Affiliation:
1School of Pharmaceutical Sciences, Lovely Professional University, Phagwara 144411, Punjab, India
ORCID: https://orcid.org/0000-0003-1634-4206
Affiliation:
1School of Pharmaceutical Sciences, Lovely Professional University, Phagwara 144411, Punjab, India
ORCID: https://orcid.org/0009-0009-9116-8426
Affiliation:
1School of Pharmaceutical Sciences, Lovely Professional University, Phagwara 144411, Punjab, India
ORCID: https://orcid.org/0009-0007-4368-2282
Affiliation:
2Department of Pharmaceutics & Pharmaceutical Technology, Yarmouk University, Irbid 21163, Jordan
Email: alaaj@yu.edu.jo
ORCID: https://orcid.org/0000-0002-9519-6338
Affiliation:
3School of Bioengineering and Biosciences, Lovely Professional University, Phagwara 144411, Punjab, India
Email: yachanamishra@gmail.com
ORCID: https://orcid.org/0000-0002-3467-0518
Affiliation:
1School of Pharmaceutical Sciences, Lovely Professional University, Phagwara 144411, Punjab, India
ORCID: https://orcid.org/0000-0001-6542-2464
Explor Immunol. 2025;5:1003203 DOI: https://doi.org/10.37349/ei.2025.1003203
Received: January 11, 2025 Accepted: June 01, 2025 Published: July 16, 2025
Academic Editor: Wenping Gong, The Eighth Medical Center of PLA General Hospital, China
The article belongs to the special issue Old and New Paradigms in Viral Vaccinology
Advancements in viral vaccine development have revolutionized public health by reducing the burden of infectious diseases worldwide. The development of vaccinology started with Jenner’s smallpox vaccine and Salk’s polio vaccine among other live attenuated and inactivated vaccines before shifting to modern platforms that include subunit, protein-based, and viral vector vaccines as well as messenger RNA (m-RNA) vaccines. Subunit and protein-based vaccines are the ones that protect specific subpopulations and contain low risks; reverse vaccinology, built on genome sequencing and using computational methods for identification of the antigens, helps to cut the time for vaccination development. The COVID-19 experience by itself has shown the feasibility of faster and easily scalable m-RNA development that provides a very strong immunogenicity and safety profile. These advancements are crucial in the fight against new and resurging pathogens, for example, severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), human immunodeficiency virus (HIV), and influenza. They allow the creation of vaccines for highly mutable pathogens or those that evolve strategies to avoid the immune system. Truly innovational approaches in delivering vaccines are lipid nanoparticles, microneedle patches, and thermostability that improve the stability, accessibility, and administration of vaccines in low- and middle-income countries (LMICs). Furthermore, computational immunology, artificial intelligence, and bioinformatics are involved in creating precision vaccines that are likely to suit different populations in society. This review presents solutions to critical barriers including vaccine refusal among the population and unequal distribution systems and transportation requirements along with clinical trial gender bias. Recent strategies employing nanotechnology-based delivery methods and universal vaccines receive assessment regarding their solutions to present challenges. The need for joint public-private collaborations combined with strong health programs and systematic research investments stands essential for developing extensive scalable vaccination strategies. These findings present a detailed guide for improving both the effectiveness and accessibility of vaccines as well as readiness against current and future viral infections.
Viral disease epidemiology is a scientific discipline focused on identifying, investigating, and analyzing the patterns, rates, distribution, and determinants of viral disease occurrence in human populations. The characteristics of the virus and host population, as well as behavioral and external factors that influence epidemiological transmission in a community, all influence the chance of infection or disease in a population [1]. As a result, vaccines are required to prevent new viral infections and put an end to the transmission of diseases. Viral vaccinology has evolved significantly over time, transitioning from traditional approaches to innovative modern paradigms. These vaccines work towards enabling the immune system to search out the viruses and destroy them, however not causing the disease to spread out. Vaccination is an economical and effective intervention that can greatly reduce the risk of the spread of infection-associated sicknesses. Informing people about vaccination has made the prevention of diseases possible, significantly reducing and in some cases eradicating several types of diseases. There are already respective human vaccinations that help to avoid deadly viruses which are the reason for numerous diseases and fatal cases reaching tens of millions of people. As diseases such as smallpox are eradicated, or the occurrence of others like polio or measles is greatly reduced, viral vaccinations are indispensable tools in global health [2]. This review highlights viral infections of human populations by investigating their geographic spread and identifying the factors that contribute to disease transmission patterns. New virus outbreaks along with worldwide pandemic diseases including severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), Ebola, and Middle East respiratory syndrome (MERS) reveal the necessity of developing effective vaccines to manage disease spread and decrease death rates. Through time viral vaccinology shifted from standard live attenuated and inactivated vaccines to recombinant, messenger RNA (m-RNA), and viral vector-based platforms which led to enhanced vaccination effectiveness and improved production capabilities. Several challenges hinder progress despite recent developments since vaccine refusal limits accessibility and the possibility of developing vaccine-resistant viral strains. The pursuit of global vaccine research encounters multiple persistent barriers that involve the study of cost-effective methods as well as fair distribution practices and vaccine strategies tailored to individual patients. The promise of future vaccines using nanotechnology-based vaccines and universal vaccines needs additional research to achieve development. It also explores three major gaps in current research by investigating viral vaccine development patterns and testing barriers alongside an examination of emerging vaccine creation methods for addressing current limitations. This research adds to existing studies that work to enhance vaccine performance together with worldwide accessibility and readiness against health emergencies.
The diseases brought to humans by bacteria, viruses, and parasites which spread through vectors are termed vector-borne. The Global Vector Control Response (GVCR) 2017–2030 was endorsed by the World Health Assembly in 2017 [3]. Because of the research findings and utility to the global population health, several viral diseases affect global health immensely and are, thus, classified as viral infectious diseases of poverty (vIDPs). The 2021 vIDP associated estimated global mortality and disability-adjusted life years (DALYs) were 8.7 million and 259.2 million, accounting for 12.8% and 9%, of total all-cause mortality and DALYs, respectively. Among all vIDPs, COVID-19, which highlights its connectivity on a world level, belongs to them. Similarly, human immunodeficiency virus (HIV)/AIDS cannot be considered a controlled disease as it is still a person-to-person infection of the pulmonary type demanding strict global measures. Severe hepatitis, dengue fever, and rabies are some examples of vIDPs that received much attention and pose certain challenges to the public health systems. Due to high mortality rates, new threats, including Ebola virus disease (EVD), also gain much attention. Furthermore, while they are not currently transmissible from human to human, some subtypes of influenza viruses including H5N1 and H7N9 cause severe and often fatal illnesses. SARS and MERS are two examples of coronaviruses that have led to severe acute respiratory syndromes. SARS was almost an epidemic until it was controlled, although MERS is still a threat of transmission among people. All the illnesses prove the need for constant learning, monitoring as well as innovative intervention methods [4, 5].
The approval of SARS-CoV-2 vaccines has highlighted a critical issue in clinical trials—the lack of consideration for sex differences in vaccine response. Historically, males and females exhibit different immune reactions to vaccines, yet many phase III trials and real-world studies failed to stratify results by sex. Additionally, pregnant and lactating individuals were excluded, raising concerns about regulatory guidelines for their vaccination [6]. Analysis and synthesis of data examined female participation rates in 398 randomized controlled trials (RCTs) executed in multiple medical specializations. Among 300 eligible RCTs, the median female enrollment rate reached 41% yet only trials involving immune system diseases reached above 50% participation for women. The research showed that older patients demonstrated lower enrollment in studies as the female participation decreased from 47% for those ≤ 45 years old to 33% for subjects ≥ 63 years old (P < 0.001). The lack of documentation regarding sex distribution in 11 studies underscores fundamental gaps in experimental study design. Clinical trial data about treatment safety and effectiveness remains insufficient because women receive inadequate representation, particularly for older women. Medical research requires both the elimination of female participation barriers and sex-stratified analytical techniques to achieve more accurate findings and full research inclusivity [7]. Table 1 demonstrates the global progress of viral vaccinations, showcasing key target diseases, vaccine types, development milestones, and their global impact. By eliminating illnesses like smallpox, containing epidemics like COVID-19, and drastically lowering the death rate from polio, measles, and human papillomavirus (HPV), vaccines have transformed public health. While yearly updates like the influenza vaccine address issues from viral mutations, technological developments such as recombinant DNA, m-RNA, and viral vectors have sped up vaccine production. So, several approaches are employed in vaccination to enhance the immune system’s protection against viral diseases. Live attenuated vaccines, such as measles or mumps, rubella, or yellow fever, are additional examples as those vaccines contain viruses that may replicate without inciting disease. Sometimes booster doses are needed, but since inactivated vaccines do not replicate, they are safe for the consumer, for example, the polio and hepatitis A vaccines. The HPV and hepatitis B vaccines are examples of subunit, recombinant, and virus-like particle (VLP) vaccines, which have more distinct protection with minimal side effects because they target specific virus constituents like protein. For instance, the recently developed Pfizer-BioNTech and Moderna COVID-19 vaccines are an example of this type of vaccine where instead of using dead viruses, genetic instructions are used to win over the cells to produce viral proteins which then simulate immunity. Viral vector vaccines like vaccines for COVID-19 from AstraZeneca and the Ebola virus are harmless viruses that transport genetic information from target diseases. The last one is DNA vaccines which are still in the research phase; these vaccines employ DNA to initiate immune reactions and have promising prospects in the treatment of various sorts of viral diseases [8, 9]. Virosomes are essentially reconstituted viral envelopes. This means they consist of the lipid membrane of a virus, embedded with viral surface proteins, but they lack the virus’s genetic material. Virosomes present antigens to the immune system in a way that mimics a natural viral infection, stimulating a strong immune response [10].
Global progress of viral vaccinations, showcasing key target diseases, vaccine types, and development milestones
| Vaccine | Target virus/disease | Type of vaccine | Development milestone | Global impact | Reference |
|---|---|---|---|---|---|
| Smallpox vaccine | Smallpox | Live attenuated (cowpox virus) | 1796 (Edward Jenner) | Eradicated smallpox in 1980 | [11] |
| Inactivated polio vaccine (IPV) | Poliovirus | Inactivated vaccine (IPV) | 1955 (Jonas Salk) | Reduced polio incidence worldwide | [12] |
| Oral polio vaccine (OPV) | Poliovirus | Oral live attenuated vaccine | 1960 (Albert Sabin) | Easy administration and improved population immunity | [13] |
| Measles, mumps, rubella (MMR) vaccine | MMR virus | Live attenuated | Mid-20th century | Significant decline in childhood diseases | [14] |
| Hepatitis B vaccine | Hepatitis B virus (HBV) | Recombinant protein | 1981 | First vaccine using recombinant DNA technology | [15] |
| Influenza vaccine | Influenza virus | Inactivated/live attenuated | Annual updates | Prevents seasonal flu but requires annual reformulation | [16] |
| Human papillomavirus (HPV) vaccine | HPV | Protein subunit | Early 2000 | Prevents HPV-related cervical cancers | [17] |
| Ebola vaccine | Ebola virus | Viral vector | 2015 | Used during outbreaks, reducing fatality rates | [18] |
| COVID-19 vaccines [messenger RNA (m-RNA)] | Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) | m-RNA | 2020 (Pfizer-BioNTech, Moderna) | Rapid development and global deployment | [19] |
| COVID-19 vaccines (viral vector) | SARS-CoV-2 | Viral vector (adenovirus) | 2020 (AstraZeneca, J & J) | Effective in managing the COVID-19 pandemic | [20] |
| Dengue vaccine | Dengue virus | Live attenuated/protein-based | Recent (Dengvaxia, Qdenga) | Reduces severe dengue cases in endemic regions | [21] |
| Rotavirus vaccine | Rotavirus | Live attenuated/oral | Early 2000 | Significant reduction in childhood diarrhea cases | [22] |
| Rabies vaccine | Rabies virus | Inactivated | 19th century | Prevents a fatal disease with post-exposure prophylaxis | [23] |
Besides enhancing the life duration of people and reducing the recurrent costs of health, pharma-vaccination has become an important research domain in medical science by hindering the spread of contagious diseases such as smallpox, poliomyelitis, and measles in the world. Live attenuated and inactivated vaccines have been administered for decades and through the COVID-19 crisis, modern m-RNA and viral vector systems have proven vaccine production safer and better [24]. However, challenges such as vaccine hesitancy, issues of equity and access, and individual choice continue to impact global vaccination efforts. Lack of economic feasibility, accessibility, and poor mandatory policy are also essential challenges within the field [25].
The manuscript provides an extensive narrative review integrating research findings about viral vaccine development from various past studies (Figure 1). It covers historical perspectives, technological innovations, and current challenges in vaccinology. The review is structured to compare traditional vaccine approaches (e.g., live attenuated, inactivated, and subunit vaccines) with modern platforms [e.g., m-RNA vaccines, viral vectors, and reverse vaccinology (RV)]. It also incorporates data from published studies and meta-analyses to illustrate global progress, as evidenced by tables summarizing key vaccine milestones and technological differences.
A systematic literature search across major databases (google scholar, PubMed) to identify relevant studies and reports on vaccine development using the search terms like “Vaccinology”, “m-RNA”, “Reverse vaccinology”, “COVID-19”, “Subunit vaccine”, “Vaccine hesitancy” etc. or in various combinations were performed. Descriptive statistics to present historical data and comparative tables to highlight differences among vaccine types. In addition, this narrative review details the application of computational approaches such as RV, structural vaccinology (SV), and machine learning (ML) techniques to predict and optimize immunogenic targets. The discussion also covers advances in delivery systems and emerging challenges like vaccine hesitancy and distribution inequities.
Vaccines have completely changed how humans and diseases interact over the last 150 years. However, more conventional approaches to vaccine development that were improved during the course of the 20th century have been particularly important for expanding vaccination access globally [26]. When Edward Jenner invented the smallpox vaccine that worked in 1796, this invention was one of the very first, but also one of the most important. Since cowpox was a less severe disease than smallpox, Jenner purposely injected a young kid with cowpox and observed that it made the boy immune to smallpox after he came across milkmaids who once infected by cowpox also could not contract smallpox. This outstanding piece of work set the stage for all subsequent vaccines by making it the first-ever use of vaccination against any viral illness. The greatest success that has ever been recorded in the history of the world public health was achieved in 1980 when smallpox was declared to be endemic due to the efficiency of the smallpox vaccine as shown in Figure 2 [11, 27, 28].
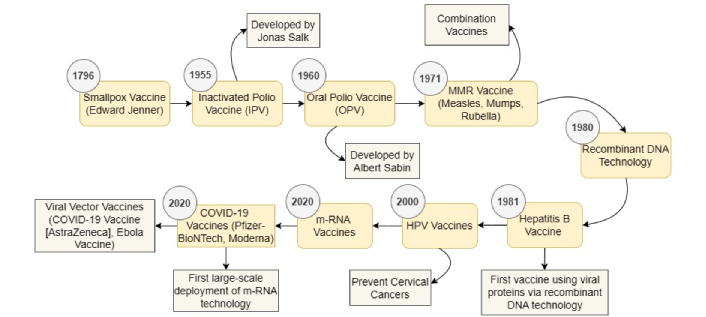
Historical and modern vaccine development. HPV: human papillomavirus; m-RNA: messenger RNA
Science in the 20th century played a fantastic role in developing quick responses to viral attacks through the invention of various vaccinations. One of the main emphases of the work on the vaccine was polio—a terrible disease that entailed both paralysis and death. Jonas Salk introduced the inactivated polio vaccine (IPV) in 1955, making it available for children. As a result of this vaccination, cases all over the world have reduced greatly thus becoming an effective weapon against the disease. That is why, in contrast to Salk’s injection vaccines, creating population immunity and easy to use, Albert Sabin’s oral polio vaccine (OPV) of the 1960 became more widely used. The use of OPV brought polio incidence down sharply through its direct reduction of polio-related mortality and disabilities [12, 13, 29]. Other vaccines that came to the world during this period include measles, mumps, rubella (MMR). One of the vaccines that provided simultaneous protection against these diseases and reduced them by a considerable margin is the MMR vaccine. The enhancement of vaccination and the middle of the 20th century transformed the overall picture of global health [14, 30]. The enhancement of recombinant DNA technology in the 1980 added value to the science of vaccinations since it allowed the generation of vaccinations that relied on particular viral proteins and not whole viruses. This was related to the concept of ensuring the effectiveness and safety of these products. The first of these vaccines used was the hepatitis B vaccine developed in 1981 which made the hepatitis B surface antigen (HBsAg) utilizing recombinant DNA technology [31]. Next in the early 2000 were HPV vaccines designed to protect women from viruses that contribute to most cervical cancers. These vaccines laid the foundation of a distinctive technology from inactivated or live attenuated viruses and are aimed at utilizing viral proteins to initiate the immune system of the organism [17].
The first vaccine employing the m-RNA approach came in the 2020 which marked the beginning of another type of vaccination research. This technique is new and focused on the fact of ensuring that, with the help of m-RNA, cells in the body develop a viral protein and trigger an immune response. The fast development of the COVID-19 vaccines by Pfizer-BioNTech and Moderna is the first that is widely known to have used m-RNA technology. These vaccinations were developed and deployed within a record time indicating that m-RNA vaccines can respond instantaneously to new viral risks [19, 20]. This effectiveness of m-RNA vaccines has promoted a curiosity for their use in other diseases including influenza, Zika, and HIV [32]. Recent research on m-RNA vaccines targeting malaria and other parasitic infections is reviewed, along with an evaluation of technical and practical factors influencing their feasibility [33]. The latter becomes another intriguing platform alongside the m-RNAs—the viral vector vaccines. These vaccines function by using harmless viruses, most frequently adenoviruses, to transfer the target virus’s genetic material into human cells. Two examples of viral vector vaccines are the COVID-19 and Ebola vaccines from AstraZeneca and Oxford University. This technology possesses the ability to fast response to viral threats and can be adjusted in treating many diseases [34]. However, there are still problems in the study of viral vaccines today even with all the breakthroughs made in recent years. Influenza, HIV, and malaria up to date are sources of serious problems. Such diseases have multiple immunity involvement hence vaccine production is a challenge. For instance, many viruses exhibit high mutation rates, which is why a flu vaccine must be reformulated annually, while malaria continues to persist owing to the extended life cycle of the parasite besides the varied immunological responses in different people [35].
There are benefits and drawbacks to conventional vaccine production techniques, such as live attenuated and inactivated vaccines and protein subunit vaccines. But in general, inactivated vaccinations are not sufficiently immunogenic or in certain cases even lead to aggravated disease processes, and on the other hand, live attenuated vaccines contain a risk of the shift to a more pathogenic strain. The number of vaccines that can be used to save lives when there is an emergency is also limited, this is because most of the pandemic vaccines still require clinical trials during an active epidemic to get enough data about the safety of the vaccine as well as its effectiveness. In case of viral vectors include poxviruses such as vaccinia virus, adenoviruses, herpesviruses, arenaviruses, retroviruses, paramyxoviruses, and flaviviruses [36]. The biggest strength of viral vectors is that they elicit a strong enough adaptive immune response even if there is no adjuvant used. But to address this issue of greater immunogenicity sometimes the actual problem that arises is the revert to virulence of the attenuated viral vector especially when the viral vector is replication competent [37]. While traditional approaches have given the world numerous effective vaccines that have served to save many people, a variety of challenges characterize this method making the process slow, expensive, and sometimes less effective at times. For example, due to the mechanisms of genetic variation of influenza viruses, the vaccines have to be redesigned annually. The need for specific facilities and procedures including the viral propagation in eggs for influenza vaccinations usually limits the production of the traditional type of vaccines [38]. For instance, it is difficult and takes a lot of time to increase the production of products to meet the global market during a pandemic. Cold chain is the storage of the vaccine since most of the traditional vaccines especially the live attenuated vaccines require refrigeration. There are challenges in maintaining the cold chain through distribution, particularly in resource-limited settings. This leads to the degradation of vaccines thus reducing their effectiveness. These problems show that, despite significant progress in the production of more traditional vaccines, new methods and technologies, innovative ideas (including the approach, used in m-RNA vaccines), and better approaches are needed to improve the effectiveness, availability, and public acceptance of vaccines [39, 40].
Firms in the vaccine industry constantly develop new advancements that range from radical to incremental solutions to stay ahead in their quickly shifting market. Current theoretical frameworks provide adequate explanations about selected aspects but do not identify the exact factors behind breakthroughs [41]. According to the study scientific developments and new disease emergence work together to drive breakthrough vaccine advances. Companies invest in creating new vaccines like m-RNA-based COVID-19 vaccines to fulfill urgent medical requirements thus they gain exclusive market power and superior position in the field. The principles of Schumpeterian innovation theory show technological transformation happens through firm-driven innovation-seeking activities. Health policies that facilitate data sharing speed up vaccine development by supporting collaboration and improving the operational efficiency of the worldwide healthcare system [42, 43].
Recent viral vaccine development has resulted in four advanced vaccine types including subunit vaccines, protein-based vaccines, RV, and m-RNA vaccines. New vaccine technologies resolve primary vaccine limitations through their speed of creating pandemic responses while ensuring protection quality and expanding disease range and their capability to scale production. The use of isolated proteins and polysaccharides in vaccines provides high safety because such vaccines contain no live pathogens and exhibit extensive characterization. The immune response from subunit vaccines tends to be weak which necessitates the addition of adjuvants while maintaining the difficulty of designing universal coverage for all possible antigens. Protein-based vaccines demonstrate excellent specificity because they utilize purified pathogen-specific proteins as well as stability properties allowing combination with adjuvants to extend their protective effects. The main drawback of these vaccines arises when pathogens rapidly mutate which results in reduced protection for multiple antigenic variants. Through RV, genome sequencing enables scientists to identify antigenic targets which enhances the speed of developing vaccines against pathogenic agents that prove difficult to combat. Computational target discovery through this method achieves quick results yet produces targets that sometimes fail to stimulate appropriate immune system responses within the body. The m-RNA vaccination system transmits genetic information which subsequently leads cells to manufacture viral components without using actual pathogens while simultaneously producing strong immune defenses. The storage requirements for m-RNA vaccines include strict cold chain protocols while their stability remains a problem. The various vaccine approaches offer unique benefits from safety to specific targeting capabilities along with quick adaptability functions although they present production challenges and potential breakdowns against novel virus strains (Table 2) [44–47]. The adoption of new vaccine platforms happens through problem-driven innovation after pandemics such as COVID-19 and SARS and Ebola resulting in improved precision together with enhanced adaptability and reduced costs. Modern biotechnology shapes public health through the replacement of traditional live attenuated and inactivated vaccines with advanced recombinant nucleic acid-based and RV methods. The development of nucleic acid vaccines serves as a potential candidate to prevent the Marburg virus (MARV) according to scientific research because Marburg hemorrhagic fever has no approved treatments or vaccines. Researchers improved MARV glycoprotein sequences for DNA and m-RNA vaccine development following a process of codon optimization before testing immune response in mice. Tests revealed DNA vaccines produced outstanding antibody responses though m-RNA vaccines created better interferon-gamma (IFN-γ) and interleukin-4 (IL-4) levels which demonstrated superior cellular immunity. Rapid viral genome sequencing combined with target identification and scalable vaccine production capabilities have revolutionized vaccine research and enabled faster product release during health crisis response. Although major progress has occurred there remain obstacles before universal health coverage can be attained because of delivery challenges and equitable access issues [48].
Differences between subunit, protein-based, reverse vaccinology, and messenger RNA (m-RNA)-based vaccines
| Vaccine type | Mechanism | Advantages | Limitations | Examples | Reference |
|---|---|---|---|---|---|
| Subunit vaccines | Use isolated components (e.g., proteins, polysaccharides) | Safe; no live pathogens | Lower immunogenicity; often requires adjuvants; challenging to produce epitopes for all strains | Hepatitis B [recombinant hepatitis B surface antigen (HBsAg)], human papillomavirus (HPV) (Gardasil®) | [44] |
| Protein-based vaccines | Utilize pathogen-specific proteins (often purified or recombinant) | Highly specific; can be combined with adjuvants; stable | Limited efficacy against pathogens with rapid mutation | Novavax COVID-19 vaccine (protein subunit) | [45] |
| Reverse vaccinology | Uses genomic sequencing to identify antigenic targets | Enables vaccine development for difficult pathogens; faster discovery | Computational predictions may not always yield effective antigens | Multi-epitope-based peptide vaccine against avian rotavirus (AvRV) strains | [47] |
| m-RNA vaccines | Deliver m-RNA encoding viral or pathogen-derived proteins | Rapid development; no need for live pathogens; strong cellular/humoral immunity | Requires cold chain storage; risk of instability | Pfizer-BioNTech and Moderna COVID-19 vaccines | [46] |
Inadequate vaccine solutions for emerging infectious diseases (EIDs) represented by chikungunya and Zika have created a major worldwide health security concern. The development of EID vaccines encounters two fundamental obstacles which include research development readiness before epidemics and research development emergency testing during outbreaks. By establishing the Coalition for Epidemic Preparedness Innovations (CEPI) in 2016 the organization seeks to prevent EIDs from developing into humanitarian crises through improved preparedness and response mechanisms. The mission of CEPI demonstrates how private industry partnership combined with risk-sharing strategies provides the most efficient solution to address these problems. The worldwide COVID-19 vaccine response required immediate manufacturing acceleration based on vaccine platform selection and dosage requirements alongside production capacity assessment of different manufacturers. Vaccine access depended on initial production hurdles alongside nation-specific vaccine policies. The Developing Countries Vaccine Manufacturers Network (DCVMN) accomplished greater vaccine distribution by performing technology transfers to vaccine companies in India, Korea, and Bangladesh. Vaccine research and development (R & D) efforts by public-private sectors should focus on creating new vaccine technologies including m-RNA and RV and viral vector approaches to address future disease outbreaks. The partnerships create technological advances while developing essential production and distribution systems vital for worldwide health defense [49, 50].
Subunit vaccines derived from pieces of microbes can get past such barriers. Only the antigenic substances of the pathogen that are necessary to elicit successful immune responses are included in subunit vaccinations. Antigens might be proteins, polysaccharides, or nucleic acids. These vaccines elicit an immunological response by using certain viral components, often proteins. There is no chance of disease development because they do not employ the entire pathogen. Protein subunits containing a specific virus product, not the full viral particle, are responsible for immune responses [51]. Viral structural proteins are important for vaccine development because of high antigenicity and immunogenicity, of which the spike (S) protein is the most important. The S protein is the primary vaccine antigen, with m-RNA (Pfizer-BioNTech; Moderna) and viral vector vaccines (AstraZeneca) targeting the viral S protein that binds with the angiotensin-converting enzyme 2 (ACE2) receptor. The other structural proteins include membrane (M), envelope (E), and nucleocapsid (N) proteins, which are also involved in viral assembly and immune reactions, but they elicit lesser immunogenic responses on their own. The integration of conserved proteins such as N in next-generation vaccines may generate wider immune responses and enhanced protection against emerging variants [52].
Hepatitis B is still one of the most dangerous diseases in the world, even after the human hepatitis B virus (HBV) was discovered more than 40 years ago. The development of a preventative vaccination using the HBV surface (envelope) protein (HBsAg) today represents a significant advancement in the fight against this virus. Both the isolation of HBsAg subviral particles (SVPs) from the blood of asymptomatic HBV carriers as antigens for the first-generation vaccines and the availability of recombinant HBsAg SVPs produced in yeast as the antigenic components of the second-generation vaccines could be regarded as advances in biotechnology and medicine. HBV contains four open reading frames (C, P, S, and X) and encodes seven proteins [polymerase, X protein, hepatitis B core antigen (HBcAg), hepatitis B e antigen (HBeAg), HBsAg-large (HBsAgL), HBsAg-middle (HBsAgM), and HBsAg-small (HBsAgS)]. The polymerase’s reverse transcriptase, RNaseH, and priming functions make it necessary for several replication pathway stages. The X protein facilitates effective in vivo infection and reproduction, which may identify and combat the real virus if it is subsequently encountered. Chronic viral infections may be addressed by novel vaccination approaches, such as structurally altered subunits to alter antigen processing or enhance immunogenicity. Chimeric vaccines have demonstrated potential in eliciting immunological responses, such as those that use HBsAgS SVPs as carrier platforms for foreign antigens. Combining HBsAgS SVPs with other antigens, such as HBcAg, may help next-generation vaccines achieve their therapeutic goals by eliciting widespread T cell responses [53].
The HPV vaccine which covers the HPV employs VLPs, which are formed from the HPV protein coat but are not composed of viral DNA. One of the most prevalent viruses is the HPV, which causes skin-to-skin contact during sexual encounters. Currently, it is estimated that there are more than 99 different variants of HPV in humans. Of these kinds, thirty are genital and mucosal HPV viruses. These are referred to as low-risk; HPV types can be HPV-6, 11, 72, 81, and others while the high-risk HPV types are HPV 16, 18, 31, 33, and 35, among others. Thus, cervical carcinoma is associated more frequently with the types of high-risk HPV, mainly with HPV 16, 18, 31, 33, and 35 [54]. Most cervical malignancies are caused by HPV-16 L1 major capsid protein and HPV-18, two of these HPV strains. By neutralizing antibodies, HPV preventive vaccinations work to create humoral immunity and protect against HPV infection. However, because the current vaccination lacks a number of genes involved in the integration of the viral genome into the host genome, including early genes (E1, E2, E4, E5) and late genes (L1, L2), this does not protect an organism that is already infected. The best option for this vaccination was determined by predicting and assessing the B cell and T cell antigenic sites of HPV-66’s L1 main capsid protein using a bioinformatics technique. The HPV-66 L1 main capsid protein was discovered to be hydrophilic and unstable, with a secondary structure that includes 31.80% loops, 17.97% β-sheets, and 39% α-helices. Five B cell epitopes and fourteen T cell epitopes [10 helper T lymphocyte (HTL) and 4 cytotoxic T lymphocyte (CTL)] were among the 19 dominant epitopes found in the protein. The framework for the creation of a multi-epitope vaccine that targets HPV infection is laid by these discoveries, which offer important molecular insights into the HPV-66 L1 protein [55].
Subunit vaccines are similar in some ways to protein-based vaccines but could contain other things such as polysaccharides or certain bacterial or viral antigens. The hepatitis A vaccine is an inactivated subunit of the virus that enhances the body’s immune system to fight against hepatitis A by administering the body with a dead version of the hepatitis A virus. In the same way, the influenza vaccine, often referred to as the flu vaccine, is often a subunit-based vaccine, which contains proteins such as neuraminidase or hemagglutinin or inactivated virus particles. Major antigens of the influenza virus, those proteins assist in the identification of the virus by the immune system. Protein subunit vaccines also have high specificity for immunogenicity since they only recognize those proteins specific to the pathogen. These vaccines target specific proteins that are able to strongly stimulate immune response with high capacity; they may be capsid or surface proteins. They are often incorporated with adjuvants with a view of enhancing their immunogenicity; a component that enhances the body’s immune response to various vaccines [56]. Subunit vaccines are more flexible than other types up to the possibility of containing proteins and non-protein substances such as polysaccharides. Since polysaccharides from the bacterial cell wall are important in immunity, they are very useful for bacterial vaccines. Besides this subunit vaccines can provide a versatile approach to the protection from the infectious diseases owing to the possibility of combining large numbers of antigen types [57].
The immense amount of data generated by whole genome sequencing projects and the development of bioinformatics has led to the development of a “third generation” of vaccinations, which are based on the application of vaccinomics research to vaccinology. Reverse vaccination is the oldest known example of such an approach [58]. By centering on the genetic sequence, this approach forecasts which antigens are most likely to be viable candidates for immunization. This approach not only enables the identification of all antigens identified via previous methods, but it also enables the discovery of new antigens that function on a completely different paradigm. Consequently, this method facilitates the discovery of novel immune intervention mechanisms [59]. Additionally, by rapidly identifying immunogenic targets, it speeds up the development of vaccines for illnesses including COVID-19, Ebola, and Zika. The study demonstrates that evidence-based vaccine creation functions as a critical factor for creating efficient vaccines for combating infectious diseases such as COVID-19. Computational approaches through RV and SV function as essential tools to discover and enhance vaccine antigens. The protegen database provides a standard for identifying protective antigens that represent vaccine targets [60]. Compared to vectored or attenuated live vaccinations, epitope-based immune-derived vaccines (IDV) are usually considered to be effective. Furthermore, epitope-based IDV may provide timely T cell help needed for antibody-directed vaccinations. Since the choice of the components may eliminate unwanted side effects shown with vaccinations that include both protein subunits and the whole pathogen, such vaccines may have a major advantage over previous work in the development of vaccines. Epitope can be defined as an antigenic site that is very important to an immunological system. These are located on the surface of objects from which the antibody can distinguish the pattern [61]. A study focuses on developing an epitope-based vaccine against marine birnavirus (MABV), a major pathogen affecting marine and shellfish species. Since MABV lacks effective prevention and control measures, an immunoinformatics approach was used to design a vaccine targeting virulent proteins VP2, RNA-dependent RNA polymerase (RdRp), and polyprotein. To optimize vaccine production, codon adaptation and in silico cloning into the E. coli K12 pET28a (+) vector were successfully performed. The study concludes that this epitope-based vaccine is a promising candidate for MABV prevention, offering a novel approach to safeguard the aquaculture industry [62]. In order to generate the MERS coronavirus (MERS-CoV) vaccine and investigate the impact of mutations in the chosen sequences on vaccine production, this work relied on employing S and E with changed S and E protein sequences with the help of the in-silico technique. S glycoprotein is defined as an envelope-anchored, trimeric, type I fusion glycoprotein that interacts with the human dipeptidyl peptidase 4 (DPP4) receptor. To mediate viral entrance, it is composed of two subunits: S2, which contains the cell fusion machinery, and S1, which possesses the receptor-binding domain and regulates cell tropism. E protein was thought to be a component of the virus cell membrane [63]. The field of RV involves the computer study of the genome to predict surface protein epitopes. Therefore, while developing a potential vaccination, the epitopes are crucial. B and T lymphocytes play a key function in the immune system. B cells play a crucial role in identifying the antigen epitopes that the antibody’s paratopes can detect. When administered in a T cell environment, processed antigenic peptides interact with T cells, so these can occasionally be involved in cell-mediated immunity. A vaccine against a variety of illnesses, including meningitis, endocarditis, malaria, and anthrax, has been prepared using the RV technique. In this method, computer analysis is used to compare the genomes of several isolates of the same organism. The initial pan-genome strategy was used to combat Streptococcus pyogenes [64]. The application of RV in designing vaccines therefore follows a stepwise plan as shown in Figure 3. The first step entails whole-genome sequencing of the pathogen to form the basis of computational studies for proteins that are secreted and translocated to the pathogen surface. The B cell and T cell epitopes are then determined by immunoinformatics approaches to check whether these epitopes are accessible and are effective in inducing immune response. Such predictions include the identification of helper T cell (Th) epitopes, CTL epitopes, analytical assessment of reactions between three factors: Th, CTL, and major histocompatibility complex (MHC), and study of immunodominance. The extent of variability in the detected epitopes is then determined, and the universality of epitopes to changes in the human leukocyte antigen (HLA) is determined. To decrease off-target reactivity and allergenicity, multi-epitope vaccination candidates are constructed through the linking of epitopes using appropriate linkers. Immunogenicity and reactivity are tested using animals while molecular epidemiology is used to ensure the results are ported across populations. At the population level, safety and protection assessments are performed; this leads to vaccine use permission and advice. The vaccine is then brought into phase IV during which it is used in practice in order to ensure it can work and is safe in the real world [65, 66].
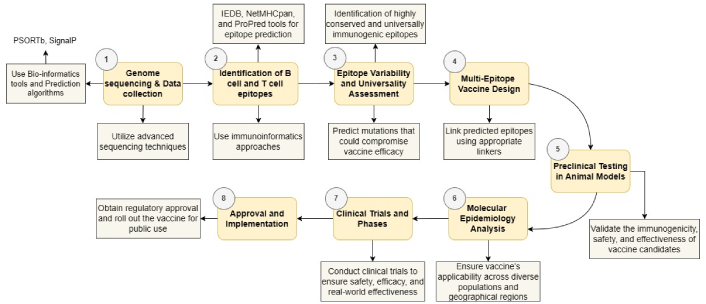
Process overview of reverse vaccinology. PSORTb: protein subcellular localization prediction tool-bacterial version; IEDB: immune epitope database and analysis resource
A new approach to vaccine design known as immunoinformatics has emerged, which uses software to identify the most immunogenic parts of the organisms (epitopes). This is because the previous MERS-CoV vaccine could be either an inactivated coronavirus, live attenuated coronavirus, S protein-based, DNA, or combination vaccines against coronaviruses [63].
Prediction is the cornerstone of the RV strategy, and methods based on ML and deep learning hold great promise for forecasting the positive or negative consequences of vaccination. The ML module may integrate inputs such as mass cytometry, cytokine profile, structural characteristics, direct experimental data from proteomics and metabolomics, and more for training, ultimately establishing a classification or prediction feature. This rule’s output forecasts the presence of either nonspecific reactogenicity (such as allergenicity) or immunogenicity (such as the ability to catalyze B/T cell epitopes, proteasomal processing, and MHC presentation) [67]. An ML algorithm supported the investigation of COVID-19 vaccination campaigns and their impact on fatality rates. The data shows that nations achieving high vaccination success demonstrated rapid decreases in COVID-19 mortality statistics which helps policymakers create stronger future outbreak prevention plans. This study contains various limitations because vaccination data is incomplete and case reporting is insufficient and there are undisclosed confounding elements from non-pharmaceutical controls and healthcare systems. Next-generation COVID-19 variants will potentially lower vaccine effectiveness which means ongoing evaluations become necessary [68].
Biological networks broadly fall under two categories known as data-driven networks and knowledge-driven networks. Knowledge-derived networks are extracted from source literature and may be present as interaction databases and indicate well-defined pathways or molecular signaling pathways. Using the information that describes their interactions, one may determine the association scores between genes, making it possible to build data-driven networks like co-expression networks. Depending on the topology between the nodes, which constitutes the network, different functional relationships are then subsequently inferred using the principles of graph theory. Such networks comprising data and knowledge may be exploited to deduce other relations and at the same time learn from prior tries. To increase understanding of how the genes in the network work, additional biological information sources, including the projected transcription factor targets, may be integrated into the data-driven networks. Collecting the data for training, selecting possible vaccines, with the right ML algorithm with the necessary level of selectivity, specificity, accuracy, and precision with model validation and testing are the four basic steps when using artificial intelligence (AI) for such a prediction. Researchers use different ML methods to develop predictive classifier systems that find new immunogenic targets during vaccine development. Various ML algorithms including support vector machines (SVM), artificial neural networks (ANN), random forest (RF), and k-nearest neighbors (KNN) clustering and boosting techniques aid accuracy levels in vaccine candidate predictions. Bioinformatics technologies hold a vital position in vaccine research by focusing on identifying genes since surface-exposed or secreted proteins often create immune responses. The VaxiJen together with Vaccine Investigation and Online Information Network (VIOLIN) serve as leading in silico tools that assist scientists in identifying vaccine antigens, selecting proper targets, and assessing immunological responses. Computational approaches simplify vaccine development by controlling experimental expenses as well as speeding up the identification of targets and boosting vaccine performance by using predictive data models [66, 69].
Informing an immune response by the genetic material, m-RNA vaccines are brand new in the field of vaccine research [70]. Recent innovations in m-RNA optimization, biomaterial-based delivery, and thermostable designs present promising solutions [71]. These vaccines introduced a new concept in vaccine science. They transmit synthetic molecular blueprints to cells, which synthesize viral proteins that generate immunity rather than relying on whole viruses or protein fragments as in conventional vaccines. Thus, being an alternative to a natural infection, or a live microbe vaccine but not actually using infectious agents it can be seen as a revolutionary approach to immunization. It does this by bringing in a transcript into the host’s cytoplasm that codes for the immunogens, where they are subsequently synthesized into immunogenic proteins that may be processed intracellularly, secreted, or inserted into the membrane. A study concludes that codon deoptimization is a promising approach for developing safe and effective m-RNA vaccines against the white spot syndrome virus (WSSV) in shrimp disease management [72]. Once the genetic sequence of the target antigen is identified, vaccines could be developed in a matter of weeks because m-RNA can be synthesized rapidly, on a large scale, and in a cell-free system derived through in vitro transcription of a DNA template. This simplified procedure ensures that one can easily switch from one goal to the other using what is available around or with minimal infrastructure changes [73]. m-RNA vaccines differ from traditional vaccines in ways that offer notable strengths in safety, effectiveness, and ease of manufacturing. These vaccines elicit strong humoral and cellular immunological performances such as activation of CD4+ and CD8+ T cells, while having no potential of integrating into the human genome since the m-RNA degrades upon translation. MHC class I and II pathways effectively process immunogens for presentation, improving immune recognition. The use of various constructs based on the same technology and repeated delivery are also made possible by m-RNA vaccines’ avoidance of anti-vector immunity. Because of these characteristics, m-RNA vaccines are a very effective and adaptable tool for battling infectious illnesses and other health issues [74]. Another study highlights key technological advancements that enabled the success of COVID-19 m-RNA vaccines, particularly nucleoside modification and ionizable lipid nanoparticles (LNPs), which enhanced vaccine potency and safety. Alternative m-RNA vaccine platforms are emerging, with providence, therapeutics and CureVac optimizing vaccine designs for improved immunogenicity. Self-amplifying RNA (saRNA) vaccines may require adjustments to minimize early immune responses for better efficacy. Additionally, LNP formulations not only aid m-RNA delivery but also influence vaccine reactogenicity and immunogenicity, though their precise mechanisms need further exploration [75].
There are two forms of m-RNA-based vaccines: self-replicating and nonreplicating, each having special advantages. Non-replicating m-RNA vaccines encompass fewer complex structures of the target immunogen with untranslated regions (UTRs), 5' cap, and poly(A) tail required for stability, efficient translation, and protection from degradation. While ML models trained on large 5' UTR datasets offer the potential for optimizing m-RNA sequences, data scarcity, and limitations in RNA 2D/3D structure prediction. The period of immunogen synthesis is significantly shorter in this approach because of the lower half-lives of nonreplicating m-RNA constructions. However, self-replicating m-RNA vaccines have an RdRp complex from alphaviruses that enables the further replication of the m-RNA. Compared to nonreplicating designs, this engenders higher immune reactions and maintains immunogen display at more reduced concentrations. Also, self-amplifying m-RNA can synthesize multiple immunomodulatory molecules through having multiple gene sequences; thus, increasing vaccine efficiency. These structures however are relatively big that inhibit internalization, stability, and production [74, 76, 77]. Future studies need to develop integrated sequence optimization strategies that examine m-RNA stability at both global and local levels to optimize translation speed and degradation management. The addition of extensive experimental data about translation, stability, and immune response in m-RNA vaccine-related databases will enhance computational accuracy. Also, advanced optimization algorithms that balance several vaccine design objectives will lead to better vaccine effectiveness [78, 79].
m-RNA vaccines follow the natural infection process by incorporating a series of processes that produce humoral and cellular immunity. LNPs contain the m-RNA that protects the delicate m-RNA from deterioration, ensures its transport into the host cells, and acts as a template for the immunogen (viral or pathogenic protein). Any m-RNA can get access to the target cells’ cytoplasm after the vaccination as the LNPs can also merge with the cell membranes [80]. After they have entered the cytoplasm and recognized the m-RNA sequence, the host cell’s ribosomes translate the encoded viral or pathogen-derived protein (e.g., the S protein of SARS-CoV-2) as shown in Figure 4. The immune system recognizes and presents the target protein as the viral S protein once the m-RNA vaccination instructs the host cells to tap into it. In the MHC class I pathway, the intracellularly produced viral protein is degraded into small fragments called epitopes by the proteasome. After they are transported to the endoplasmic reticulum (ER), these epitopes are then cached onto MHC class I molecules visible through the cell surface. It forms a critical part of cellular immunology; it allows the CD8+ CTL to recognize and kill cells displaying the foreign antigen. In the MHC class II pathway, if the target protein is secreted or recycled, then the antigen-presenting cell (APC), including dendritic cells, takes up the extracellular proteins through a process called endocytosis. Once ingested by the APCs, endosomes or lysosomes degrade the protein into epitopes to be presented by MHC class II [81]. Figure 5 represents the pathways of MHC class I and II. After they are presented on the cell surface, these complexes are recognizable by CD4+ Ths. Some activated CD4+ T cells stimulate the proliferation and activity of CD8+ T cells and assist in the activation of B cells for the production of antibodies to the target protein. This procedure primes both the CD8+ cytotoxic T cells of cellular immunity and the B cells of humoral immunity and also generates a long-lasting immune response [82]. As well as inducing innate immunity through the detection of RNA molecules by pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I), and melanoma differentiation-associated protein 5 (MDA5), m-RNA vaccines also stimulate adaptive immunity. Upon such identification, type I IFNs (IFN-α/β) and other pro-inflammatory cytokines are produced to form organic adjuvants to increase the immune system’s efficacy [83]. Thus, the possibility of the long-term deposition of the tissue’s own genetic material into the host genome is low because the m-RNA is degraded in the cytoplasm once the translation has occurred. Memory B cells, plasma cells, and memory T cells are formed through the immune response and bring about quick identification and increased immune response against future infections caused by the pathogen [84].
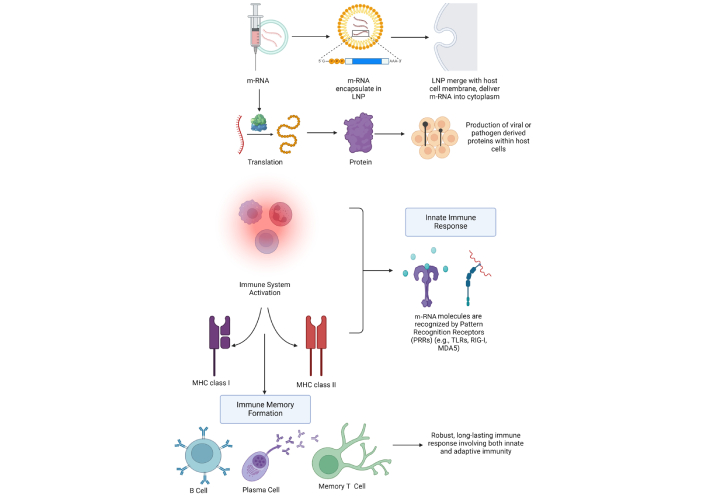
Mechanism of action of m-RNA vaccines. m-RNA: messenger RNA; LNP: lipid nanoparticle; MHC: major histocompatibility complex; TLRs: Toll-like receptors; RIG-I: retinoic acid-inducible gene I; MDA5: melanoma differentiation-associated protein 5. Created in BioRender. Sah, B. (2025) https://BioRender.com/w59v059
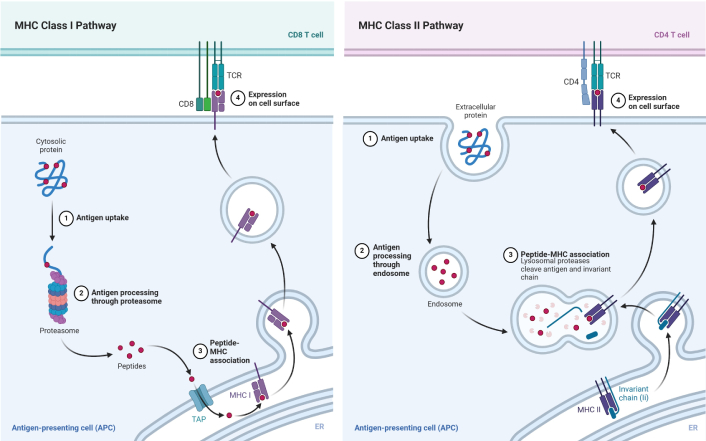
Schematic representation of MHC class I and II pathways. MHC: major histocompatibility complex; TCR: T cell receptor; TAP: transporter associated with antigen processing; ER: endoplasmic reticulum. Sah, B. (2025). Reprinted from “MHC Class I and II Pathways.” Retrieved from https://app.biorender.com/biorender-templates/details/t-5f4fb77c3b02b700b74df8c6-mhc-class-i-and-ii-pathways
The first investigations of utilizing m-RNA for vaccine development were mainly focused on cancer. It is self-evident that the known methods of vaccination cannot be effective for these non-infectious diseases. Cancer vaccines are not prophylactic, but therapeutic because they are designed to target specific antigens that are upregulated in tumor cells. This enables them to launch cell-mediated immunity that has the effect of reducing the size of the tumor. Published in 1995, Conry et al. [85] proved that immunization with m-RNA encoding carcinoembryonic antigens when injected directly into muscles might provoke the protective antitumor immunity in mice and thus, the scientific studies of m-RNA for the stimulation of the adaptive immune response to cancer began. Vaccinating patients with vaccinations that encode tumor-associated antigens (TAAs) is the simplest method of using m-RNA to prevent cancer. This strategy is exemplified by RNActive® technology (CureVac AG), which complexes protamine with m-RNA that has been tailored using exclusive techniques to optimize protein synthesis in order to stimulate Th1-type T cell responses. The resulting vaccine designs are presently being assessed in a number of clinical studies and produced humoral and cell-mediated responses in animal models [74, 86].
By focusing on patient-specific genetic mutations that contribute to the development of cancer, personalized m-RNA vaccine constructs provide a viable method of cancer vaccination. Exome sequencing of both healthy and malignant samples using next-generation sequencing identifies these mutations, which are specific to each patient’s tumor (the mutanome). A portion of such alterations produces neoepitopes that self-generated T cells can identify and are assessed for their capacity to evade central immunological tolerance, hence facilitating anticancer activity. Multiple neoepitopes can be included in a single poly-neoepitope vaccine design due to the versatility and flexibility of m-RNA synthesis [87]. For instance, a first-in-human trial merely used 13 patients with metastatic melanoma (NCT02035956) where the patients were vaccinated with an antigen that was identified to encode 10 tumor-specific neoepitopes. All vaccinated patients developed CD4+ and CD8+ T cell immune responses to the chosen epitopes; some were accompanied by anticancer immune responses with T cell infiltration and neoepitope-specific destruction of cancer cells in the metastases after vaccination. This proves the feasibility, safety, and effectiveness of personalized m-RNA cancer vaccines [88].
Currently, there is an ongoing clinical trial of m-RNA vaccines for rabies, influenza, Zika, cytomegalovirus, respiratory syncytial virus, and new coronavirus (SARS-CoV-2). Yet, none of these clinical trials have been designed beyond the phase one study, except for the one on SARS-CoV-2. SARS-CoV-2 is a newly emerging coronavirus that began at the end of 2019 and rapidly became a global pandemic early in 2020. Some asymptomatic young individuals in good health may present with no disease markers of SARS-CoV-2 infection, while the elderly and those with comorbidities may present with severe disease features including fever, pneumonia, and acute respiratory distress. Even though the infection hardly causes severe symptoms, a strong immunological reaction takes place and provokes tissue and organ alteration for a long time [89]. An estimated 4% of the population has died due to the pandemic, although people of higher ages are more vulnerable. To the best of our knowledge, more research is needed to confirm such effects, but SARS-CoV-2 S protein mutations have been identified across the world, and there is evidence that the mortality incidence is higher and the vaccine is less effective. This is solved by m-RNA vaccines, namely the ones developed by Moderna and Pfizer-BioNTech where the body is introduced to synthetic m-RNA to induce an immune response. These vaccines build the immune response that can identify the virus and is capable of killing it while these new vaccines utilize genetic engineering to guide the body to produce the S protein, which is a component of the virus that facilitates entry into human cells [90].
Both the m-RNA-1273 vaccine from Moderna and the BNT162b2 vaccine from Pfizer-BioNTech are nucleoside-modified m-RNA vaccines that encode the stabilized prefusion S protein of SARS-CoV-2 and are made with LNP. With 95% effectiveness against COVID-19, BNT162b2 triggers robust neutralizing antibody responses and Th1-type CD4+ and CD8+ T cell activation. Nevertheless, an allergy rate of 11.1 instances per million doses has been documented, and it has to be stored at a temperature of –70°C. However, new guidelines permit storage between –25°C to –15°C for up to two weeks. On the other hand, the m-RNA-1273 vaccination from Moderna is 100% effective against severe instances of COVID-19 and 94.1% effective against symptomatic patients. More stability is provided by its 30-day viability at 2°–8°C. CureVac’s CVnCoV vaccine induces robust humoral and cell-mediated immunity in non-human primates (NHPs) by encoding the full-length S protein using proprietary non-chemically modified m-RNA technology. Notably, because of its densely packed LNPs and lack of nucleoside alterations, CVnCoV offers better storage stability, with a three-month shelf life at 5°C [91–93].
An inventive strategy to counteract the reemergence of this zoonotic illness brought on by the monkeypox virus (MPXV) is the development of m-RNA vaccines for Mpox, formerly known as monkeypox. Mpox m-RNA vaccines are made to encode certain viral antigens, including the E or M proteins, that elicit a strong immune response [94]. They are made using the same m-RNA platform that was successfully used for COVID-19. Moderna’s m-RNA Mpox vaccine is undergoing Phase I/II research to assess the immunogenicity, safety, and tolerability of m-RNA-1769 in healthy persons. Clinical studies for m-RNA-1769, an m-RNA vaccine to prevent smallpox and Mpox, are now underway. According to Moderna, the information will be utilized to guide a critical vaccination trial that is being carried out at several sites across the United Kingdom (UK). The expected date of the study completion is June 2025 [95]. Moderna’s m-RNA technologies that emerged during the COVID-19 outbreak have the potential to be useful in treating Mpox and other diseases according to preclinical findings. Importantly, m-RNA vaccine recipients, especially those in vulnerable populations, experienced fewer Mpox lesions and better survival than non-vaccinated participants. As evident from their safety, the m-RNA vaccines may be adapted to other diseases even though they only present short-term protection. BioNTech SE is also using its previous technology in a potential m-RNA vaccine named BNT-166A. The Phase I/II trial aims to evaluate the immunogenicity, safety, tolerability, and reactogenicity of BNT-166A for active Mpox vaccination in individuals aged 18 to 65. The United States and United Kingdom locations are included in the research, which is anticipated to be completed in May 2025 [94, 96, 97].
Promising results have been observed with the use of m-RNA vaccines for some diseases during preclinical studies and phase I trials; therefore, they are being considered for use as therapeutic interventions. Recent studies involving an experimental group of mice with m-RNA influenza vaccines demonstrated sufficient levels of B- and T cell responses, as well as some degree of cross-reaction to conserved viral proteins, including nucleoproteins, which evidence broad immunity to not only influenza viruses [98]. These vaccinations also demonstrated resistance to heat stress. Currently, Moderna is going through the Phase 1 and Phase 2 trials with the m-RNA vaccine candidates for the Zika virus (ZIKV). To overcome this challenge, Richner et al. [99] developed a formulation of m-RNA that encoded ZIKV structural proteins that were encapsulated in LNP and brought about robust neutralizing antibody production in mice. For enhancing translation some mechanical base substitutions along with other changes in the UTRs were applied. When administered in adult mice, the m-RNA vaccination was more effective and safer than both DNA and inactivated vaccinations. Fetal neutralizing antibody levels were significantly lower although it was safe for pregnant mice, meaning that more research is required [100].
The specific characteristics of the targeted product remain the key goal throughout the process of developing a vaccine. The nature of vaccination doses depends on the following factors: types of antigenic preparations where the known classes of the vaccines such as live attenuated, inactivated ones, subunit, and the newest m-RNA based vaccines. Sex-based differences in immune responses should be considered when discussing vaccines and immunogenicity. Women generally exhibit stronger innate and adaptive immune responses than men, which can lead to higher vaccine efficacy but also increased adverse reactions. These differences are influenced by hormonal regulation (e.g., estrogen enhancing immunity, testosterone suppressing it), genetic factors (X-chromosome-linked immune genes), and microbiome variations [101]. Its effectiveness is also followed by the formulation development process. One of the most significant concerns throughout the formulation development process is the administration’s planned mode. When selecting the type of vaccine delivery method, the impact on both mucosal and systemic immunity has always been a consideration. Mucosal immunity requires tolerance while on the other hand, systemic immunity requires preparedness or sensitivity. Vaccines are given either parenterally or mucosal. The parenteral sites consist of intradermal, intramuscular, subcutaneous, and intravenous [102]. There are several ways that guide the choice of the route of vaccine administration including the site of infection, mode of transmission, type of vaccine, and expected immunogenicity. Mucosal immunization would prove effective for a localized immune response, particularly because it imitates the process of natural infection. Mucosal immunization also elicits systemic and/or other mucosal immune responses [103]. Further, specialized mucosa-associated lymphoid tissue (MALT), an extensively developed lymphoid tissue is located at the mucosal boundary. MALT includes the innate and adaptive immune systems [104]. Compared to the parenteral approach, mucosal vaccination offers a number of benefits, including patient compatibility, lack of sharp waste generation, and no need for a medical expert. Nasal vaccine administration, a kind of mucosal delivery, stimulates the production of immunological responses, particularly innate immunity and IgA humoral and mucosal antibodies, by the nasal-associated lymphoid tissues (NALTs), which contain specialized M-cells [105]. There is just one approved nasal spray flu vaccine available for purchase, FluMist Quadrivalent® (live attenuated influenza vaccination), which protects against influenza B and influenza A (H1N1, H3N2) [106]. Tolerance is produced via the oral mucosal vaccination. Oral vaccinations that are presently on the market include the rotavirus vaccine live oral Rotarix™ (GlaxoSmithKline Biologicals), the typhoid vaccine live oral Ty21a Vivotif™ (Berna Biotech, Ltd.), and the cholera vaccine live oral Vaxchora™ (PaxVax Bermuda Ltd.). These have been proven to be efficient in stimulating the immune system and preventing diseases. Some recent studies concerned the vaginal route for delivering the vaccine for genital infections and malignancies such as cervical infections and HPV. Topical vaccination would be possible only for a restricted immunological capability in genital infections. After being immunized through inactivated and live attenuated vaccines, the genital mucosa has specific types of immune reactions. The parenteral method is used for administering the largest part of the vaccines available at the moment. The intramuscular approach is applied in the administration of the newly developed Pfizer-BioNTech and Moderna COVID-19 vaccines. It is usually possible to develop a higher systemic immune response through IgM, IgG, and IgA antibodies to these innovative m-RNA vaccines [107–109].
To administer drugs to the epidermal layer, microneedle arrays are minimally invasive instruments made up of micron-sized needles (50–900 microns in height) that are intended to pierce the stratum corneum of the skin. Microneedles, which are made of silicones, metals, and polymers, produce tiny aqueous holes that allow drugs to diffuse. They provide a painless, patient-compliant, and self-administered substitute for hypodermic needles, having been introduced decades ago and receiving substantial study attention in the mid-1990s. Numerous medications and formulations may be precisely delivered via microneedles to certain tissues, such as the skin or eye. They may be divided into four categories: solid, coated, dissolving, and hollow. Using microneedle-based vaccines can reach more people in areas where the cold chain is not feasible. To be used in routine vaccination programs, stable vaccines that do not require refrigeration, call for further research on the thermostability of microneedle patches. Microneedle-based vaccines must follow general WHO standards, for instance, the vaccines must have a vaccine vial monitor (VVM) category 7 or more; higher categories like VVM14 (stable up to 14 days at 37°C) or VVM30 (stable up to 30 days at 37°C) are encouraged for room temperature vaccines. The fact that the microneedle matrix has a low moisture content and a shapeless structure helps to achieve this stability. Furthermore, in the field of 3D printing, discoveries lead to individualized, large-scale production of microneedles used in vaccines [108, 110].
NP delivery systems are being considered promising vaccine adjuvants or carriers. To achieve long-lasting immunity NPs can incorporate various types of vaccines including antigens, proteins, peptides, or nucleic acids and directly deliver them to immune cells. Table 3 provides a comprehensive analysis of different NP-based drug delivery systems (NDDS) used in vaccine development, categorizing them by their composition, mechanism of action, advantages, and applications. NDDS also provides targeted or sustained vaccination payloads bypassing the biological constraints of the target site. These systems target specifically the humoral and cellular immunity meanwhile traditional vaccines might not be able to do so. Although trust in nano vaccine platforms has increased due to the success of LNP-based COVID-19 vaccines, there remain worries regarding possible cytotoxicity because NPs have a short safety history. With these reservations, NPs have demonstrated remarkable potential in the treatment of infectious illnesses and may be modified for other difficult disorders such as cancer [111].
With simple and safe components, polymeric NPs administered through the mucosal route showed a robust immune response against the hemorrhagic septicemia virus (HSV). When administered nasally, gold NPs in the influenza A virus vaccination increased immune system function, demonstrating increased availability and decreased toxicity. Compared to conventional techniques, hepatitis B vaccinations using polymeric NPs greatly increased antibody levels and enabled targeted administration. A regulated, non-invasive, and extremely biocompatible nanogel administration technology enhanced the immune response to general viral infections. Also, the HPV vaccine provided targeted delivery with a great safety and biocompatibility profile since it was made using particles that resembled viruses [112].
Different types of nanoparticle-based drug delivery systems used for vaccines
| Nanoparticle type | Composition | Mechanism of action | Advantages | Examples/Applications | Reference |
|---|---|---|---|---|---|
| LNPs | Lipids (e.g., phospholipids, PEG) | Encapsulate and deliver m-RNA to cells; LNPs fuse with cell membranes to release the m-RNA into the cytoplasm | Protect m-RNA, ensure targeted delivery, biocompatible, scalable | Pfizer-BioNTech and Moderna COVID-19 vaccines | [113] |
| Polymeric nanoparticles | Biodegradable polymers (PLGA, PLA) | Controlled release of antigens or genetic material; degradation triggers antigen release and immune response | Biodegradability, controlled release, high stability | Vaccines for TB and influenza (research stage) | [112, 114] |
| Liposomes | Phospholipid bilayer vesicles | Encapsulate antigens or adjuvants; merge with host cell membranes to deliver the payload intracellularly | Biocompatible, customizable, can co-deliver drugs and antigens | Virosome-based vaccines, e.g., Epaxal® (Hep A) | [115] |
| Inorganic nanoparticles | Gold, silica, carbon nanotubes | Serve as carriers or adjuvants; enhance the immune response by targeted antigen delivery or stimulating immune cells | High stability, strong immune activation, tunable size | Gold nanoparticles in cancer vaccines (preclinical) | [116] |
| Protein-based nanoparticles | Viral capsids, ferritin, or albumin | Self-assemble into nanoscale structures; mimic natural pathogens to enhance immunogenicity | High immunogenicity, biocompatibility, scalable | Virus-like particles (e.g., Gardasil® for HPV) | [117] |
| Hybrid nanoparticles | Combination of materials (lipid-polymer) | Integrate the benefits of multiple types of nanoparticles, e.g., stability and targeting | Versatility, enhanced stability, and functionality | Cancer and viral vaccines (preclinical) | [118] |
LNPs: lipid nanoparticles; m-RNA: messenger RNA; PEG: polyethylene glycol; PLGA: poly(lactic-co-glycolic acid); PLA: polylactic acid; TB: tuberculosis; HPV: human papillomavirus
As per WHO, immunizations against cervical cancer, cholera, coronavirus, COVID-19, diphtheria, hepatitis B, influenza, Japanese encephalitis, malaria, measles, meningitis, mumps, pertussis, pneumonia, polio, rabies, rotavirus, rubella, tetanus, typhoid, varicella, yellow fever are used in several countries depending on the prevalence of these diseases. These include over 20 human illnesses preventable through vaccination; among these, only the vaccines for pertussis, influenza, measles, and diphtheria have been estimated to save between 3.5 and 5 million lives per year. Therefore, the Immunization Agenda 2030, or IA2030 is meant to vaccinate the people of the world to improve health [119]. 24 million people might avoid poverty by 2030 if IA2030 is achieved. But problems are getting worse; for example, malaria alone might cause 60,000 more fatalities annually by 2050 due to climate change. The complex of antigens, persistent infections, high sequence variability pathogen evolution, and new and re-emerging diseases pose problems for conventional vaccinations. Significant obstacles to the effective creation of vaccines include a lack of knowledge about the development of immunity, host and pathogen diversity, safety of novel vaccines, and nonheritable variables [35, 120].
The issues of immunization in cases of newly emerging communicable diseases are multifaceted and include issues of vaccine production, availability, and biological issues. The control of new and old vaccinations is limited by health care in developing countries, and some people refuse to be immunized due to safety issues. Because of the time taken to develop a vaccine; the disease may have spread when the vaccine is available [121]. Additionally, immunosenescence in older people reduces vaccine efficacy, increasing the risk of developing an infection, while genetic variability in RNA viruses complicates the development of vaccines. The creation of vaccinations against such diseases as HIV, malaria, and tuberculosis (TB) is very difficult because these pathogens and the mechanisms that destroy them interact with the immune system in the human body. Public adaptation, adjuvant effectiveness, societal expectations, and business model constraints are some of the elements that can worsen these problems. Addressing vaccination hesitancy, distribution disparities, and newly and re-emerging viral infections are also major obstacles, especially in low- and middle-income countries (LMICs) [122]. Strong surveillance, quick vaccine development, and flexible vaccination plans are essential given the ongoing introduction of novel viruses like SARS-CoV-2 and the resurgence of illnesses like measles, Ebola, and Mpox. There are serious ethical issues with accelerated vaccination studies, such as the ones carried out during the COVID-19 epidemic. Researchers conducted a study about vaccination participation and societal vaccine resistance during pandemic outbreaks, particularly COVID-19, to measure voluntary inoculation levels without mandatory regulations. Research performed on 150 countries demonstrates that vaccination rates rise as income per capita increases until they reach a natural population vaccination limit of approximately 70%. Pushing for 90% vaccination coverage through harsh policies would undermine democratic principles and possibly generate social difficulties that exceed the consequences of the pandemic. The Understanding of population vaccination behavior helps create successful vaccination plans that promote health policies to handle upcoming widespread outbreak protection despite restrictions [123].
The human challenge trials also present a positive ethical concern since healthy participants are intentionally exposed to infections. To ensure that both genders, minorities, and other potentially exploitable participants are not overrepresented, non-biased participant sampling should also be seen as critical [35, 120, 124]. Furthermore, vaccine barriers including misinformation, perceived risk, and cultural beliefs slow down immunization at a global level and therefore require a specific health promotion campaign to fight against misinformation to increase vaccine uptake and confidence. Moreover, LMICs continue to face challenges with vaccine distribution because they have less-developed health systems, fewer funds, and many practical challenges that prevent them from getting vaccines fast and fairly [125]. Concerns about viral vaccinology across the globe require the implementation of multiple approaches focusing on affordability, efficacy, and variation. To raise the level of understanding of immune responses, systems biology, and the determination of non-immunoglobin markers of protection are important. Ideally, vaccines must also generate humoral and cellular immunity, and the effector mechanisms related to rapid convalescence from infection should be explored. While working on vaccine production and deployment, the issue of population growth and genetic differences must be addressed to get multitarget and multispecific products and reagents [122]. Vaccine hesitancy remains a significant challenge in global immunization efforts, influenced by a complex interplay of sociocultural, psychological, and informational factors. The decision to accept vaccines depends on knowledge levels alongside past experiences and perceived importance rating with subjective norms and risk perception together with religious beliefs and all factors emerge from historical, political, and socio-cultural settings. This decision faces influence from three external elements including public health policies which promote vaccination programs and safety monitoring and health professional recommendations based on expert medical trust, while social media and digital platforms enhance misinformation exposure, which requires immediate intervention to address [126]. Social media and digital platforms allow real-time collection of vaccine hesitancy data, which fuels new analytical techniques to study how publications about vaccines affect both disease prevention numbers and vaccine acceptance rates. The academic collaboration between Facebook and educational institutions resulted in user surveys about COVID-19 vaccine attitudes, which produced extensive timely data sets that match findings from other studies although potential participant biases exist. The vaccine hesitancy data allowed researchers to create ZIP code-based geographic maps of hesitancy across the USA that displayed important geographical patterns within county areas. A global survey indicates physicians alongside healthcare providers as the most trusted medical advisors because a majority of respondents (73%) and up to 90% in higher-income nations choose them as their primary healthcare advisors. Effective vaccine acceptance promotion depends on healthcare providers delivering support while listening attentively to patient concerns and correcting misinformed ideas [127, 128]. Techniques of effective communication need to be used because they prevent vaccine hesitancy. Public health authorities need to provide clear communication regarding vaccines while simultaneously reaching audiences through culturally appropriate methods by involving respected community members. During the COVID-19 vaccine campaign healthcare staff partnered with both social media influencers and religious leaders to prove the vaccination worth and defeat wrong information about vaccines. Global HPV vaccine programs achieved success by implementing vaccination programs in schools while providing education for parents and obtaining healthcare provider support. The acceptance of vaccines increases through proven methods such as incentive-based programs and workplace vaccination mandates along with real-time misinformation tracking systems. Regulatory bodies must enhance their authority to control false vaccine claims and conspiracy theories because public trust depends on it. Chronic diseases, which are more exacerbated by new emerging viral diseases and inequitable distribution of healthcare, require approaches that include the fusion of public-private partnerships (PPPs), equal distribution of vaccines, and global surveillance [129]. Global surveillance and response efforts are providentially managed by international health organizations such as the CDC and WHO. During the incidence of public health crises, these organizations support countries’ collaboration, provide consultation, and disseminate essential information. As member states learn to identify, assess, and promptly address epidemics, for instance, the WHO’s Global Outbreak Alert and Response Network (GOARN) mobilizes expert teams. Infectious disease identification and early detection depend on surveillance systems. Through data acquisition, analysis, and dissemination processes, these systems are capable of responding rapidly to epidemic events and assessing their potential impacts. Predictive modeling and real-time data sharing are examples of the methods used in the timely development of proactive reaction capacities for such threats. For instance, AI algorithms and digital monitoring technologies could look at patients’ data feeds and use analytics algorithms to analyze patterns that signify disease outbreaks for an early containment process [130, 131].
PPPs effectively manage global health issues by integrating business, academics, and government resources and capabilities. Universities provide scientific information, research capacity, and innovation, while government provides funding, regulation, and laws. To accelerate the R & D and the deployment of vaccines and medical technologies, industry partners contribute technological tools, manufacturing facilities, and market channels. Successful PPPs have made progressive improvements in the research, development, and distribution of vaccines. Thus, the coordination of polio vaccines in different countries is done by grouping: the Global Polio Eradication Initiative (GPEI), governments of affected countries, WHO, the United Nations Children’s Fund, the Rotary Club, and CDC. Teamwork is well exemplified in this program since it has greatly reduced instances of polio globally [131, 132]. Research investigated healthcare costs against COVID-19 death rates throughout European nations to develop functional crisis management strategies. Analyses conducted through regression analysis and descriptive methods show enhanced healthcare expenses per person together with preventive care spending and increased hospital infrastructure and medical worker availability decrease COVID-19 death tolls. The mortality rate for COVID-19 decreases by 0.74% when authorities increase their healthcare expenditure by 1% per capita across all populations. Eastern European regions with lower healthcare budgets faced higher mortality rates than Western European territories with higher spending levels. Continuous funding in healthcare services with medical technologies and information technology infrastructure represents the key requirement to enhance pandemic readiness and emergency response capabilities [133].
That COVID-19 vaccines were created and rolled out so quickly demonstrated the importance of global cooperation and the importance of cooperative science. Scientists evaluated how quickly strategic COVID-19 vaccination strategies help prevent new cases and deaths from the pandemic. The vaccination threshold needed for maximum results grew from 46.75 doses per 100 people in March 2021 up to 90 doses in May 2021 following changes to the pandemic. The implementation of high vaccination rates reduced hospital deaths which proved that public health actions must be implemented quickly. Global elimination of COVID-19 becomes difficult because of multiplexed barriers like unequal vaccine distribution and hesitation from people and new virus versions appearing alongside questions regarding immunity duration. Vaccination campaigns require good governance together with strong public health policies and efficient crisis management systems for their success. Vaccination coverage has dropped during the last ten years and the current pandemic has shown major problems within immunization programs despite continuous work. The gaps in vaccination coverage have triggered the return of diseases such as polio, measles, diphtheria, and pneumonia because these healthcare systems need more robust vaccination approaches [134]. The development of IA2030 contains a goal to establish vaccination accessibility for all citizens from prenatal through adult and elderly stages regardless of geographical location or socioeconomic circumstances. This vision establishes equal vaccine accessibility by integrating immunization delivery into primary healthcare and supports universal healthcare as well as the sustainability development goals. One of the major goals of IA2030 works through seven essential priorities which build up immunization programs and secure fair vaccine distribution across all communities while preventing upcoming health threats using the pandemic’s momentum to protect worldwide health [135].
This review presents an investigation into viral vaccinology evolution and infection outbreaks combined with vaccine applications toward public health for removing smallpox alongside polio along with measles and COVID-19. Conventional vaccines, such as live attenuated, inactivated, and subunit vaccines, have been effective but are limited in production time, storage, and response to emerging pathogens. Emerging platforms such as m-RNA, viral vectors, and recombinant vaccines provide fast development, scalability, and increased immunogenicity, as seen with COVID-19 vaccines.
Current vaccine development faces multiple obstacles which include vaccine reluctance among certain populations as well as unequal vaccine distribution needs and human health restrictions from new vaccine variants. The study acknowledges that women are underrepresented in clinical trials thus blocking access to safety and effectiveness data. Multiple strategies for future vaccines include nanotechnology-based approaches as well as universal vaccine development and personalized vaccination approaches in addition to enhanced international cooperation for global health protection. The research promotes the ongoing development of inclusive research and policy adjustments to raise vaccine effectiveness together with expanded accessibility and pandemic readiness [136]. The development history, progress, and challenges of viral vaccine development over the last 150 years reflect a remarkable journey of scientific innovation, global health impact, and evolving technological strategies. Smallpox vaccination by Edward Jenner in 1796 launched vaccinology through major breakthroughs which have included polio vaccine (IPV and OPV) and MMR and hepatitis B vaccines. Research into recombinant DNA technology during the 1980 enabled scientists to create new vaccines with enhanced safety characteristics that included HPV and hepatitis B vaccines. During 2020, m-RNA vaccines emerged as a novel vaccine platform to develop and deploy vaccines swiftly which resulted in the COVID-19 vaccines produced by Pfizer-BioNTech and Moderna. EIDs received critical support through viral vector vaccines developed by AstraZeneca for both COVID-19 and Ebola virus prevention. Challenges, however, remain, such as with influenza, HIV, and malaria, which demand complex immune responses and high mutation rates. Throughout human history, vaccine development occurred during worldwide health emergencies which resulted in four core vaccine types namely subunit vaccines, protein-based vaccines, RV, and m-RNA vaccines [137]. They have been briefly described below:
Subunit vaccines use selected viral components to elicit an immune response without containing the entire pathogen. They offer high safety but may require adjuvants to boost immunogenicity.
Protein-based vaccines rely on purified viral proteins and provide high specificity but may not be effective against rapidly mutating viruses.
RV leverages genomic sequencing to identify antigenic targets, speeding up vaccine development for difficult pathogens.
m-RNA vaccines instruct cells to produce viral proteins, inducing strong humoral and cellular immunity with rapid scalability. However, challenges include cold chain storage and stability issues.
Researchers developed Marburg hemorrhagic fever T cell and humoral immune responses in mice through DNA and m-RNA vaccines containing optimized glycoproteins’ codons. Recombinant vaccines made with VLPs and engineered HBsAg SVPs have proven to be critical breakthroughs in managing chronic viral infections. Global pandemic response experienced revolutionary changes with Pfizer-BioNTech and Moderna’s m-RNA vaccines that now create opportunities for disease prevention against influenza along with HIV and Zika.
Medical R & D efforts during times without epidemics remain crucial because they create the foundation for future vaccine development against EIDs such as chikungunya and Zika. The international initiative CEPI motivates private-sector companies to fill these gaps. Low-resource settings face difficulties with storing vaccines that need cold chain facilities, especially those using m-RNA technology. Through technology transfers, the DCVMN has effectively expanded vaccine availability. PPPs with educational programs must be established to overcome vaccine accessibility barriers and public confidence issues that hamper vaccination rates. New third-generation vaccines are emerging because of advances in whole-genome sequencing and bioinformatics alongside ML. RV has transformed specific antigen analysis by using predictive computational methods which speed up the development of vaccines for COVID-19 along with Ebola and Zika virus. Immune-derived epitope-based vaccines demonstrate better accuracy along with milder adverse effects during vaccination and quicker immune response than classic live attenuated or subunit vaccines. The vaccine design methodology has resulted in successful vaccine development for meningitis, malaria, anthrax, and MABV. The assessment of MABV vaccine development showed how codon optimization and in silico cloning together increased E. coli K12 antigen expression while validating their effectiveness for aquaculture security. ML and AI technologies now predict three vital vaccine attributes: efficacy levels along with antigenic properties and immunological responses. The development of vaccine candidates becomes optimized through ML models that merge structural data with proteomics and metabolomics. The vaccine research is enhanced through predictive capabilities by implementing algorithms consisting of SVM, RF, ANN, and KNN. VaxiJen and VIOLIN represent two computational tools that simplify vaccine selection processes. Research showed that greater vaccine coverage numbers produced major decreases in mortality numbers which proves that data-centric vaccine approaches work effectively [66, 92, 138].
The development of m-RNA vaccines brought an innovative transformation to immunizations because of their quick production speed alongside their flexible and easily scalable regenerative technique. The operating mechanism of m-RNA vaccines differs from conventional immunizations since they transmit genetic information to produce immunogenic proteins which trigger powerful immune system responses from both cellular and humoral components. When LNPs transport synthetic m-RNA inside host cells, these molecules get converted into viral antigen proteins. Uptake of these antigens from MHC class I and II pathways activates CD8+ cytotoxic T cells and CD4+ Ths which produce antibodies and create long-lasting immune memory. Post-translational degradation occurs quickly to m-RNA which eliminates its ability to integrate into the genome. The two vaccines produced by Pfizer-BioNTech and Moderna reached 95% effectiveness against severe disease which confirms m-RNA vaccines’ potential application during pandemics. The ZIKV vaccine developed by Moderna using m-RNA technology generated substantial neutralizing antibody production according to preliminary laboratory experiments. Both Moderna with m-RNA-1769 together with BioNTech using BNT-166A work on Phase I/II Mpox infection vaccine trials [139, 140].
Key advancements that have enabled the success of m-RNA vaccines include:
nucleoside modification (e.g., pseudouridine) to enhance stability and reduce immune overactivation
ionizable LNPs for efficient delivery and intracellular uptake
codon optimization and AI-driven design to improve translation efficiency and immunogenicity
saRNA technology, which increases antigen production and immune response longevity.
The development of vaccines depends upon three key components including antigen type, delivery method, and immune response activation. The delivery approach for vaccines determines the effectiveness of vaccines as well as immune system activation and the degree to which patients adhere to vaccination protocols. The traditional vaccine administration methods involve intramuscular and subcutaneous and parenteral and mucosal routes which offer different benefits to vaccine delivery. The design of mucosal vaccines matches the course of typical infections by activating MALTs along with parenteral vaccines that produce robust systemic immunity responses. The benefits from nanovaccines include better delivery methods alongside extended drug retention and harmless substance interactions, yet questions exist about toxic potential and prolonged health risks. More investigation is essential to create improved NP formulations and strengthen vaccine stability and distribution techniques. The combination of 3D printing technology and nanotechnology could transform the way vaccines are delivered as it creates new potential for improved effectiveness and global accessibility in vaccination programs. The success of immunization programs is now thought to be threatened by a lack of trust in vaccinations. Reduced vaccination coverage and a higher chance of vaccine-preventable disease outbreaks and epidemics are thought to be caused by vaccine hesitancy [126, 141, 142].
IA2030 targets equitable access to vaccines worldwide, potentially preventing 24 million individuals from entering poverty by 2030 [135]. Although gains have been made, threats remain in the guise of climate change-facilitated disease resurgence, pathogen mutation, and vaccine refusal. Malaria, measles, and polio continue to pose health risks to the world, particularly in LMICs with fragile healthcare systems. The research emphasizes the need for rapid, strategic COVID-19 vaccination campaigns, with the optimal level increasing from 46.75 to 90 doses per 100 individuals as the pandemic worsened. Results indicate that increased healthcare spending is associated with reduced COVID-19 mortality, reinforcing the need to continue investing in medical technology and crisis preparedness. PPPs among governments, academia, and industry are critical to vaccine research, development, and equitable access. Initiatives such as the GPEI and WHO’s GOARN illustrate the value of partnership in the fight against infectious diseases. Lastly, IA2030 targets integrating immunization into primary healthcare systems, with lifelong access to vaccines from infancy to old age, enhanced pandemic preparedness, and extended universal health coverage to safeguard global populations against impending health emergencies. Table 4 highlights the findings of the studies related to the m-RNA vaccine development.
Summarized findings of the studies related to the m-RNA vaccine development
| Parameter | Findings |
|---|---|
| Vaccine types | Traditional (live attenuated, inactivated), subunit, protein-based, viral vector, and modern m-RNA vaccines were reviewed |
| Efficacy & immune response | m-RNA vaccines demonstrated high immunogenicity and rapid response, while subunit vaccines had stable but lower efficacy |
| Computational approaches | Reverse vaccinology, structural vaccinology, and AI-driven machine learning enhances antigen identification and vaccine design |
| Safety & scalability | Modern vaccine platforms, particularly m-RNA and viral vector vaccines, have revealed improved safety and faster production scalability |
| Delivery methods | LNPs, viral vectors, and NP-based carriers emerged as efficient vaccine delivery strategies |
| Challenges identified | Vaccine hesitancy, global accessibility, high production costs, and the need for more inclusive clinical trials were major obstacles |
| Future prospects | Advances in nanotechnology-based delivery, AI-driven vaccine design, and saRNA vaccines hold promise for improved efficiency and accessibility |
m-RNA: messenger RNA; AI: artificial intelligence; LNPs: lipid nanoparticles; saRNA: self-amplifying RNA
This review demonstrates how solution-oriented innovation together with technological progress has resulted in outstanding advances in viral vaccine development. The field of viral vaccinology has achieved remarkable breakthroughs but continues to face substantial difficulties in biological, technical, logistical, social, economic, and ethical domains. The biological challenges in vaccine creation stem from viral mutations and complex immune system responses and low levels of antigen response by the body. Manufacturing alongside logistical limitations creates issues regarding large-scale manufacturing capacity together with strong temperature control needs and high operating expenses. Social and behavioral challenges emerge because people resist vaccination and there are problems with equal health service availability and inadequate public knowledge about vaccines. Most LMICs face two major economic barriers which include high R & D expenses along with scant resources available for funding projects. Successful advancement of viral vaccinology and global health protection demands persistent innovation alongside collaborative efforts together with a devoted commitment to solving complex barriers.
The review establishes important contributions to theories within both innovation studies and public health research. The study establishes powerful empirical data that supports the model of innovation as a response to problems during technological development. A detailed analysis shows how advanced medical breakthroughs starting with smallpox vaccine development by Jenner up to the present m-RNA revolution stem directly from important global health emergencies. History emphasizes that vital societal requirements that demand rapid solutions make both revolutionary and small-step innovations possible for essential breakthroughs like vaccines. The development of vaccine platforms through subunit and protein-based and viral vectors along with m-RNA technology shows that flexible technological solutions enable quick deployment to tackle various challenges. Platform technologies successfully demonstrated their power to speed up innovation and deliver adaptable solutions in active disease outbreak situations through their establishment with m-RNA vaccines. The movement from traditional vaccination methods toward computation and nanotechnology-based vaccine development creates a substantial shift in vaccine science. Modern vaccine development relies on RV and immunoinformatics alongside AI/ML to help scientists accelerate scientific advances through predictive modeling and computational simulations of data analysis.
Governments and funding agencies must invest strategically in developing and sustaining flexible vaccine platform technologies, especially m-RNA, viral vector, and advanced subunit platforms. Such visionary investment, and not just crisis-driven reactive funding, is necessary for pandemic preparedness and the capability for a quick response. This involves financing not just basic research but also large-scale manufacturing infrastructure and speedy deployment of the platforms. R & D strategies must adopt interdisciplinary research, combining virology, immunology, bioinformatics, computational biology, nanotechnology, and social science skills. Policymakers must actively encourage and enable PPPs, inducing private sector participation in the R & D of neglected diseases and pandemic preparedness. In addition, strong international collaboration, involving technology transfer, sharing of data, and concerted research, is needed to confront global health problems effectively. Managerial and policy interventions must put at the top of the agenda the creation of public confidence in vaccines and vaccine hesitancy. Ethical considerations must remain at the heart of rapid vaccine development and rollout, with equal access, informed consent, and managing data privacy and possible side effects. Policies must induce the creation of flexible and scalable vaccine manufacturing plants that can be easily repurposed to manufacture different types of vaccines during pandemics.
Future research requires sustained intensity to build universal vaccines that protect people from major viral families such as influenza viruses, coronaviruses, and HIV, while supporting the ongoing development of IA2030. Researchers need to develop individualized vaccination plans by combining AI with data analytical methods to match strategies with patients’ genetic makeup and risk profiles. Research necessary now involves making improvements to advanced vaccine delivery methods that consist of microneedle array products along with oral and nasal vaccine presentations as well as NP platforms. The research priorities should include both enhancing stability under varying temperatures and improving patient engagement alongside the development of minimally invasive delivery systems that specifically target immune areas to achieve better performance and wider population coverage. Evidence-based communication methods that specifically address public concerns and foster trust must be developed and assessed to boost worldwide vaccine adoption rates. Such research should investigate how vaccines affect overall socioeconomic conditions in addition to their roles in economic advancement and poverty alleviation as well as social equality measures.
AI: artificial intelligence
CEPI: Coalition for Epidemic Preparedness Innovations
CTL: cytotoxic T lymphocyte
E: envelope
EIDs: emerging infectious diseases
HBsAg: hepatitis B surface antigen
HBsAgS: hepatitis B surface antigen-small
HBV: hepatitis B virus
HIV: human immunodeficiency virus
HPV: human papillomavirus
IA2030: Immunization Agenda 2030
IFN-γ: interferon-gamma
LMICs: low- and middle-income countries
LNPs: lipid nanoparticles
MABV: marine birnavirus
MALT: mucosa-associated lymphoid tissue
MERS: Middle East respiratory syndrome
MHC: major histocompatibility complex
ML: machine learning
MMR: measles, mumps, rubella
Mpox: monkeypox
m-RNA: messenger RNA
NP: nanoparticle
OPV: oral polio vaccine
PPPs: public-private partnerships
R & D: research and development
RV: reverse vaccinology
S: spike
SARS-CoV-2: severe acute respiratory syndrome-coronavirus 2
SVPs: subviral particles
Th: helper T cell
UTRs: untranslated regions
vIDPs: viral infectious diseases of poverty
VLP: virus-like particle
VVM: vaccine vial monitor
ZIKV: Zika virus
AK and FA: Conceptualization, Data curation, Formal analysis, Writing—original draft, Writing—review & editing. BKS: Formal analysis. AAAA, YM, and VM: Validation, Visualization, Methodology, Formal analysis, Supervision, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
All datasets analyzed for this study are included in the manuscript.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Marc H.V. Van Regenmortel
Christine Jacomet
Vladimir N. Uversky
Brent Brown ... Ingo Fricke
Chittaranjan Baruah ... Bhabesh Deka
Brent Brown ... Enrique Chacon-Cruz
Om Saswat Sahoo ... Subhradip Karmakar
Mikolaj Raszek ... Alberto Rubio-Casillas
Yulia Desheva ... Irina Isakova-Sivak
