Affiliation:
1Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Yarmouk University, Irbid 21163, Jordan
Email: alaaj@yu.edu.jo
ORCID: https://orcid.org/0000-0002-9519-6338
Affiliation:
1Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Yarmouk University, Irbid 21163, Jordan
ORCID: https://orcid.org/0000-0003-2396-9132
Affiliation:
2Department of Clinical Pharmacy and Pharmacy Practice, Faculty of Pharmacy, Yarmouk University, Irbid 21163, Jordan
ORCID: https://orcid.org/0000-0001-8801-2652
Explor Immunol. 2025;5:1003204 DOI: https://doi.org/10.37349/ei.2025.1003204
Received: January 29, 2025 Accepted: July 04, 2025 Published: July 29, 2025
Academic Editor: Shubhada Chiplunkar, Homi Bhabha National Institute (Deemed University), India
Oncolytic virotherapy (OVT) employs genetically engineered or naturally occurring viruses to selectively replicate within tumor cells, leading to direct lysis and induction of systemic anti-tumor immune responses. This dual mechanism distinguishes OVT from conventional therapies and positions it as a promising candidate in precision oncology. This review synthesizes recent advancements in understanding the molecular mechanisms underlying OVT efficacy, including viral entry, replication kinetics, immunogenic cell death, and modulation of the tumor microenvironment. We highlight innovations in viral engineering, such as promoter targeting, microRNA control, and immune-modulatory gene insertions that enhance tumor specificity and therapeutic safety. Clinically, OVT has shown measurable benefits in various solid tumors, with several viruses, such as talimogene laherparepvec, entering regulatory approval and others progressing through late-phase clinical trials. When combined with immune checkpoint inhibitors, OVT has demonstrated synergistic effects by improving antigen presentation and reversing immunosuppressive signaling. Integration with targeted therapies and nanotechnology-based delivery systems has further refined viral biodistribution and pharmacodynamics. However, therapeutic resistance, immune clearance, stromal barriers, and heterogeneous tumor responses remain key limitations. Overcoming these challenges requires optimized delivery routes, predictive biomarkers, and combination strategies tailored to immune and genetic tumor profiles. As OVT evolves from proof-of-concept to a platform-based therapeutic strategy, its integration into multimodal cancer treatment protocols will depend on refined bridge oncolytic activity with durable immunotherapy effects.
Cancer is one of the most common causes of death worldwide, highlighting the urgent need for new and effective treatments [1, 2]. Oncolytic viruses (OVs) are designed to selectively replicate in and lyse tumor cells while minimizing harm to healthy tissues, offering a dual role of direct tumor destruction and immune activation [3–5]. In contrast to earlier reviews that primarily focused on individual viral platforms or immune mechanisms, this manuscript delivers a unified, mechanistically detailed, and translationally oriented analysis highlighting underrepresented vectors such as baculoviruses, engineering innovations like CRISPR-guided modifications, and the convergence of nanotechnology and immunotherapy in clinical pipeline design [6, 7].
Cancer remains a formidable foe to human health, and new strategies are necessary to hold it at bay and eliminate it. Despite improvements, traditional cancer treatments often have limited efficacy in advanced disease and can cause systemic toxicity [8, 9].
The combination of nanotechnology with biotechnology has significantly increased the efficacy, specificity, and safety of OVs, offering practical solutions to the persistent difficulties in cancer treatment [10]. Nanotechnology has enabled the delivery of OVs through nanocarriers, such as liposomes, polymeric nanoparticles, and exosome-encapsulated systems, thereby shielding viral vectors from immune neutralization and enhancing tumor selectivity via ligand-directed targeting or environmentally responsive release platforms [11, 12].
Nanoparticles also co-deliver OVs with chemotherapeutic agents or immunomodulators, inducing synergistic therapeutic effects while enabling real-time imaging and tracking of OV biodistribution with theranostic nanoparticles [13, 14]. In parallel, biotechnology enables the genetic modification of OVs, allowing cancer-selective replication via tumor-selective promoters and the insertion of therapeutic genes, such as cytokines or immune-activating molecules, to potentiate anti-tumor immunity [15, 16]. Technical developments in synthetic biology, such as CRISPR-Cas9 and artificial gene circuits, have enhanced the specificity and activity of OVs [17]. Emerging clinical trials, AI-enabled viral design, and exosome-based delivery platforms represent the leading edge of translational oncolytic virotherapy (OVT) [18–20]. Recent preclinical and clinical studies have demonstrated that OVT can generate durable antitumor responses by combining direct tumor lysis with immune activation, particularly when integrated into multimodal treatment strategies [21, 22]. Additional immunotherapeutic platforms, including adoptive cell transfer and checkpoint blockade, are under active investigation in combination with OVs to further improve therapeutic efficacy [23, 24].
OVs represent a promising avenue in cancer immunotherapy, designed to selectively replicate within cancer cells and induce their destruction while sparing healthy tissues [16]. Engineered to exploit the defective antiviral defenses characteristic of many cancer cells, these viruses selectively target and lyse tumors [25]. During viral replication in cancer cells, tumor-associated antigens initiate an immune response that primes antitumor T-cells and systemic antitumor immunity [26, 27]. Further refinements include genetic modifications that enhance tumor selectivity and promote expression of immunostimulatory transgenes, thereby enabling synergy with complementary immuno-oncology strategies such as checkpoint blockade [28, 29]. By leveraging the innate and adaptive immune responses triggered by viral infection, OVT offers a rational framework for developing highly selective and durable anticancer modalities [30]. Figure 1 provides a comprehensive overview of the molecular mechanisms and design strategies underlying OV therapy, highlighting selective targeting, immune stimulation, and genetic engineering approaches.
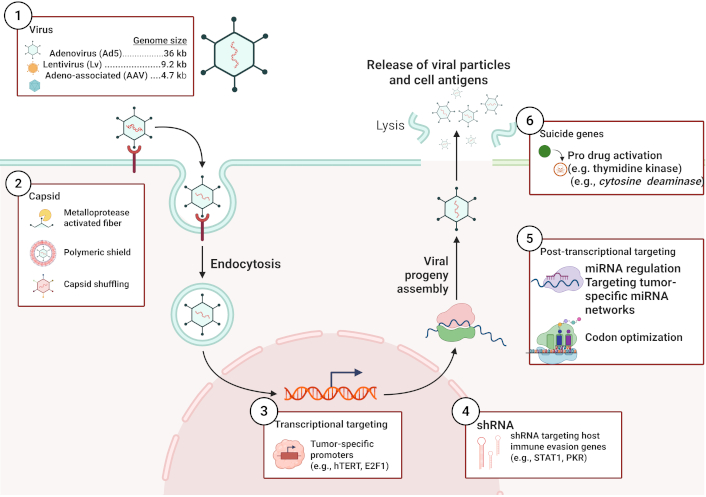
Overview of oncolytic virus engineering and infection pathway. The schematic depicts receptor-mediated endocytosis as a primary entry mechanism, typical of non-enveloped vectors such as adenovirus. Alternative pathways (e.g., macropinocytosis, membrane fusion, transcytosis) may also be employed by other virus families depending on envelope status, receptor specificity, and target cell type. Created in BioRender. Bashatwah, R. (2025) https://BioRender.com/y69z2op
The dual mechanism of action, direct oncolysis and immune activation, positions OVs as highly valuable tools in cancer therapy, particularly in combination with other immunotherapeutic modalities [31]. The mechanisms by which OVs exert antitumor activity are multifaceted [32]. Most importantly, these viruses selectively infect tumor cells, resulting in direct oncolysis [33]. For instance, the oncolytic poxvirus JX-594 selectively replicates in tumor cells through genetic pathways that are commonly activated in cancers, thus causing extensive tumor lysis without damaging normal tissues, as shown by Parato et al. [34]. This selectivity in replication is critical, as it facilitates amplification of the viral load within the tumor microenvironment, enhancing the therapeutic effect in general [35, 36]. Moreover, OVs can induce immunogenic cell death (ICD), which eliminates tumor cells, promotes dendritic cell (DC) maturation, and activates cytotoxic T lymphocytes (CTLs) to enhance anti-tumor immunity, as has been reported [37–40].
Recent advances in OV therapy have engineered the expression of immune-modulating agents [3]. For example, oncolytic adenoviruses are armed with PD-L1 inhibitors, which have been previously shown to induce neoantigen-specific T-cell responses against tumors, thus enhancing immune-mediated tumor elimination [41, 42]. Similarly, a combination of OVs and immune checkpoint inhibitors has shown promising activity in preclinical models, suggesting that such combinations might significantly improve therapeutic efficacy by overcoming the immunosuppressive tumor microenvironment [43, 44]. This bimodal strategy is also in concordance with data showing that the antitumor immune response following oncolytic viral treatment is an important component of the therapeutic efficacy of such interventions [3, 45].
Clinical trials have begun to demonstrate the potential of OVs in a range of tumor types. For example, in the case of liver cancer, patients were treated with the oncolytic vaccinia virus JX-594; this was not only safe but also elicited immune responses to tumor antigens [3, 46]. A recent systematic review of randomized controlled trials stressed the efficacy and safety of OVs, reinforcing their role as viable candidates for cancer immunotherapy [47, 48]. While such findings are promising, further challenges remain to be addressed to maximize clinical efficacy. The presence of neutralizing antibodies, and the immunosuppressive tumor microenvironment, for example, may impair viral replication and antitumor immune engagement [49]. Current research has optimized viral constructs and combination strategies to enhance the therapeutic window and overcome biological resistance mechanisms. As viral engineering and combination therapies advance, offering new avenues for enhanced therapeutic efficacy, OVs remain a key research direction for the development of next-generation therapies against tumors [11, 27, 42].
Clinical trials involving OVs are underway for several types of cancer, some of which have been approved by the US FDA [4]. OVT has emerged as a clinically validated and mechanistically distinct approach within modern cancer immunotherapy [50]. OVT involves the use of live replicating viruses that selectively infect and destroy tumor cells, leading to tumor regression [51]. Viruses such as talimogene laherparepvec (T-VEC) and Imlygic have received FDA approval for use in melanoma, and numerous active trials are evaluating novel viral platforms and combinatorial regimens across multiple cancer types [4, 52]. Challenges related to intratumoral delivery, host resistance, and biosafety are being actively addressed through ongoing efforts to optimize viral design, identify novel viral candidates, and elucidate virus–immune system interactions [53, 54].
OVs represent a rapidly advancing domain in cancer treatment, integrating nanotechnology and biotechnology through rational design. Through genetic engineering, these viruses selectively infect and destroy malignant cells, leaving surrounding healthy tissues intact, and their engineered delivery systems amplify therapeutic efficacy [3, 25]. For example, T-VEC, a genetically modified oncolytic herpes simplex virus type 1 (HSV-1), has demonstrated significant clinical utility in clinical trials because of its specificity in replicating cancer cells, while also expressing granulocyte-macrophage colony-stimulating factor (GM-CSF) [55]. This not only enhances the specificity of tumor targeting but also strengthens the immune response against cancer. Polymer-coated adenoviruses, like PEG and PEI-coated adenoviruses, were also developed for stabilization and targeting the tumors specifically at the same time [56]. These enhancements reduce hepatic sequestration and augment antitumor immunomodulatory functions of oncolytic adenoviruses [57].
The emergence of OVs with nanoparticles, such as gold nanoparticles conjugated to the oncolytic vaccinia virus, has resulted in increased ICD and improved antitumor immunity [27]. Similarly, magnetic nanoparticle-guided delivery of oncolytic adenovirus has enabled targeted accumulation at the tumor site, improving viral distribution in tumors and enhancing therapeutic efficacy [58]. Furthermore, the development of an oncolytic Newcastle disease virus (NDV) that expresses tumor-associated antigens exemplifies the dual benefit of direct tumor lysis while stimulating potent antitumor immune responses [59, 60]. These integrated biotechnological and nanotechnological strategies underscore a paradigm-shifting approach in cancer therapy, promising further innovations and broader clinical applications in the future [6].
Several OVs, such as adenovirus, vaccinia virus, herpesvirus, coxsackie A virus, NDV, and reovirus, have been developed as oncolytic therapeutics. Clinical trials have shown promising results, and some OVs have entered late-stage clinical development for the treatment of various cancers. The success of OV therapy has led to increased interest and ongoing research in this field [61–63]. OVs mediate antitumor effects in several ways, including infecting cancer cells, presenting tumor-associated antigens, activating damage-associated molecular patterns (DAMPs) to generate a less immune-tolerant tumor microenvironment, and serving as transduction vehicles for the expression of inflammatory and immunomodulatory cytokines. The combination of OV therapy with other antitumor therapies, such as CAR T-cell therapy, has shown the potential to induce immunogenic cancer cell death and improve treatment outcomes [5, 64].
Biotechnology and nanotechnology are two rapidly advancing fields that have the potential to transform the development and delivery of OVs. Biotechnology enables the precise genetic engineering of OVs, enhancing their safety, tumor selectivity, and immunogenicity. Nanotechnology facilitates the creation of targeted delivery platforms that transport OVs into cancer cells while shielding them from immune clearance [65–68].
Research on OVs in nanotechnology and biotechnology is evolving rapidly. Numerous new approaches have been investigated, some of which have already reached the level at which clinical trials are possible [69, 70]. Some of the most promising areas of research are as follows:
Gene editing: Gene editing technologies can be used to produce new OVs that are safer and more effective. Examples include the knockout of genes using CRISPR-Cas9, specifically those that limit viral replication or contribute to off-target toxicity. Researchers have also used CRISPR-Cas9 to add new genes to OVs that make them more effective or to add therapeutic genes to cancer cells [71, 72].
Targeted delivery: Researchers are currently developing new methods for targeting OVs to specific cancer cells. This is achieved by engineering OVs to express ligands or binding domains that recognize and attach to receptors overexpressed on tumor cells. This targeted approach enhances therapeutic precision, increases intratumoral viral accumulation, and minimizes off-target effects and systemic toxicity [73, 74].
Immunostimulation: New methods are being developed to engineer OVs to stimulate an immune response against tumor cells. They do this by expressing proteins that can activate immune cells, including T cells and natural killer (NK) cells. Such immunostimulatory modifications can potentiate OV efficacy, particularly in tumors that are refractory to conventional therapies [75, 76].
Effective delivery systems currently under development for OVs using nanotechnology include nanoparticles, exosomes, and 3D-printed scaffolds. These platforms are designed to improve viral stability, enhance tumor-specific delivery, and evade premature immune clearance. Their core objectives include maximizing OV accumulation within tumor tissues while minimizing systemic inactivation by the host immune system [3, 77].
Although substantial progress has been achieved, OV therapy still has to overcome delivery constraints, immune neutralization, and the development of tumor cell resistance [5]. These limitations necessitate precise retargeting strategies, next-generation genetic engineering, and rationally designed combinatorial regimens. Emerging research directions include the application of nanoparticles for targeted delivery, the enhancement of immune modulation, and the exploration of synergy with immune checkpoint inhibitors and other immunotherapies [15].
Despite the significant advances in the field, there are still several critical challenges that must be overcome before OVs can become standardized components of clinical oncology. One such challenge is the difficulty of OVs are often difficult to deliver to tumor cells. Another challenge is that OVs can be neutralized by the immune system. Finally, OVs can cause side effects such as inflammation and fever [61]. Oncolytic viral therapy for cancer has advantages and disadvantages. The advantages include the ability of OVs to selectively target and kill cancer cells, present tumor-associated antigens, activate DAMPs to generate a less immune-tolerant tumor microenvironment, and serve as transduction vehicles for the expression of inflammatory and immunomodulatory cytokines. OV therapy has shown promising results in preclinical and clinical trials, and some OVs have entered late-stage clinical development for the treatment of various cancers [62, 78].
However, oncolytic viral therapies have several disadvantages. One of the main challenges is the potential for the virus to cause toxicity and adverse effects in healthy cells [78]. Another challenge is the development of viral resistance in cancer cells, which can limit the effectiveness of therapy. Additionally, the immune system recognizes and eliminates the virus before it achieves sufficient intratumoral replication [79]. Finally, the high cost of developing and producing OVs can limit their accessibility to patients [4]. In summary, OV therapy has both advantages and disadvantages. While it represents a promising modality in cancer immunotherapy, further research is needed to optimize its therapeutic effectiveness and safety for the treatment of human and animal cancers [5, 80].
Table 1 presents a SWOT analysis of OVT, highlighting weaknesses such as delivery complications and immune neutralization; strengths in tumor selectivity and immunogenicity; opportunities in combination immunotherapy; and threats including regulatory and manufacturing barriers.
Integrated evaluation of oncolytic virus therapy: strengths, weaknesses, opportunities, and limitations
| Category | Content |
|---|---|
| Strengths |
|
| Weaknesses |
|
| Opportunities |
|
| Limitations |
|
This table provides a consolidated overview of the advantages, barriers, and strategic opportunities associated with oncolytic virus therapy in cancer treatment. Strengths and weaknesses represent current biological and clinical performance characteristics, while opportunities and limitations reflect future directions and implementation challenges. ECM: extracellular matrix
While adenoviruses, herpesviruses, and reoviruses are well-established oncolytic platforms, this review devotes focused attention to baculoviruses due to their emerging potential in oncolytic applications. Their unique biological properties non-replicative nature in mammalian cells, high transgene capacity, and low cytotoxicity make them promising candidates for safe and versatile therapeutic delivery systems in cancer therapy.
OVs are a class of viruses that exhibit a unique ability to target and infect cancer cells while sparing healthy cells [81]. This is partly due to the altered physiology of cancer cells, and partly attributable to genetic alterations in the viruses themselves. Once inside a cancer cell, an OV replicates selectively, ultimately inducing tumor cell lysis. Their natural tropism for malignant cells has made them highly attractive therapeutic candidates [81, 82].
Among heterogeneous assortments of OVs, baculoviruses represent a novel and unlikely ally in the fight against cancer [83, 84]. Baculoviruses, members of the Baculoviridae family, are double-stranded DNA viruses that infect insect cells but do not replicate in mammalian hosts. While they do not meet the definition of replicating OVs, their large transgene capacity, safety profile, and lack of pre-existing human immunity make them attractive tools for cancer gene therapy [85, 86]. They have been studied as non-replicative vectors capable of delivering therapeutic genes, including pro-apoptotic factors and immunostimulatory cytokines, into tumor cells. However, their clinical translation has been limited due to complement-mediated inactivation and poor transduction efficiency in vivo [83, 87]. Current efforts involving surface modification, encapsulation, and nanoparticle co-delivery are ongoing to overcome these barriers and harness baculoviruses as supporting vectors in cancer immunotherapy [88, 89].
OVs have various benefits as treatment modalities. Foremost among these is their ability to selectively replicate in malignant cells while sparing normal tissues. This reduces side effects, and the drug is more tolerable to patients, which is a consideration in the treatment of cancer [3, 90]. Moreover, OVs have been found to trigger immune responses against tumors. Viral replication within tumor cells typically induces an immunogenic cascade that synergizes with host antitumor immunity. This immunomodulatory role can enhance the long-term efficacy of oncolytic viral treatments [91, 92]. One of the most valuable attributes of OVs is their genetic flexibility. Researchers can perform genetic engineering to enhance tumor-targeting efficiency or even add therapeutic payloads, such as genes that make cancer cells sensitive to other forms of treatment. This flexibility paves the way for personalized approaches to cancer therapy [90].
Although the prospects of OVs are promising, some problems and drawbacks must be considered. The development of resistance in cancer cells poses a serious obstacle. Over time, cancer cells may acquire mechanisms that enable them to evade viral entry or replication, thereby compromising the efficacy of oncolytic treatment [3, 79]. The therapeutic window for OVs can be restricted by the host immune response. Host antiviral immunity may eliminate the virus before it achieves sufficient tumor-selective replication. Strategies for circumventing the immune response or enhancing viral persistence in tumors are under active investigation [4, 93].
A challenge comes in the safety profile of OVs; while they are engineered for tumor-specific targeting, there is always going to remain the possibility of an off-target effect. The safety of the virus needs to be rigorously investigated to minimize collateral toxicity in healthy tissues [3].
OVs eliminate cancer cells through various mechanisms. They can cause direct lysis and replication in cancer cells until they burst and release new viruses that can infect other malignant cells [94]. Another method through which OVs may act is the induction of the host immune response against tumor cells. This may be achieved through the presentation of tumor antigens, that is, proteins specifically expressed by malignant cells, or through the activation of immune cells, including T cells and NK cells [95].
OVs can kill tumor cells by one or more of the following mechanisms: (1) Direct lysis, where OVs can replicate within tumor cells, which can finally burst to release new viruses, subsequently resulting in the death of the tumor cells and release of tumor antigens that might stimulate the immune system [96]. (2) Immune activation: OVs can stimulate an immune response in tumor cells. This may occur through the release of tumor antigens or by activating immune effector cells such as T lymphocytes and NK cells [37, 97]. (3) Gene therapy: Most OVs can be designed to deliver therapeutic genes to tumor cells. These transgenes may encode cytotoxic proteins that promote tumor cell death or immunostimulatory molecules that enhance antitumor immune responses [3].
This review provides an overview of OVs and highlights some of their unique mechanisms of action within the broad context of cancer therapy. Given the complexity and heterogeneity of cancer, novel targeted interventions are urgently required to address therapeutic resistance and delivery barriers [19, 50, 79]. By exploring the characteristics of baculoviruses and their potential to selectively kill cancer cells, this review not only deepens our understanding of OVT but also indicates potential avenues for subsequent research and clinical applications. This synthesis aims to bridge key knowledge gaps, offering a conceptual foundation for the development of more personalized and effective cancer therapeutics [8, 27, 54].
The antitumor effects of OVs are mediated through multiple, distinct biological mechanisms, which are summarized here to consolidate mechanistic explanations and reduce repetition in subsequent sections. Emerging therapeutic strategies are redefining the architecture of cancer treatment, and several hold the potential to significantly alter current clinical paradigms [98]. Among these, OVs have gained prominence as a powerful modality in tumor-selective immunotherapy [3]. In particular, baculoviruses traditionally recognized for their applications in insect pathology have garnered scientific interest due to their distinct, multifunctional properties as non-replicating gene delivery vectors in OVT. This review delineates the mechanistic underpinnings by which baculoviruses exert therapeutic effects, highlighting their potential integration into broader cancer treatment frameworks [99]. The postulated mechanisms of action of baculoviruses in killing tumor cells include the following.
Baculoviruses can replicate within tumor cells, causing cell lysis and releasing new viruses that can infect other tumor cells [100, 101]. They hijack host cellular machinery to support viral replication, ultimately leading to tumor cell destruction via direct lysis. Their rapid replication kinetics in malignant cells underlie their potential as potent cytolytic agents [102]. The foundation of baculovirus oncolytic potential is based on its capacity for direct oncolysis. Once internalized, baculoviruses initiate a lytic cascade that leads to membrane rupture and virion release [3]. OVs selectively enter and replicate into malignant cells, taking advantage of the compromised antiviral defenses in such cells. In lytic infection, virion proliferation ends with programmed cell death (apoptosis) or direct lysis of the malignant cell membrane, liberating infectious virions that can attack nearby malignant cells. In a recurring mechanism, direct oncolysis not only locally destroys tumor tissue, but also multiplies the therapeutic payload. In addition, during cell lysis, delivery of tumor antigens and DAMPs can evoke a systemic antitumor immune reaction in response to persistent or metastatic malignant cells [103–105].
Beyond direct cytolysis, OVs disrupt tumor-supportive processes critical to cancer progression. Several OVs express angiogenesis-inhibitory genes or induce interferon signaling that downregulates VEGF pathways, thereby impairing neovascularization. In parallel, viral infection perturbs tumor metabolic homeostasis, interfering with glycolytic flux, lactate accumulation, and mitochondrial reprogramming that are hallmarks of tumor metabolism. Furthermore, certain OVs degrade or remodel the extracellular matrix (ECM), weakening the structural barriers that limit immune infiltration and drug penetration. These secondary mechanisms enhance the therapeutic impact of OVs, particularly when combined with agents targeting hypoxia, metabolism, or ECM stiffness.
Baculoviruses recruit and stimulate the immune system against tumor cells. This may occur through tumor antigen release or by activating immune effector cells such as T lymphocytes and NK cells [85, 106]. These baculoviruses can be engineered to deliver therapeutic genes to cancer cells, thereby sensitizing them to radiotherapy or chemotherapeutic agents [83].
However, in addition to their prowess in direct oncolysis, they exhibit additional immunostimulatory functions that enhance their therapeutic impact. Once internalized by tumor cells, baculoviruses trigger a cascade of immune-relevant events, including the release of tumor-associated antigens and DAMPs [107]. These signals serve as molecular cues that activate antigen-presenting cells and initiate downstream immune responses. This leads to the recruitment of CTLs and NK cells into the tumor microenvironment, where they contribute to tumor clearance. Immune stimulation amplifies the therapeutic action of baculoviruses by augmenting direct oncolysis with host-mediated immune destruction of malignant cells [108, 109].
Baculovirus can be modified to introduce therapeutic genes into tumor cells. Therapeutic genes are genes that either directly induce tumor cell death or increase susceptibility to other cancer therapies [110]. Examples include modification of baculovirus to introduce genes encoding proteins that can kill cancer cells, such as pro-apoptotic proteins or proteins that induce ICD. ICD is a type of cell death that releases tumor antigens and DAMPs that trigger downstream immune activation [88, 110]. The genetic plasticity of baculoviruses enables precise customization for therapeutic gene delivery applications in OVT. They can be engineered to deliver transgenes encoding immunomodulators, cytotoxic proteins, or sensitizers to enhance responsiveness to existing cancer treatments [87, 111]. This includes the insertion of therapeutic genes into the viral genome, allowing baculoviruses to act as gene delivery vectors. These genes encode proteins that render cancerous cells sensitive to standard treatments such as chemotherapy or radiation therapy, thereby improving treatment efficacy. This integration of gene transfer and viral therapy represents a promising direction for personalized oncology and precision medicine [112].
OVs differ substantially in structure, replication behavior, and immunological interaction, influencing their application across tumor types. For example, HSV (T-VEC) is highly engineerable and well-suited for intratumoral injection in cutaneous or accessible tumors such as melanoma, while reovirus (pelareorep) naturally targets Ras-activated pathways, making it promising for pancreatic and colorectal cancers [52, 55, 113]. Adenoviruses (e.g., DNX-2401, CG0070) have robust tumor selectivity and scalable production profiles, often preferred for gliomas and bladder cancers [114]. In contrast, newer vectors like baculoviruses are non-replicating in mammalian cells, offering high transgene capacity and safety for delivery applications, especially when immunogenicity must be tightly controlled. Similarly, OVs have shown promising therapeutic activity in a range of tumor types beyond liver cancer. T-VEC (a modified HSV-1) is approved for advanced melanoma; DNX-2401 has demonstrated immune activation in glioblastoma; pelareorep has been tested in pancreatic and breast cancer; and CG0070 is under evaluation for non-muscle invasive bladder cancer [115, 116]. These differences justify the development of next-generation vectors optimized for tumor microenvironment, delivery route, and therapeutic payload integration [83, 117–120].
Baculoviruses, while sharing general OV properties, offer unique platform-specific benefits in design flexibility and targeting, discussed below in the context of clinical translatability [86, 121]. They demonstrate strong tumor selectivity, minimizing off-target effects and reducing toxicity to normal tissues. Their activity is largely confined to the tumor microenvironment, where they initiate targeted cytotoxicity without disrupting systemic immune homeostasis [122]. Moreover, the genetic tractability of baculoviruses allows investigators to modify their behavior to suit the specific requirements of patients, thereby enabling personalized cancer therapies [123].
However, there are challenges to this scientific frontier. The delivery of baculoviruses to cancer cells remains a challenge and requires the development of advanced delivery platforms and tumor-specific targeting strategies. Host immune surveillance can rapidly neutralize baculoviruses, thereby compromising their systemic bioavailability and therapeutic efficacy prior to tumor localization. In addition, the therapeutic window for baculoviruses is tenuous because their potent immunostimulatory activity can result in inflammation and fever, which must be carefully managed to avoid off-target immunotoxicity [124, 125].
This review critically evaluates the mechanistic potential of baculoviruses as oncolytic agents, focusing on their functional roles in cancer therapy. The integration of direct oncolysis, immune modulation, and transgene delivery underscores both the therapeutic promise and the biological limitations of baculoviruses in oncology [126, 127]. It synthesizes recent advances in baculovirus-mediated OVT and contextualizes them within broader oncological research frameworks. These findings aim to inform ongoing discussions regarding the translational value and clinical feasibility of baculoviruses in future cancer therapy. Figure 2 illustrates key therapeutic mechanisms of OV therapy, including selective viral replication in cancer cells, immune activation, and transgene expression, highlighting its multifaceted antitumor activity.
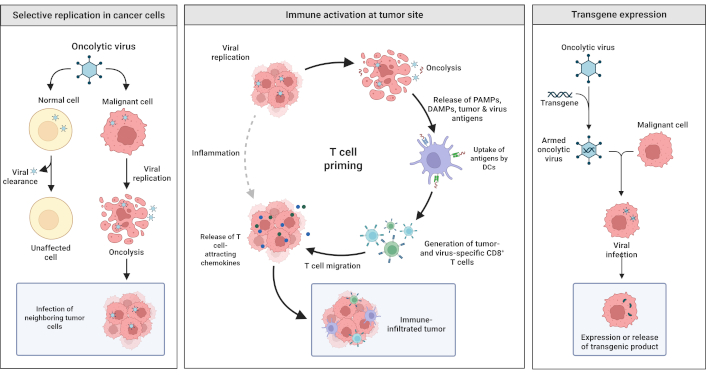
Mechanisms underlying oncolytic viral therapy. The following figure illustrates the most important mechanisms through which oncolytic viruses produce therapeutic activity: selective replication in cancer cells. Oncolytic viruses selectively replicate in and lyse malignant cells, but spare normal cells. Viral infection and replication cause lysis of tumor cells and infection of adjacent tumor cells. Activation of immunity at the tumor: Lysis of tumor cells releases pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and tumor antigens, which are captured by dendritic cells (DCs) and stimulate T cells to produce tumor-specific immune responses. The mechanism involves the migration of T cells to the tumor site, creating an immune-penetrated tumor microenvironment. Transgene expression: Engineered oncolytic viruses introduce therapeutic transgenes into malignant cells. Transgene expression maximizes therapeutic activity through direct toxicity and/or immune stimulation. Created in BioRender. Bashatwah, R. (2025) https://BioRender.com/16kdso1
Oncolytic baculoviruses have several important advantages as tools for cancer therapy in terms of efficacy, selectivity, and versatility. They exhibit selective tropism for malignant cells, enabling targeted infection with minimal toxicity to normal tissues and reduced systemic side effects [128]. After entering malignant cells, baculoviruses replicate with high efficiency, producing strong oncolysis through proliferation and secondary lysis [129]. This dual mechanism enables both direct tumor cell destruction and the release of tumor-associated antigens and DAMPs, initiating systemic immune responses against residual or metastatic malignancies [130]. In addition, baculoviruses can be engineered to encode therapeutic cargo, including prodrug-converting enzymes and immunostimulatory genes, for personalized therapy of a variety of malignancies and individual patient requirements [131]. For instance, gene modifications for the expression of suicide genes (e.g., thymidine kinase) induce selective cytotoxicity in tumor cells while preserving healthy tissue [132]. These properties position baculoviruses as a versatile and customizable platform for precision oncology.
Thus, the transition from preclinical promise to clinical reality is a critical point in the development of OVs as viable cancer therapeutics. The following section reviews key clinical trials involving OVs, highlights the most promising candidates under evaluation, and identifies major challenges and future directions for clinical translation [5, 35, 80, 112]. Within the past 20 years, there has been a significant development in clinical trials testing OVs for cancer treatment. To date, more than 500 clinical trials have been conducted with OVs, and more trials are ongoing [43, 46, 78, 99, 103, 133]. Several candidates under clinical investigation have demonstrated notable efficacy and safety across diverse malignancies, including but not limited to the following.
Reolysin® (pelareorep), a naturally occurring reovirus, has been tested across a range of malignancies. In metastatic breast cancer, a phase II trial demonstrated improved progression-free survival when combined with paclitaxel. Similarly, in pancreatic cancer, Reolysin® has shown immune priming and improved response rates when paired with chemotherapy. ONYX-015, an E1B-55k-deleted adenovirus, has been clinically evaluated in head and neck squamous cell carcinoma (HNSCC), where intratumoral administration showed partial responses and disease stabilization. Combination with cisplatin and 5-FU has further enhanced its efficacy. These virus-tumor pairings illustrate the importance of tailoring viral vectors to specific tumor microenvironments and treatment contexts.
T-VEC: T-VEC is the first genetically engineered HSV approved for the treatment of melanoma. T-VEC has shown promising results in clinical trials for other tumor-based cancers such as breast cancer and head and neck cancer [134, 135]. In a phase III clinical trial for melanoma, T-VEC demonstrated significantly improved overall survival compared to chemotherapy [52, 136].
Reolysin (rebastinib): Reolysin is a naturally occurring reovirus that has exhibited antitumor activity across multiple cancer types. It is currently under evaluation in phase III trials for the treatment of pancreatic cancer and glioblastoma [119, 137, 138].
NV1020: NV1020 is a genetically modified strain of NDV with demonstrated clinical efficacy against solid tumors. It is presently undergoing phase II clinical evaluation for multiple myeloma and non-small cell lung cancer [139–141].
The clinical field of OV therapy has undergone substantial advancements over the past decade. Clinical trials of these new agents have been conducted across diverse tumor types and have yielded encouraging efficacy data, along with critical insights into safety, dosing, and delivery challenges [77, 141]. Notably, trials involving adenoviruses, HSVs, and baculoviruses have demonstrated disease-specific applicability. These trials span from phase I studies assessing safety and tolerability to phase III trials evaluating therapeutic efficacy [5].
Early-stage trials primarily aim to define safety profiles, identify maximum tolerated doses, and monitor adverse events. These trials serve as foundational assessments for subsequent efficacy evaluations. Later-stage trials incorporate randomized control groups and larger sample sizes to evaluate clinical efficacy and therapeutic outcomes [142]. Several ongoing and completed trials have demonstrated the feasibility and efficacy of OVT in solid tumors. T-VEC, based on HSV-1, improved durable response rates in advanced melanoma and was the first OV approved by the FDA. DNX-2401 has shown immune activation and tumor shrinkage in recurrent glioblastoma. Pelareorep has demonstrated synergy with paclitaxel in metastatic breast cancer, while CG0070 has produced high complete response rates in BCG-unresponsive bladder cancer. To provide a structured overview, Table 2 summarizes selected clinical trials evaluating the therapeutic efficacy of OVs across various cancer types.
Representative clinical trials of oncolytic viruses across cancer types
| Virus | Cancer type | Trial phase | Combination strategy | Key outcome | Reference |
|---|---|---|---|---|---|
| T-VEC (HSV-1) | Melanoma | Phase III | + Pembrolizumab | ORR: 48.6% vs. 22.2% (mono); no OS benefit | [143] |
| DNX-2401 (Adenovirus) | Glioblastoma multiforme (GBM) | Phase II | + Nivolumab | Increased CD8+ infiltration; prolonged survival in responders | [114] |
| Pelareorep (Reovirus) | Metastatic breast cancer | Phase II | + Paclitaxel | Higher ORR; improved PFS | [144] |
| Pelareorep (Reovirus) | Pancreatic cancer | Phase II | + Gemcitabine | Immunologic priming; improved disease control rate | [119] |
| CG0070 (Adenovirus) | Non-muscle invasive bladder cancer | Phase II | Monotherapy (GM-CSF expressing) | CR rate: ~47% in BCG-unresponsive patients | [116] |
| Pexa-Vec (Vaccinia) | Hepatocellular carcinoma | Phase IIb | + Sorafenib | No OS benefit; early immune activation noted | [145] |
This table presents a comparative overview of key clinical trials involving oncolytic viruses, summarizing the virus platform, cancer indication, trial phase, combination strategy (if applicable), and reported outcomes. It highlights the therapeutic landscape of oncolytic virotherapy in solid tumors, with emphasis on immune modulation, synergistic combinations, and objective response rates observed in peer-reviewed studies. HSV-1: herpes simplex virus type 1; T-VEC: talimogene laherparepvec; ORR: objective response rate; OS: overall survival; PFS: progression-free survival; GM-CSF: granulocyte-macrophage colony-stimulating factor; CR: complete response; BCG: Bacillus Calmette-Guérin (intravesical immunotherapy for bladder cancer)
Several OV candidates have demonstrated strong clinical potential, signaling meaningful advances in cancer treatment outcomes [3]. Among them, adenoviruses have been engineered to selectively infect cancer cells while sparing normal tissues. Their robust immunogenicity, which triggers an intense immune response against cancer cells, makes them promising candidates [16].
Herpesviruses also show therapeutic potential because of their natural predilection for infection and replication within tumor cells. HSV-based vectors have been evaluated in clinical trials for melanoma and other solid tumors, with partial success and ongoing efforts to enhance efficacy [146].
Baculoviruses are emerging vectors in OVT, recognized for their multifunctional properties and novel mechanisms of action. Early-phase clinical studies are underway to evaluate their therapeutic applicability across diverse cancer types [83–87, 110].
Enhancement of antitumor immune function: Recent trials have revealed the clinical potential of several OV candidates across multiple malignancies, although efficacy outcomes vary by tumor type and combination strategy. T-VEC, an HSV engineered to produce GM-CSF, is used in combination with pembrolizumab in the MASTERKEY-265 trial to enhance response (objective response rate: 48.6% vs. 22.2% with pembrolizumab alone), though without significant improvement in overall survival [143, 147]. In phase I/II trials, HNSCC has shown that T-VEC induces durable responses, particularly in PD-L1-negative tumors [148]. DNX-2401 is a modified adenovirus used for glioblastoma multiforme (GBM) in combination with immunotherapy, such as nivolumab, and has also been engineered to deliver cytokines or immune-activating genes, resulting in enhanced intratumoral immune cell infiltration [114]. Reolysin (pelareorep) has been evaluated in various solid tumors, including breast and pancreatic cancers, showing improved outcomes when combined with chemotherapy [119, 144, 149]. Figure 3 illustrates the mechanism of action of T-VEC, highlighting its tumor-selective replication, induction of apoptosis, and stimulation of both local and systemic antitumor immunity.
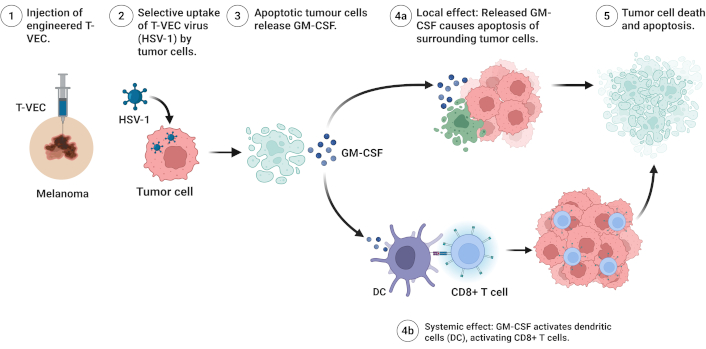
Mechanism of action of talimogene laherparepvec (T-VEC) in cancer therapy. The following figure describes the sequential mechanism of T-VEC, an engineered HSV-1, for melanoma therapy. (1) Locally, T-VEC is delivered to a melanoma site, specifically melanoma cells. (2) The virus selectively enters melanoma cells via specific uptake. (3) Viral infection in melanoma cells triggers apoptosis and releases granulocyte-macrophage colony-stimulating factor (GM-CSF). (4a) Local effect: GM-CSF triggers the entry of surrounding melanoma cells into apoptosis with an increased therapeutic effect. (4b) Systemic effect: GM-CSF triggers the activation of DC and subsequently CD8+ T cells for systemic antitumor immunity. (5) Widespread melanoma cell death and apoptosis occur, and the burden of tumor is reduced, and immune-mediated clearance is stimulated. Created in BioRender. Bashatwah, R. (2025) https://BioRender.com/svnj11w. HSV-1: herpes simplex virus type 1
The combination with immunotherapies has demonstrated potential in overcoming tumor immune evasion mechanisms. Synergistic effects between checkpoint inhibitors and OVs have been observed, including pembrolizumab and T-VEC, which showed a significant boost in CD8+ T-cell infiltration in tumors [149, 150]. LOAd703, a CD40L- and 4-1BBL-expressing adenoviral vector administered with atezolizumab, showed activity in pancreatic carcinoma (NCT02705196). In CAR T-therapy, engineered OVs with expression of chemokines, including CXCL11, have augmented recruitment of T-cells in solid tumors, a critical issue in adoptive cell therapy [151, 152]. However, stromal-rich tumor microenvironments continue to impair viral dissemination and limit OV penetration in solid tumors [153]. Immune neutralization is an issue, with the presence of antiviral antibodies in subjects interfering with OV efficacy [154]. Safety concerns have been reported, including cytokine release syndrome and other grade ≥ 3 adverse events in trials combining OVs with CAR T-cells [155]. Moreover, the manufacturing of genetically stable and scalable viral vectors remains technically demanding and cost-prohibitive, limiting widespread clinical deployment [156].
Future advancements in OV therapy include next-generation engineering approaches, biomarker-guided therapies, and new delivery modalities. OVs armed with bispecific T-cell engagers, such as VG161, have entered early-phase clinical trials for hepatocellular carcinoma [27]. Promoters regulated by microRNA-responsive elements with tumor-selective activity provide enhanced specificity for malignant tissues [157]. Biomarker-guided selection of patients is increasingly becoming a reality, with a specific direction towards selection for immunologically “cold” tumors for OV-facilitated immune stimulation [158]. To counteract immune neutralization, new delivery platforms such as nanocarrier-coated OVs and mesenchymal stem cell-based carriers are in development [159]. Meanwhile, worldwide trials expand OV indications, with RP1 (HSV-1) with nivolumab reporting a 62.5% response in phase II trials for malignancies in the skin [160], and CAN-2409 (an adenovirus) moving towards phase III trials for carcinoma of the prostate (NCT05070047) [161]. In conclusion, OVT is redefining cancer treatment paradigms by transforming immunologically resistant tumors into inflamed, immunoresponsive lesions. However, persistent challenges related to delivery, immune evasion, and scalable manufacturing must be resolved to ensure clinical success. Future breakthroughs will depend on enhanced viral engineering, biomarker-driven patient stratification, and rational combination strategies that maximize therapeutic synergy. Breakthroughs in the future will rely on increased viral engineering, biomarker-stratified therapies, and combinatorial approaches that maximize therapeutic synergy [140, 158].
The administration route of OVs plays a decisive role in shaping therapeutic efficacy, biodistribution, and immune response. Intratumoral injections offer high local concentration and are ideal for accessible tumors like melanoma. Intravenous delivery, essential for metastatic or deep-seated tumors, is limited by rapid neutralization and non-specific uptake. Regional routes, such as hepatic artery infusion or intracavitary delivery, balance access and safety in cases like liver or ovarian cancers. Each approach has specific trade-offs regarding immune clearance, systemic exposure, and delivery precision, and must be matched to tumor type, location, and disease burden [58, 162–164].
Although recent advancements in OV therapies reflect cautious but meaningful progress, numerous challenges still stand in the way of clinical translation [46, 78]. Delivery to tumors continues to be a problem and requires novel delivery strategies that enhance tumor specificity and overcome physical and immunological barriers. The immunological environment, although potentially a valuable partner, can also neutralize viral activity, necessitating the development of immune evasion mechanisms such as shielding or transient immunosuppression. The safety of the treatments and the avoidance of potential side effects are significant concerns [47, 48, 142]. There is also an economic need for the scalability and affordability of oncolytic viral therapies. Potential regulatory hurdles could increase development and manufacturing costs, ultimately limiting patient access [41, 114, 130].
The clinical development of oncolytic viral therapies marks the beginning of a new era in cancer therapy. With many trials, promising candidates, and a growing understanding of both challenges and opportunities, the future of OVs holds significant promise for advancing therapeutic strategies in oncology [50, 51, 79]. However, as we enter the clinic, it is essential to address key translational challenges with scientific precision and to leverage emerging opportunities to optimize therapeutic outcomes for patients [27, 123, 125].
OVs represent a novel and scientifically validated class of cancer therapeutics. They exert antitumor effects through direct oncolysis, activation of tumor-specific immune responses, and targeted gene delivery within malignant tissues. Although oncolytic viral therapies are not yet widely adopted in clinical practice, several issues need to be resolved [61, 79, 104]. Ongoing research continues to address these barriers, and OVs are positioned to become integral components of future cancer treatment strategies.
Safety issues regarding the development and clinical application of OVs must balance potential benefits with intrinsic risks. Clinical trial data suggest that most OVs exhibit an acceptable safety profile, with adverse events typically limited to mild or moderate flu-like symptoms [165].
Key studies such as the systematic review by Macedo et al. [166] reported that serious adverse events were rare across 97 clinical trials, with the majority of side effects classified as moderate. Genetic modification is pivotal in this regard, such as the deletion of neurovirulence factors, such as ICP34.5, in HSV-1-based OVs, including T-VEC. This change significantly reduces the risk of serious adverse events [167].
The residual effect of preferential replication in cancer cells reduces injury to normal tissues, which is an important characteristic [168]. Although the efficacy of these viruses is compromised by immune responses these responses often enhance therapeutic efficacy without inducing severe adverse effects [169]. Both viral shedding and transmission are concerns that are alleviated by studies like those on T-VEC, which have reported low viral shedding with no cases of transmission to close contacts. Long-term safety profiles, although still unfolding, are favorable, as seen in follow-up studies on T-VEC [117].
However, the safety profiles can be altered when OVs are combined with other cancer therapies, particularly immunotherapies [55, 113]. Combinations can enhance activity but require careful monitoring to manage immune-related adverse events. In general, OVs are a promising approach for cancer treatment, with a generally encouraging toxicity profile. However, further investigations are warranted, and follow-up remains essential [25, 34]. Continued improvements in genetic engineering, delivery techniques, and adequate follow-up for safety will further optimize these therapies for clinical benefit.
As the field progresses, the risk/benefit ratio will increasingly guide oncologists will further guide physicians toward making appropriate clinical decisions in pursuit of maximum therapeutic benefits. OVs remain among the most versatile investigational platforms in oncology, due to their tumor-selective replication, cytolytic activity, and capacity for therapeutic gene delivery. Efficiently kill cancer cells and can be engineered to deliver therapeutic genes to cancer cells. However, continued research is required to further reduce toxicity and enhance therapeutic potency. With sustained translational progress, OVT is poised to become a core modality in future cancer treatment algorithms.
As the field of OV therapies matures, future directions are charting a course toward enhanced efficacy, improved safety, and synergistic integration with complementary cancer therapies. This section outlines emerging strategies and therapeutic innovations aimed at maximizing the translational potential of OVs. Key research priorities include the development of precision engineering, immune modulation, and advanced delivery technologies [47, 57].
The search for optimized therapeutic index has driven ongoing research into OV therapies. Several strategies have been developed to increase the therapeutic impact of viruses, including the following:
Precision engineering: Current efforts are directed at enhancing tumor-specific targeting by modifying viral surface proteins or tropism determinants. These modifications improve viral binding affinity for tumor-associated receptors while minimizing off-target effects [11, 29, 124].
Immune modulation: OVs are being engineered to reshape the tumor immune microenvironment, thereby enhancing antitumor immune responses and overcoming immunosuppressive barriers [30, 44, 61, 75, 94, 97, 112].
Combinatorial strategies: Combining OVs with checkpoint inhibitors, chemotherapies, or targeted therapies has shown potential for additive or synergistic effects, particularly in resistant or immunologically “cold” tumors [15, 43, 64, 119, 160].
Gene editing: CRISPR-Cas9 and other genome editing technologies are being utilized to delete viral genes associated with toxicity and to introduce therapeutic transgenes that enhance immune activation or tumor selectivity. This includes both loss-of-function modifications to eliminate negative regulators and gain-of-function insertions for payload delivery [17, 71, 72].
Targeted delivery: Targeted delivery platforms are under active investigation to enhance intratumoral localization while minimizing systemic exposure. This includes engineering viral vectors to express ligands for overexpressed tumor receptors or using encapsulated systems such as nanoparticles and exosomes [14, 88, 89, 104, 159, 170].
Immunostimulating induction: OVs are being modified to express immunostimulatory cytokines, costimulatory ligands, and chemokines to recruit and activate effector immune cells within the tumor microenvironment. This approach has shown promise in enhancing response rates in tumors unresponsive to conventional immunotherapies [106].
The OV development pipeline continues to expand, with novel candidates entering preclinical and early clinical phases at a steady pace. Below are selected candidates currently in preclinical or early clinical evaluation, reflecting the next generation of translational innovation in cancer virotherapy.
Several new OVs are undergoing phase I/II trials or advanced preclinical testing, each engineered with distinct design advantages. VCN-01 (an oncolytic adenovirus) incorporates hyaluronidase to degrade ECM and improve tumor penetration. CF33-hNIS-antiPDL1, a chimeric orthopoxvirus, expresses both imaging and immune checkpoint-modulating genes for theranostic applications. MG1-Maraba virus has been designed for potent replication in RIG-I-defective cancer cells and is under evaluation in breast cancer. SVV-001, a picornavirus, shows natural tropism for neuroendocrine tumors with minimal modification. Each candidate leverages rational design to overcome delivery barriers, immune clearance, or tumor selectivity challenges.
Parvoviruses are small, single-stranded DNA viruses that can be genetically engineered to selectively infect and replicate in malignant cells. Early-phase clinical studies are evaluating its antitumor efficacy across multiple cancer types. It is a naturally occurring parvovirus that has demonstrated preclinical activity across a broad spectrum of tumor models. PVH-1 is currently being evaluated in phase I/II clinical trials for lung cancer, HNSCC, and other solid tumors [91, 103, 153].
Measles virus: The measles virus is currently being engineered as an oncolytic platform due to its inherent tumor tropism and immunostimulatory properties. Preclinical efforts have concentrated on enhancing tumor selectivity and minimizing neurotoxicity through genome modifications and receptor retargeting strategies [171].
Vesicular stomatitis virus (VSV): VSV, a negative-strand RNA virus, is currently being evaluated in early-phase clinical trials due to its potent lytic activity in a variety of tumor models. Ongoing research focuses on genetic modifications to improve tumor selectivity and reduce neurotoxicity, which remains a key safety concern in clinical development [172, 173].
Adenovirus 5-E1A-F (Ad5-E1A-F): Ad5-E1A-F is a genetically engineered adenovirus that has demonstrated robust antitumor activity in multiple preclinical tumor models. It is currently undergoing clinical evaluation for the treatment of pancreatic cancer, glioblastoma, and other solid tumors [174, 175].
VSV is a naturally occurring, negative-strand RNA virus that has demonstrated broad-spectrum oncolytic activity in multiple preclinical tumor models. It is also being evaluated in clinical trials targeting multiple myeloma, glioblastoma, and various solid tumors [173, 176, 177].
These OVs can be combined with other types of cancer treatment, such as immunotherapy or targeted therapy, to achieve synergistic therapeutic outcomes [38, 61, 159]. For instance, when used alongside immune checkpoint inhibitors, OVs can enhance tumor antigen presentation and promote T-cell infiltration into the tumor microenvironment. In other cases, OVs may sensitize tumor cells to targeted therapies by modulating apoptotic pathways or altering the expression of molecular targets [7, 45, 134, 167].
Clinical trials are still underway to assess the application of OVs in combination with other approaches for cancer therapy. Clinical trials are currently underway to evaluate T-VEC in combination with the immunotherapeutic drug pembrolizumab for melanoma treatment [52, 55, 134]. The future of oncolytic viral treatment is inherently linked to its use in combination with other forms of cancer treatment. These OVs can be combined with immunotherapies such as checkpoint inhibitors to enhance immune responses against cancer. Among current combination strategies, pairing OVs with immune checkpoint inhibitors such as anti-PD-1 or anti-CTLA-4 antibodies has shown the greatest potential to transform immunologically “cold” tumors into “hot”, inflamed, and responsive phenotypes. For instance, the combination of T-VEC with pembrolizumab in melanoma (MASTERKEY-265 trial) yielded improved objective response rates compared to monotherapy. Similarly, DNX-2401 combined with nivolumab in glioblastoma has shown promising intratumoral immune activation and durable clinical responses. These combinations leverage the ICD triggered by OVs to prime T-cell responses that are then sustained by checkpoint blockade, thereby creating a synergistic antitumor effect [16]. Moreover, the combination of OVs with targeted therapies directed against the inhibition of certain signaling pathways in cancer cells creates an attack against malignancies from many sides. Simultaneously, targeting several weaknesses of cancer cells can lead to more effective treatment outcomes [178].
The future of oncolytic viral treatment is bright and innovative. Currently, new strategies are being developed to achieve maximum efficacy without compromising safety. New candidates are poised at the gate, ready to enter the clinical arena and further diversify the array of alternative therapies. The integration of OVs into multimodal regimens represents a paradigm shift in the treatment of cancer, potentially leading to synergy that might become key to better patient outcomes [16].
While venturing into this evolving field, it is important not to lose sight of ongoing research, clinical trials, and the dynamic interactions of OVs with other modalities. It is envisioned that by fully harnessing the capabilities of these viral warriors and their strategic combinations, a new face in cancer therapy will emerge, bringing new hope to patients and a brighter future in the fight against cancer [16].
OVs have become a new hope in fighting cancer, with a selective mechanism that specifically destroys malignant tissue while sparing healthy tissue. Genetically engineered and naturally derived viruses have been in trials, with already gained approval by the FDA [25, 34]. Efficacy in a range of malignancies has been demonstrated, with successful patient experiences in trials. In one such case, a 72-year-old male with metastatic melanoma took part in a clinical trial for T-VEC, a gene-altered form of an HSV engineered to cause an anti-tumoral immune reaction in humans. After four infusions of intertumoral T-VEC, two infusions a week apart, for four weeks, significant shrinking of the tumor, was observed, with more than 50% reduction in tumor size. Following the trial, after the trial, and two years and three months later, the patient remained in remission with restored quality of life [52, 55, 113, 134]. In a similar case, a 55-year-old female with metastatic pancreatic carcinoma took part in a phase II clinical trial for reolysin, a naturally derived reovirus with a high level of activity in searching out and attacking malignant tissue. She took off Reolysin® in two infusions a week apart, for two weeks, through an intravenous route, and during week two, her tumors started shrinking. By the conclusion of the study, there was more than a 70% reduction in tumor volume. Over a year later, the patient remained in remission, returned to work, and resumed international travel for both personal and professional activities [179].
Resistance to OVT arises from both tumor-intrinsic and host-mediated mechanisms. Tumor cells may evade infection by downregulating viral entry receptors or upregulating antiviral signaling pathways such as interferon-stimulated genes (ISGs) [180]. Additionally, intact autophagy and apoptosis resistance pathways can hinder viral replication and spread. From the host side, innate immune clearance, activation of DCs, and rapid production of neutralizing antibodies can restrict systemic OV dissemination. The tumor microenvironment further impairs efficacy through dense ECM components, abnormal vasculature, and immunosuppressive stromal elements. Overcoming these barriers will require rational design of OVs with enhanced evasion, immune modulation, and delivery capabilities [181, 182].
Beyond safety and efficacy, the clinical application of OVs raises several ethical considerations. These include the risk of viral shedding and unintended transmission to close contacts or immunocompromised individuals, particularly when using replication-competent or genetically engineered viral platforms [183]. Ethical trial design also demands rigorous informed consent, given the experimental nature of many OV-based therapies. Long-term monitoring is necessary to assess delayed adverse effects, immune consequences, or viral persistence. Additionally, equitable access to potentially costly, individualized OV-based therapies poses challenges in resource-limited settings. Regulatory frameworks must balance innovation with biosafety and public trust, especially in trials involving viral genome modifications or combinatorial immunotherapy [184, 185].
Combination strategies involving OVs are emerging as highly promising in clinical oncology. OVs can stimulate anti-tumor immunity by inducing ICD and releasing tumor-associated antigens, thereby priming the tumor microenvironment for response to immune checkpoint inhibitors. For instance, intratumoral injection of T-VEC has been shown to increase CD8+ T cell infiltration and PD-L1 expression, enhancing sensitivity to anti-PD-1 therapy. When combined with chemotherapy or radiotherapy, OVs can also disrupt tumor vasculature, increase cellular stress, and upregulate type I interferon pathways, improving therapeutic responsiveness. However, challenges such as optimal sequencing, pre-existing antiviral immunity, vector clearance, and toxicity overlap must be addressed through better patient selection, combination timing, and engineering of virus-host interaction.
OV therapy is a new and exciting modality in cancer therapy, leveraging the natural propensity of viruses to selectively target and kill malignant cells and induce an antitumor immune reaction. Sitting at the nexus of virology, oncology, and immunology, such a modality holds tremendous potential in both preclinical and clinical trials, with a variety of OVs approved for use in humans and several receiving regulatory approval, including by the FDA. Expanded therapeutic use in a variety of tumor types continues in ongoing and planned trials, with exciting candidates such as adenoviruses, herpesviruses, and reoviruses effective in melanoma, glioblastoma, and pancreatic carcinoma, respectively. Despite such success, a variety of key impediments, including efficient targeting of tumors, immune neutralization, and the development of viral resistance, must be overcome. Overcoming such obstacles through new genetic engineering, immune manipulation, and nanotechnology-based delivery systems will be critical for maximizing therapeutic efficacy.
The future of OVT will involve integration with current cancer therapies. Combinations of OVs with immunotherapy, chemotherapy, and radiation have already proven synergistic, most notably in reprogramming immunologically “cold” tumors to become “hot” and sensitive to immune attack. Personalized and multimodal therapies with OVs have the potential to maximize patient prognosis, most notably for resistant and refractory malignancies. In addition, new candidates, such as parvoviruses, measles virus, and vesicular stomatitis virus, will soon enter the early phases of clinical development, further extending the therapeutic envelope of OVT.
However, ethical controls must follow scientific breakthroughs. OVs with potential for off-target toxicity, immune-related toxicity, and theoretical use in bioterrorism must have stringent controls placed over them. Patient safety in children requires strong regulatory environments and ethical controls. Clinical trials must be highly concerned about safety, efficacy, and selection to maximize therapeutic gain with minimum danger.
The future holds great hope for OVT to become the norm in precision medicine in oncology. As virologists become increasingly specific, more effective at immune manipulation, and move towards new types of viral vectors, the future for OVs to revolutionize cancer therapy is enormous. The future will require both scientific integrity and ethics; however, with continued development and collaboration, OVs can soon become flagships in oncology, offering new hope for patients worldwide.
Ad5-E1A-F: adenovirus 5-E1A-F
CTLs: cytotoxic T lymphocytes
DAMPs: damage-associated molecular patterns
DCs: dendritic cells
ECM: extracellular matrix
GM-CSF: granulocyte-macrophage colony-stimulating factor
HNSCC: head and neck squamous cell carcinoma
HSV-1: herpes simplex virus type 1
ICD: immunogenic cell death
NDV: Newcastle disease virus
NK: natural killer
OVs: oncolytic viruses
OVT: oncolytic virotherapy
T-VEC: talimogene laherparepvec
VSV: vesicular stomatitis virus
AAAA: Conceptualization, Writing—original draft, Writing—review & editing, Data curation. RB: Data curation, Writing—review & editing, Validation, Resource. OG: Writing—original draft, Writing—review & editing, Data curation. All authors have read and agreed to the final version of the manuscript.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
