidemia was considered when the levels of total cholesterol and triglycerides exceeded the reference values (for total cholesterol, the reference values were 3.90–6.20 mmol/L).
FVL and FII mutations were examined by polymerase chain reaction.
Body mass index (BMI) was calculated for each patient at the time of inclusion in the study. BMI of 30 was considered obesity.
The study of aPL involved the determination of IgG/IgM aCL, IgG/IgM anti-β2-GPI and IgG/IgM aPS/PT by ELISA, IgG/IgM/IgA aCL, IgG/IgM/IgA anti-β2-GPI, and IgG anti-β2-GPIDI by CLA.
IgG/IgM aCL, IgG/IgM anti-β2-GPI were determined by ELISA on an automatic analyzer for laboratory diagnosis of autoimmune diseases Alegria (Orgentec Diagnostika GmbH, Germany) with a reagent kit for antibody determination by Orgentec Diagnostika GmbH, Germany. IgG aCLs were measured in the phospholipid-binding activity of IgG aCLs per 1 IU/mL in GPL units, and IgM aCLs were measured in the phospholipid-binding activity of IgM aCLs per 1 IU/mL in MPL units. IgG/IgM anti-β2-GPI was measured in IU/mL. Values > 25.00 GPL for IgG aCL, > 24.70 MPL for IgM aCL, > 15.30 IU/mL for IgG anti-β2-GPI, and > 17.00 IU/mL for IgM anti-β2-GPI were considered positive [6].
IgG/IgM aPS/PT was determined by ELISA using a Tecan sunrise absorption microplate spectrophotometer (Austria) with an AESKULISA Serin-Prothrombin-GM reagent kit for antibody determination. IgG/IgM aPS/PT were measured in IU/mL. Based on the mean values of the control group for the determination of IgG/IgM aPS/PT the positivity levels were identified according to the formulas: arithmetic mean + 3 standard deviations (SDs) or 5 SDs: mean + 3 SDs and mean + 5 SDs. The diagnostic significance of the isolated levels for positivity and the levels proposed by the reagent manufacturers was assessed, as a result of which the positivity levels were determined: for IgG aPS/PT > 73.6 IU/mL (mean + 5 SDs) and for IgM aPS/PT > 18.0 IU/mL (the data from the reagent’s manufacturer).
The patients included in the study were tested for IgG/IgM/IgA aCL, IgG/IgM/IgA anti-β2-GPI by CLA using a BIO-FLASH® analyzer (Biokit S.A., Spain). The reagent kits were AcuStar (Spain) for the detection of IgG/IgM anti-β2-GPI and IgG/IgM aCL and QUANTA Flash® (USA) for determination of IgA aCL, IgA anti-β2-GPI, and IgG anti-β2-GPIDI. The tested aPLs were measured in chemiluminescent unit (CU). Based on the mean values of the control group for the determination of IgG/IgM/IgA aCL, IgG/IgM/IgA anti-β2-GPI, and IgG anti-β2-GPIDI, the positivity levels were identified according to the formulas: arithmetic mean + 3 SDs or 5 SDs: mean + 3 SDs and mean + 5 SDs. The diagnostic significance of the isolated levels for positivity and the levels proposed by the reagent manufacturers was assessed, as a result of which the positivity levels were determined: for IgG aCL > 25.9 CU (mean + 5 SDs), for IgM aCL > 19.5 CU (mean + 3 SDs), for IgA aCL > 18.9 CU (mean + 5 SDs), for IgG anti-β2-GPI > 32.0 CU (mean + 5 SDs), for IgM anti-β2-GPI > 6.9 CU (mean + 3 SDs), for IgA anti-β2-GPI > 20.0 CU (the data from the reagent’s manufacturer), and for IgG anti-β2-GPIDI > 20.0 CU (the data from the reagent’s manufacturer).
The following indicators were used to describe quantitative variables: arithmetic mean, SD, median, 25 and 75 percentiles, as well as the frequency for qualitative variables. Differences were considered to be statistically significant at P ≤ 0.05. The frequency differences for two independent study group objects were calculated using one-factor logistic regression. Odds ratio (OR) and confidence interval (CI) were calculated using the Hooke-Jeeves and quasi-Newton one-factor logistic regression method and by constructing forest-plot diagram. Receiver operating characteristic (ROC) curves were used to analyse the relationship between the sensitivity and specificity of aPL in relation to recurrent thrombosis. ROC curves were generated using the IBM SPSS Statistics 13.0 for Windows software package (IBM Corporation, USA).
Recurrent thrombosis was reported in 34 (65%) out of 52 patients and 18 (35%) did not have it. One case of thrombosis recurrence was in 16 (47%) out of 34 patients, 2 cases in 8 (23%), 3 cases in 2 (6%), 4 cases in 4 (12%), 5 cases or more in 4 (12%). The main reason for the recurrence of thrombosis was the lack of anticoagulant therapy: in 18 (52.9%) out of 34 patients with recurrent thrombosis. Eight patients with PAPS did not receive anticoagulant therapy before diagnosis (Table 1). They were diagnosed for the first time when they were included in the study. Three patients were taking warfarin at the time of thrombosis recurrence, but target international normalized ratio (INR) levels were not achieved. Other patients with recurrent thrombosis were taking DOACs.
The total number of recurrent thromboses was 74: 41 (56%) recurrences were without anticoagulant therapy, 22 (30%) recurrences on DOAC therapy, 9 (12%) recurrences on warfarin, 2 (3%) recurrences on low molecular weight heparin (LMWH). The duration of warfarin therapy was 60.0 [24.0; 192.0] months and the duration of DOAC therapy was 10.5 [3.0; 48.0] months.
Patients were divided into two groups depending on the presence or absence of recurrent thrombosis. The first group included patients with recurrent thrombosis (n = 34), and the second group—without recurrence with one thrombotic event (n = 18).
As shown in Table 2, recurrent thrombosis was observed in 31 patients with IgG aCL by ELISA, whereas IgG aCL-negative patients had recurrent thrombosis in 3 (P = 0.002, OR = 10.33). The risk of recurrent thrombotic events with IgG aCL-positive by CLA was 21 times higher (P = 0.007). A lower probability recurrence of thrombosis was observed with positive IgG anti-β2-GPI and IgG aPS/PT values in ELISA: 7.56 and 7.25, respectively. A high risk of thrombosis recurrence (OR = 32.0) was noted when IgG anti-β2-GPI was determined by the CLA method (Table 2): 33 (97%) out of 34 patients with recurrent thrombosis were positive for IgG anti-β2-GPI and against 9 (50%) with IgG anti-β2-GPI out of 18 patients without recurrent thrombosis (P = 0.002).
Relationship between aPLs and recurrent thrombosis in patients with PAPS
| aPL | Recurrent thrombosis | P | OR (95% CI) | ||
|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | ||||
| IgG aCL, n = 52; ELISA | + | 31 (91) | 9 (50) | 0.002 | 10.33 (2.20–48.37) |
| – | 3 (9) | 9 (50) | |||
| IgM aCL, n = 52; ELISA | + | 7 (21) | 4 (22) | 0.89 | 0.90 (0.21–3.76) |
| – | 27 (79) | 14 (78) | |||
| IgG anti-β2-GPI, n = 52; ELISA | + | 29 (85) | 9 (50) | 0.004 | 7.50 (1.79–31.36) |
| – | 4 (15) | 9 (50) | |||
| IgM anti-β2-GPI, n = 52; ELISA | + | 8 (23) | 4 (22) | 0.91 | 1.07 (0.26–4.36) |
| – | 26 (77) | 14 (78) | |||
| IgG aPS/PT, n = 52; ELISA | + | 29 (85) | 8 (44) | 0.003 | 7.25 (1.85–28.35) |
| – | 5 (15) | 10 (56) | |||
| IgM aPS/PT, n = 52; ELISA | + | 13 (38) | 7 (39) | 0.96 | 0.97 (0.29–3.23) |
| – | 21 (62) | 11 (61) | |||
| aCL with anti-β2-GPI, n = 52; ELISA | + | 29 (85) | 9 (50) | 0.009 | 5.79 (1.48–22.58) |
| – | 5 (15) | 9 (50) | |||
| IgG aCL with IgG anti-β2-GPI and with IgG aPS/PT, n = 52; ELISA | + | 27 (79) | 7 (39) | 0.005 | 6.06 (1.66–22.09) |
| – | 7 (21) | 11 (61) | |||
| IgG aCL, n = 52; CLA | + | 33 (97) | 11 (61) | 0.007 | 21.00 (2.17–202.78) |
| – | 1 (3) | 7 (39) | |||
| IgM aCL, n = 52; CLA | + | 13 (38) | 6 (33) | 0.72 | 1.23 (0.36–4.23) |
| – | 21 (62) | 12 (67) | |||
| IgA aCL, n = 49; CLA | + | 21 (66) | 5 (29) | 0.01 | 4.58 (1.23–16.94) |
| – | 11 (34) | 12 (71) | |||
| IgG anti-β2-GPI, n = 52; CLA | + | 33 (97) | 9 (50) | 0.002 | 32.00 (3.33–307.02) |
| – | 1 (3) | 9 (50) | |||
| IgM anti-β2-GPI, n = 52; CLA | + | 13 (38) | 6 (33) | 0.72 | 1.23 (0.36–4.23) |
| – | 21 (62) | 12 (67) | |||
| IgA anti-β2-GPI, n = 49; CLA | + | 18 (37) | 5 (29) | 0.07 | 3.08 (0.84–11.20) |
| – | 14 (63) | 12 (71) | |||
| IgG anti-β2-GPIDI, n = 48; CLA | + | 29 (93) | 8 (47) | 0.001 | 16.31 (2.77–96.03) |
| – | 2 (7) | 9 (53) | |||
| aCL with anti-β2-GPI, n = 52; CLA | + | 32 (94) | 11 (61) | 0.008 | 10.18 (1.74–59.24) |
| – | 2 (6) | 7 (39) | |||
| IgG aCL with IgG anti-β2-GPI and with IgG anti-β2-GPIDI, n = 48; CLA | + | 29 (93) | 7 (41) | 0.0006 | 20.71 (3.48–123.00) |
| – | 2 (7) | 10 (59) | |||
+: positive aPLs; –: negative aPLs. P: reliability. P value ≤ 0.05 represents significance difference
The combination of IgG aCL with IgG anti-β2-GPI and with IgG anti-β2-GPIDI is more informative with respect to the risks of thrombosis recurrence compared to double positivity for IgG aCLwith IgG anti-β2-GPI. Triple positivity for IgG aCL with IgG anti-β2-GPI and with IgG aPS/PT was associated with a significant risk of thrombosis recurrence compared to double positivity IgG aCL with IgG anti-β2-GPI (Table 2).
No correlation between IgM aPL positivity and recurrent thrombosis was observed (Table 2). The Figure 1A and 1B show the OR and CI of the incidence of aPL in groups of patients with recurrent thrombosis and without recurrent thrombosis.
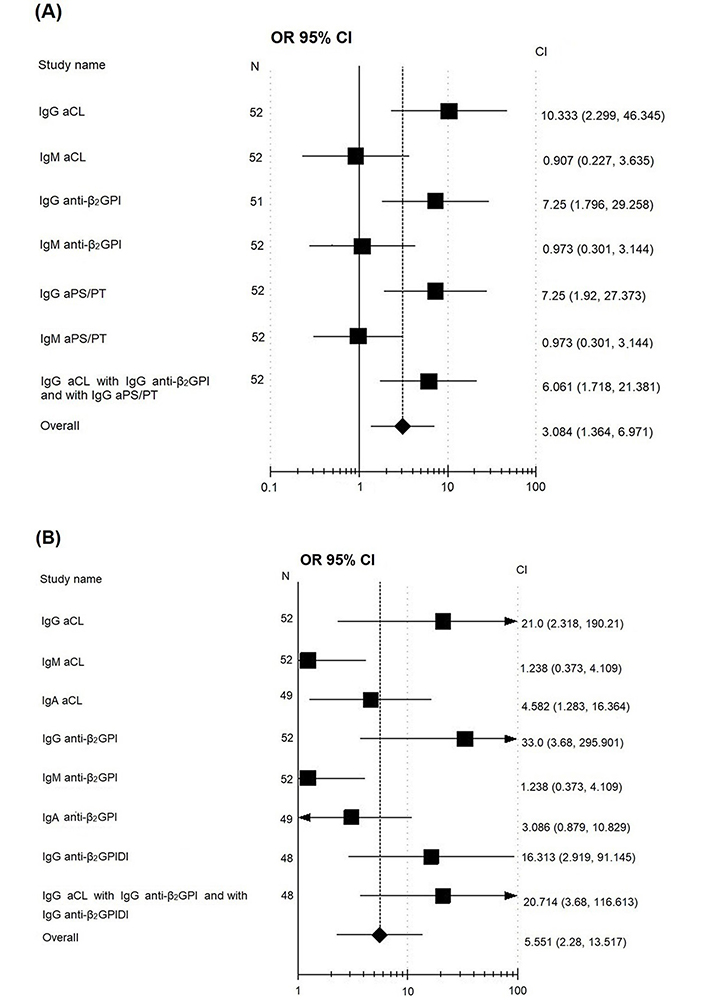
OR and CI of the incidence of aPL in their determination by ELISA (A) and CLA (B) in groups of patients with recurrent thrombosis and without recurrent thrombosis. Analysis of the influence of independent factors (recurrent thrombosis) on the studied variables (aPL) used the method of drawing a forest-plot diagram, based on the data of PAPS with and without recurrent thrombosis; squares denote the features (aPL), located to the right of the vertical line passing through 1; rhombus at the bottom—the average parameter for all indicators, if it crosses the vertical line, it means that the result including data from all the studies is statistically insignificant, if the rhombus is shifted (and does not cross the vertical line) to the right, it means there are more events in the patients with recurrent thrombosis, if to the left, then in the patients without recurrent thrombosis
The diagnostic efficiency of aPL depending on the presence of recurrent thrombosis was evaluated according to the ROC curves (Figure 2) in determination of IgG aPL by ELISA (Figure 2A) and IgG aPL and IgA aCL CLA (Figure 2B).
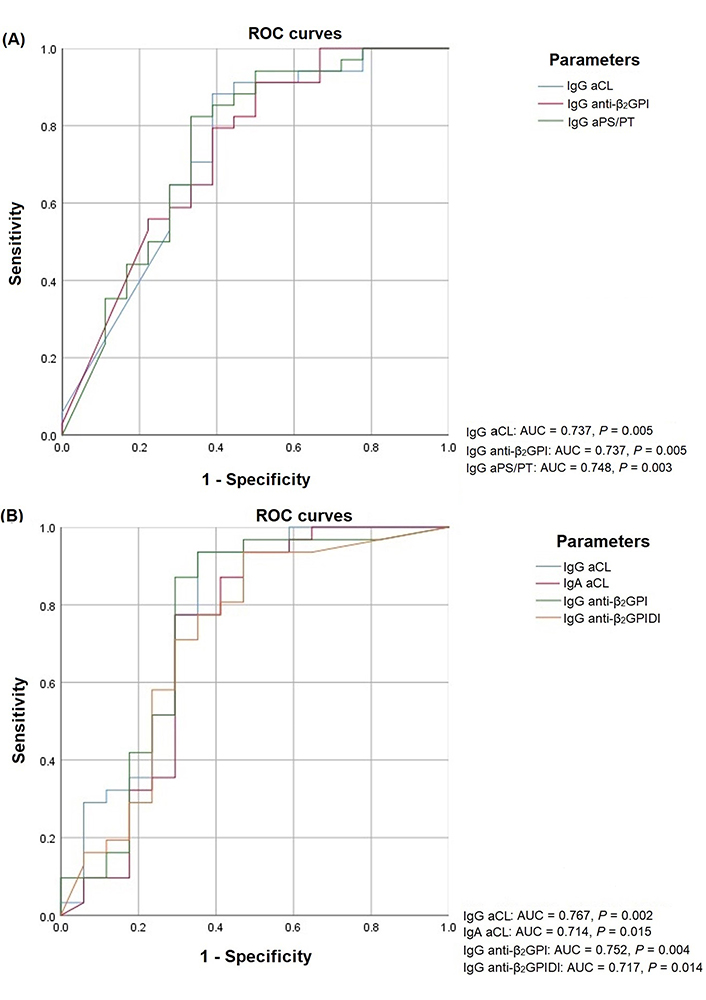
ROC curves aPL depending on recurrent thrombosis in the determination of IgG aPL by ELISA (A) and IgG aPL/IgA aCL by CLA (B). AUC: area under curve
The area under the ROC curve for all tested antibodies is given in Table 3. The area under the ROC curve in recurrent thrombosis for IgG aCL by ELISA was 0.737 (0.583–0.891) and 0.767 (0.607–0.927) by CLA, for IgG anti-β2-GPI was 0.737 (0.587–0.887) by ELISA and 0.752 (0.583–0.922) by CLA. The area under the ROC curve of more than 0.700 is also noted for some non-criteria—aPL: IgG aPS/PT, IgA aCL, IgG anti-β2-GPIDI (P < 0.05).
The area under the ROC curve for all tested antibodies
| Method of determination | aPL | AUC | P | 95% CI |
|---|---|---|---|---|
| ELISA | IgG aCL | 0.737 | 0.005 | 0.583–0.891 |
| IgM aCL | 0.582 | 0.336 | 0.405–0.758 | |
| IgG anti-β2-GPI | 0.737 | 0.005 | 0.587–0.887 | |
| IgM anti-β2-GPI | 0.557 | 0.501 | 0.390–0.724 | |
| IgG aPS/PT | 0.748 | 0.003 | 0.596–0.901 | |
| IgM aPS/PT | 0.555 | 0.519 | 0.369–0.740 | |
| CLA | IgG aCL | 0.767 | 0.002 | 0.607–0.927 |
| IgM aCL | 0.578 | 0.377 | 0.400–0.755 | |
| IgA aCL | 0.714 | 0.015 | 0.535–0.894 | |
| IgG anti-β2-GPI | 0.752 | 0.004 | 0.583–0.922 | |
| IgM anti-β2-GPI | 0.557 | 0.383 | 0.398–0.756 | |
| IgA anti-β2-GPI | 0.674 | 0.049 | 0.492–0.856 | |
| IgG anti-β2-GPIDI | 0.717 | 0.014 | 0.549–0.885 |
P value ≤ 0.05 represents significance difference
AH occurred in 22 (42%) of 52 patients with PAPS. AH was associated with recurrent thrombosis in PAPS patients: 18 (53%) out of 34 with recurrent thrombosis had AH versus 4 out of 18 without recurrent thrombosis (P = 0.003; Table 4). The risk of recurrent thrombosis in PAPS patients was 3.93 times higher in the presence of AH. There was no significant association between smoking and recurrent thrombosis. There was a trend toward recurrent thrombotic events in obese patients: 13 (38%) of 34 patients with recurrent thrombosis were obese versus 2 (11%) of 18 patients who were obese but without recurrent thrombosis (P = 0.053).
Relationship between comorbidities and recurrent thrombosis in patients with PAPS
| Comorbidities | Recurrent thrombosis | P | OR (95%CI) | ||
|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | ||||
| AH, n = 52 | + | 18 (53) | 4 (22) | 0.03 | 3.93 (1.03–14.92) |
| – | 16 (47) | 14 (78) | |||
| Hypercholesterolemia, n = 46 | + | 0 (0) | 1 (7) | 0.70 | - |
| – | 31 (100) | 14 (93) | |||
| Active smoking, n = 52 | + | 2 (6) | 3 (17) | 0.22 | 0.31 (0.04–2.17) |
| – | 32 (94) | 15 (83) | |||
| Type 2 diabetes mellitus, n = 52 | + | 1 (3) | 0 (0) | 0.74 | - |
| – | 33 (97) | 18 (100) | |||
| Obesity, n = 52 | + | 13 (38) | 2 (11) | 0.053 | 4.95 (0.93–26.22) |
| – | 21 (62) | 16 (89) | |||
+: positive comorbidities; –: negative comorbidities; -: not applicable. P value ≤ 0.05 represents significance difference
Thirty-two (61%) of 52 patients were tested for mutations in the genes of blood clotting G20210A FII and FVL. Two (6.25%) of them had a heterozygous G20210A FII mutation, and none of them had FVL mutation. Both patients with G20210A FII mutation had recurrent thrombosis. In both cases, stable positive levels of IgG and IgG anti-2-GPI were observed (triple positivity in the anamnesis).
There were no patients with prolonged use of hormonal contraceptives or in the peri-operative period in our study.
Since diagnoses of APS cannot be made without the presence of aPLs, their detection in the blood is of paramount importance in the diagnosis of the disease, since thrombosis and pregnancy pathology also occur in many other diseases [7]. In addition, the results of laboratory tests are crucial for predicting and stratifying the risk of developing clinical manifestations of APS [8–10].
In our study, LA was determined in only 8 patients, out of 52, because the anticoagulant therapy was prescribed to all other patients. Temporary withdrawal of anticoagulants for LA study is associated with high risks of thrombosis. In some countries, heparin neutralizers and DOAC-Stop® could be used to study LA even if patient take anticoagulants, but unfortunately in our laboratory heparin neutralizers and DOAC-Stop® are not used.
According to the updated criteria, in contrast to the original Sapporo criteria [11], it is recommended to classify patients with APS into those with only one type of aPL positivity and those with double/triple positivity. This classification is necessitated by the evidence that patients with only one type of aPL positive are at a lower risk of thrombosis compared to those with double and/or triple positivity [12]. Our results are consistent with those of Kearon et al. [10] who noted that patients who had more than one type of aPL positive on the same or different occasions had an approximately three-fold higher risk of recurrence than patients who had only one type.
The aim of our study was to evaluate the frequency of recurrent thrombosis in patients with PAPS and their relationship with the presence of various aPLs and known risk factors for thrombosis. A retrospective analysis showed that cases of recurrent thrombosis were noted in 65% of patients with PAPS. The relationship of each type of aPL to thrombosis recurrence in each patient with PAPS was evaluated. The main cause of recurrent thrombosis was noted to be the absence of anticoagulant therapy in 52.9% of cases. Thrombosis recurred more frequently on DOAC therapy compared to warfarin therapy (30% vs. 12%). A systematic review published previously investigated the use of DOACs in 447 patients with APS [13]. According to the review, a recurrence of thrombosis was observed in 16% of patients taking DOACs [13].
The presence of IgG aPL was found to be associated with a high risk of recurrence of thrombosis (Table 2). A significant association between IgM aPL and recurrent thrombosis was not obtained in our study. Methods of aPL research were important, as it was obtained, for predicting the risk of thrombosis recurrence. Two methods of studying classical aPL were used in our study: ELISA and CLA. The CLA method was more sensitive for predicting the risk of recurrence of thrombosis. The risk of recurrence of thrombosis in relation to the OR was 2 or more times higher compared to ELISA [for IgG aCL (OR = 21.00 vs. OR = 10.33, respectively) and for IgG anti-β2-GPI (OR = 32.00 vs. OR = 7.50, respectively)]. Higher OR in aPL studies by ELISA is most likely related to their more frequent detection by CLA compared to ELISA [14]. This is due to the advantage of the CLA method in high analytical sensitivity and performance, a wide range of detectable concentrations while maintaining high accuracy in any part of the calibration curve. The method completely eliminates the influence of interfering substances during measurement, which guarantees high accuracy of the result, unlike classical ELISA analyzers [15, 16]. According to the literature, CLA has lower sensitivity in general compared to ELISA, but is more specific than ELISA [17].
The importance of other “extra” criterion antibodies was noted, especially when combining them in different compositions. The presence of IgG anti-β2GPIDI was significantly associated with relapses of thrombosis [OR = 16.31 (2.77–96.03), Р = 0.001]. The risk stratification of thrombosis is necessary for the further management of patients with APS. Our previous work suggests the potential value of IgG anti-β2-GPIDI determination for identifying patients with APS at high risk of thrombosis [18]. This is consistent with the multicenter study where it was shown that patients with IgG anti-β2-GPIDI had a 3.5 higher risk of vascular thrombosis compared with patients without IgG anti-β2-GPIDI [19]. The relationship between anti-β2-GPIDI and thrombosis was also confirmed by a study by Mahler et al. [20] CLA. The authors concluded that the anti-β2-GPIDI detection may be a promising method for thrombosis risk assessment [21]. The prospective study also suggests that in patients with APS, an increased concentration of IgG anti-β2-GPIDI is an independent risk factor for thrombosis [21].
The significance of IgA aPL in the development of clinical manifestations of APS continues to be discussed [22, 23]. Shen et al. [22] retrospectively evaluated 472 patients with thrombosis and aPL: IgG/IgM/IgA aCL, IgG/IgM/IgA IgG anti-β2-GPI, IgG/IgM/IgA aPS. They revealed using single-factor and multivariate analyses a statistically significant risk of thrombosis in patients with elevated levels of IgA aPL in ELISA [22]. Tortosa et al. [23] published the results of a 5-year follow-up of 244 patients with positive IgA anti-β2-GPI, negative for IgG and IgM, without a history of clinical manifestations of APS. The results of the study showed that isolated IgA anti-β2-GPI positivity was an independent risk factor for the development of clinical manifestations of APS, mainly arterial thrombosis, and was independent of other cardiovascular risk factors [23]. Our data revealed an association between IgA aCL and recurrent thrombosis and no association of IgA anti-β2-GPI with recurrent thrombosis.
The significant association of antibodies to the aPS/PT is confirmed not only in our study [for IgG aPS/PT; OR = 7.25 (1.85–28.35), P = 0.003], but also in a systematic review by Sciascia et al. [24]. The study researched publications from 1988 to 2013 years [24]. The OR (95% CI) of aPS/PT for thrombosis was available in 10 studies on 1,775 patients and 628 controls. Based on the results of the analysis, 15 out of 18 analyses (83%) reported significant associations: 3/6 with arterial thrombosis, 4/4 with venous thrombosis, and 8/8 with thrombosis as a whole.
In our study, the greatest statistical significance in relation to the prognosis of thrombosis recurrence was for IgA anti-β2-GPI by the CLA method—the risk of thrombosis by OR = 32.00 (3.33–307.02). Our results are consistent with the results of other authors [25–27].
Statistically significant factors of thrombosis recurrence were a combination of different aPLs. Triple aPL positivity in the combination of IgG aCL with IgG anti-β2-GPI and with IgG aPS/PT was a significant factor in the recurrence of thrombosis in PAPS [OR = 6.06 (1.66–22.09)] and it was one-fold higher compared to patients without the combination of these antibodies. The risk of thrombosis recurrence was more than 3 times higher for the IgG aCL with IgG anti-β2-GPI and with IgG anti-β2-GPIDI combination compared to IgG aCL with IgG anti-β2-GPI and with IgG aPS/PT [OR = 20.71 (3.48–123.00), P = 0.0006]. We did not evaluate the aPS/PT combinations since these antibodies were investigated by different methods. The relationship of IgG aCL, IgG anti-β2-GPI, IgG aPS/PT, and IgG anti-β2-GPIDI with recurrent thrombosis is also confirmed by plotting forest-plot diagram and AUC according to ROC curves.
Complication risk prediction models are becoming increasingly common in both medical research and clinical practice, due in part to the increased emphasis on personalized medicine. Recently, 3 scoring systems have been developed to quantify the risk of thrombosis/obstetrical complications in APS, aimed at helping physicians stratify patients according to their risk of complications [28, 29].
Two of these scales include antibodies to prothrombin (aPT) among the variables calculated in assessing the risk of thrombosis or obstetric pathology [24, 28]. Otomo et al. [28] developed the aPL-score (aPL-S) to quantify risk based on the aPL profile. Sciascia et al. [29] formulated an alternative assessment based on a combination of independent risk factors for thrombosis and pregnancy loss: the global APS score (GAPSS). This indicator takes into account not only the APS profile (according to the criteria of APS and “extra”-criterial APS) but also includes the usual cardiovascular risk factors—hypercholesterolemia and AH into the equation. We did not evaluate GAPSS, but the study included components of this score such as cholesterol levels and AH.
The scale emphasizes the significance and necessity of determining the classical risk factors of thrombosis in patients with APS. Synthesis alone of aPL in humans cannot provoke clinically significant hemostasis disorders leading to the development of APS. This gave rise to “the two-hit hypothesis”, according to which aPL (the first hit) creates conditions for hypercoagulation, and thrombus formation is induced by additional mediators (the second hit) that enhance the activation of the clotting cascade, already caused by aPL. Indeed, there is evidence that the incidence of thrombosis in patients with aPL positivity increases in the presence of other risk factors of hypercoagulation, such as pregnancy, smoking, surgical operations, and, especially, when combined with congenital thrombophilias [30]. Thus, aPL generation is necessary, but not sufficient for the development of clinical manifestations. The second “shock” is not clearly delineated, but it is assumed that inflammation, infection, or other prothrombotic triggers contribute the development of these manifestations [31]. Traditional thrombosis risk factors together with mutations in the FII and FVL mutations were evaluated in our study.
It is worth noting that the mean age of our patients was 38.5 [31.5; 43.5]. This is a rather young age for the development of concomitant pathology. Most likely, young age was the reason for the low incidence of type 2 diabetes mellitus in our patients (2%). Also, in our patients, there was a low percentage of patients with hypercholesterolemia (2%) and smoking (10%). On the one hand, our data are consistent with the results of Navarro-Carpentieri et al. [5] which showed no connection between smoking and arterial thrombosis. These data allowed the authors to speculate that thrombotic complications in APS are largely related to aPL-mediated mechanisms.
Obesity, as it was revealed in one of the studies, was associated with venous thrombosis [32]. Our study revealed a tendency for recurrent thrombosis in obese patients (P = 0.053).
Of the classic risk factors for thrombosis, we noted an association with AH (P = 0.03). The study by Saraiva et al. [33] is in agreement with our findings. Based on an analysis of 115 patients with APS, 60% of whom had PAPS, AH was an independent risk factor associated with recurrent thrombosis [OR = 3.7 (1.6–8.5), P = 0.002]. Contradictory data were obtained by Navarro-Carpentieri et al. [5]. The authors found no relationship between increased BP and thrombosis [5].
According to the results of Berman et al. [34] patients with FII mutation had higher prevalence of venous thrombosis, with no statistical significance (80% vs. 47.9%, P = 0.35). There were no differences in the prevalence of recurrent thrombosis before or after APS diagnosis in patients with or without FII mutation. A study by Diz-Kucukkaya et al. [35] showed that the prevalence of the G20210A FII mutation was not significantly increased in patients with thrombosis with APS compared with patients without thrombosis (2.7% vs. 1.25%, P = 0.67). In our study, 2 (6.25%) of 32 patients had a heterozygous G20210A FII mutation and none of them had a FVL mutation. Both patients had recurrent thrombosis and persistently positive levels of IgG aCL and IgG anti-β2-GPI were observed (triple positivity in the history).
Tikhonova et al. [30] studied 108 patients with APS, of whom 45 (42%) were with PAPS and 63 (58%) with SLE and with APS. Authors have noted that the frequency of G20210A FII and FVL mutations in patients with APS does not exceed that in the population with thrombosis and is considered an independent risk factor for thrombosis [30].
In conclusion, recurrent thrombosis in APS is largely associated with IgG aCL, IgG anti-β2-GPI, IgG anti-β2-GPIDI, IgG aPS/PT, and IgA aCL positivity. AH was a significant risk factor for recurrent thrombosis.
aCLs: antibodies to cardiolipin
AH: arterial hypertension
anti-β2-GPI: anti-beta2-glycoprotein I
anti-β2-GPIDI: anti-domain I-beta2-glycoprotein I
aPLs: antiphospholipid antibodies
aPS/PT: antibodies to the phosphatidylserine/prothrombin complex
APS: antiphospholipid syndrome
AUC: area under curve
BP: blood pressure
CI: confidence interval
CLA: chemiluminescent assay
CU: chemiluminescent unit
DOACs: direct oral anticoagulants
ELISA: enzyme-linked immunoassay
FII: factor G20210A prothrombin
FVL: factor V Leiden
IgG: immunoglobulin G
LA: lupus anticoagulant
OR: odds ratio
PAPS: primary antiphospholipid syndrome
ROC: receiver operating characteristic
SDs: standard deviations
The study conducted within the framework of fundamental research project FURS-2022-003 [1021051402790].
FAC: Conceptualization, Validation, Writing—original draft, Writing—review & editing, Project administration. AAS: Writing—original draft, Writing—review & editing, Project administration. MVC and SIG: Validation. AML: Project administration. ELN: Project administration, Supervision. TMR: Methodology, Writing—original draft, Writing—review & editing, Project administration, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study was conducted in accordance with the Declaration of Helsinki, and approved by Ethics Committee of V.A. Nasonova Research Institute of Rheumatology (Protocol No. 25 dated December 19, 2019).
Informed consent to participate in the study was obtained from all participants.
Informed consent to publication was obtained from relevant participants.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Masahiro Ieko ... Akitada Ichinose
Gary W. Moore
Osamu Kumano ... Jean Amiral
Tiffany Pascreau ... Marc Vasse
