Editor's Picks
Open Access
Case Report
Ultrasound-guided dextrose hydrodissection for multiple peripheral entrapment neuropathies in scleroderma: a case presentation
Scleroderma, also known as systemic sclerosis, is a rare connective tissue disorder with an unclear and poorly understood pathogenesis. While it primarily affects the skin and internal organs through mechanisms involving vascular dysfunction, immune dysregulation, and fibrosis, its effects on the peripheral nervous system may also be substantial. We report the case of a 36-year-old male with a known history of scleroderma who presented with chronic, diffuse burning pain throughout the body. His symptoms included daily asthenia, dizziness, nausea, headaches, and limb pain exacerbated by cold, compression, or stretching. Diagnostic ultrasound confirmed multiple peripheral nerve entrapments, which were treated with ultrasound-guided 5% dextrose hydrodissection. This intervention provided significant relief of pain, paresthesia, and motor symptoms, which improved his quality of life. This case highlights the potential of dextrose hydrodissection as a safe, minimally invasive, and cost-effective symptomatic treatment for peripheral neuropathies in patients with scleroderma. Further studies are warranted to establish its broader therapeutic role in treating scleroderma-related neuropathies.
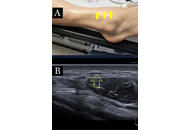
Open Access
Original Article
The ‘FebBenz’ approach for severe difficult-to-treat gout
Aim:
To evaluate the efficacy and tolerability of febuxostat (FBX) and benzbromarone (BNZ) combination therapy in patients with difficult-to-treat (D2T) gout.
Methods:
This observational study was performed at two centers and included patients fulfilling the 2015 European Alliance of Associations for Rheumatology/American College of Rheumatology (EULAR/ACR) gout classification criteria, with clinical tophi and suboptimal response to standard urate-lowering therapy. A two-step treatment regimen was implemented: a 6-month dose escalation of the FBX dose followed by add-on BNZ. Demographic, clinical, and laboratory data—including cardiovascular risk factors (CVRFs), history of nephrolithiasis, liver enzymes, and estimated glomerular filtration rate (eGFR)—were recorded. Changes in serum urate (SUA) and eGFR were analyzed using paired t-tests.
Results:
The study population comprised 15 patients (87% male, median age 59 years) with longstanding gout [median 15 years, range 3–31; interquartile range (IQR) 8–25]. Baseline SUA was 10.3 ± 1.7 mg/dL; mean eGFR was 63.7 ± 23.6 mL/min. CVRFs were common (hypertension, 93%; dyslipidemia, 73%: major adverse cardiovascular events, 13%; diabetes, 7%). At 12 months, SUA had decreased significantly to 2.9 ± 1.1 mg/dL (Δ = 7.4 mg/dL; p < 0.01), with FBX alone contributing to a Δ of 5.4 mg/dL and BNZ an additional Δ of 2.1 mg/dL (both p < 0.01). Tophi resolved in 60% of patients. No serious adverse events or significant changes in liver or renal function were observed. One unrelated death was recorded.
Conclusions:
FBX + BNZ was effective and well-tolerated in patients with severe D2T gout, achieving a substantial reduction in SUA and clinically significant dissolution of tophi.

Open Access
Review
Soft tissue sarcoma: clinical recognition and approach to the loneliest cancer
Soft tissue sarcoma (STS) is a rare malignancy with a high incidence. Early diagnosis can reduce the rate of amputations and increase survival, however, this is typically delayed. The diagnosis and treatment of smaller lesions have a better prognosis; nonetheless, patients present to physicians when the soft tissue mass is large with obvious signs of red flags. In addition, the symptoms of this disease are highly non-specific and overlap greatly with benign conditions, resulting in a lack of clinical suspicion and low awareness among practitioners and the general public. Thusly, it is entitled as “the loneliest cancer”. This can make an accurate diagnosis difficult, with a great proportion of misdiagnoses leading subsequent inadvertent to incomplete STS excision, affecting the overall prognosis of the disease and devastating consequences in the disease process. A timely and precise diagnosis is essential because half of people with STS progress toward quietly aggressive illness. The purpose of this review is to raise awareness of STSs so that early recognition, accurate work-up, overview of conventional treatment plans, and appropriate referral to a tumor center can be achieved, avoiding whoop situations, and improving patient outcomes. In addition, insight into the advances in immunotherapy, nanotechnology, and artificial intelligence (AI) can lead to STS diagnosis and treatment prognosis.
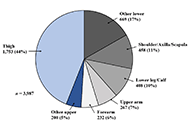
Articles
Latest
Most Viewed
Most Downloaded
Most Cited
Open Access
Meta-Analysis
Work-related musculoskeletal disorder prevalence among African nurses: systematic review and meta-analysis
Philippe Gorce, Julien Jacquier-Bret
Published: February 12, 2026 Explor Musculoskeletal Dis. 2026;4:1007116
This article belongs to the special issue Prevalence and Risk Factors of Work-related Musculoskeletal Disorders
Open Access
Editorial
Biosimilars: state of the art in the treatment of rheumatic diseases
Valderilio Feijó Azevedo
Published: February 03, 2026 Explor Musculoskeletal Dis. 2026;4:1007115
This article belongs to the special issue Biosimilars: State of the Art in the Treatment of Rheumatic Diseases

Open Access
Editorial
Magnetic Resonance Neurography: redefining the diagnostic frontier in musculoskeletal disease
Theodoros Soldatos
Published: February 02, 2026 Explor Musculoskeletal Dis. 2026;4:1007114
This article belongs to the special issue Magnetic Resonance Neurography: Advances, Techniques, and Clinical Applications

Open Access
Case Report
Xanthoma simulating gouty tophus (case report of atypical cholesterol crystal deposition)
Maxim Sergeevich Eliseev ... Maria Nikolaevna Chikina
Published: December 24, 2025 Explor Musculoskeletal Dis. 2025;3:1007113
This article belongs to the special issue Evaluation and Outcomes in the Management of Gout

Open Access
Mini Review
Lycopene supplementation in rheumatic diseases: a comprehensive review
Jozélio Freire de Carvalho, Ana Tereza Amoedo Martinez
Published: December 09, 2025 Explor Musculoskeletal Dis. 2025;3:1007112
This article belongs to the special issue Complementary and Integrative Medicine in Rheumatology: Evidence, Therapies, and Clinical Impact

Open Access
Case Report
Ultrasound-guided dextrose hydrodissection for multiple peripheral entrapment neuropathies in scleroderma: a case presentation
Helen Gharaei ... Ziba Bagherian
Published: December 08, 2025 Explor Musculoskeletal Dis. 2025;3:1007111

Open Access
Case Report
Similarities and differences between gouty arthritis and rheumatoid arthritis—an interesting case with a short look into the literature
David Kiefer ... Juergen Braun
Published: February 24, 2023 Explor Musculoskeletal Dis. 2023;1:11–19
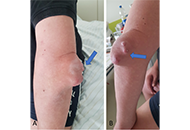
Open Access
Review
Safety and efficacy of gout treatments in people with renal impairment
Hamish Farquhar ... Lisa K. Stamp
Published: September 02, 2024 Explor Musculoskeletal Dis. 2024;2:360–374
This article belongs to the special issue Pharmacological and Non-Pharmacological Management of Gout
Open Access
Review
Soft tissue sarcoma: clinical recognition and approach to the loneliest cancer
Sujan Shakya ... Xiang Zhou
Published: February 06, 2024 Explor Musculoskeletal Dis. 2024;2:56–68

Open Access
Case Report
Return-to-play decision-making following ankle injury: a comprehensive case analysis of the functional hop test
Michael Crinion ... Michael Agnone
Published: March 06, 2024 Explor Musculoskeletal Dis. 2024;2:75–81

Open Access
Systematic Review
Role and effectiveness of surface EMG feedback in sports and orthopedic rehabilitation: a systematic review
Thomas Haab ... Paul Burkey
Published: September 12, 2024 Explor Musculoskeletal Dis. 2024;2:391–407
Open Access
Review
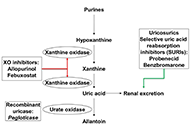
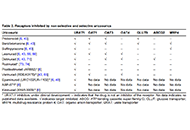
How URAT1 inhibitors can shape the future of chronic gout treatment: a narrative review of uricosurics past and present
Robert T. Keenan ... Michael H. Pillinger
Published: December 12, 2024 Explor Musculoskeletal Dis. 2024;2:529–554
This article belongs to the special issue Pharmacological and Non-Pharmacological Management of Gout
Open Access
Review
Soft tissue sarcoma: clinical recognition and approach to the loneliest cancer
Sujan Shakya ... Xiang Zhou
Published: February 06, 2024 Explor Musculoskeletal Dis. 2024;2:56–68

Open Access
Systematic Review
Role and effectiveness of surface EMG feedback in sports and orthopedic rehabilitation: a systematic review
Thomas Haab ... Paul Burkey
Published: September 12, 2024 Explor Musculoskeletal Dis. 2024;2:391–407

Open Access
Case Report
Similarities and differences between gouty arthritis and rheumatoid arthritis—an interesting case with a short look into the literature
David Kiefer ... Juergen Braun
Published: February 24, 2023 Explor Musculoskeletal Dis. 2023;1:11–19

Open Access
Review
Interstitial lung disease in patients with rheumatoid arthritis: a narrative review
Gloria Candelas Rodríguez, Virginia Villaverde
Published: October 20, 2023 Explor Musculoskeletal Dis. 2023;1:128–142
This article belongs to the special issue Comorbidities in rheumatoid arthritis
Open Access
Review
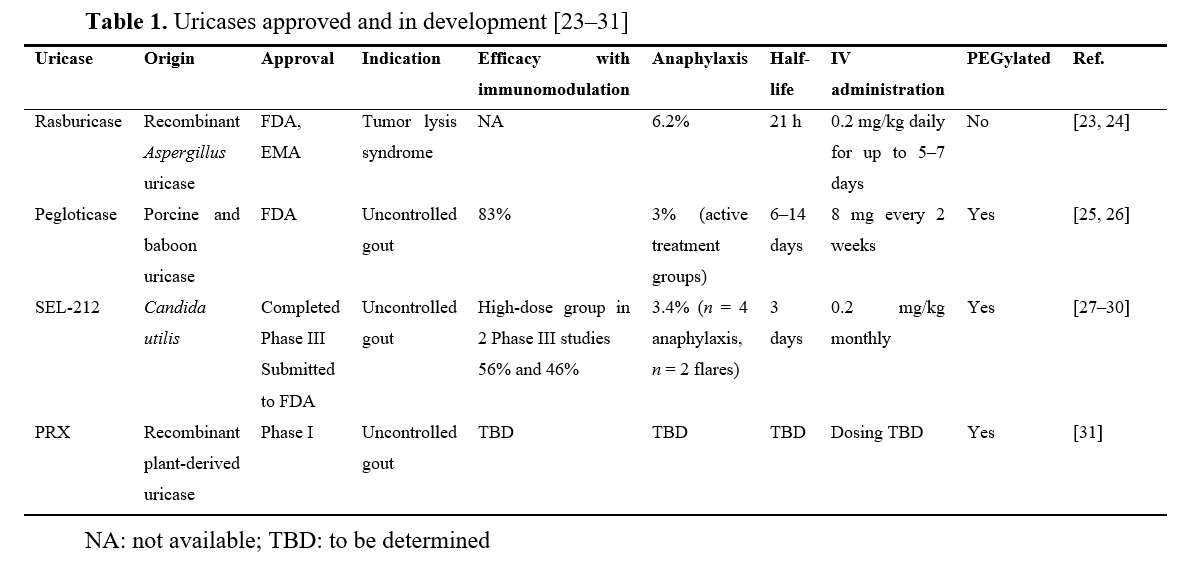
Uricases: reflections on recent developments in the management of challenging gout patients
Naomi Schlesinger, Dan Kaufmann
Published: October 02, 2024 Explor Musculoskeletal Dis. 2024;2:461–472
This article belongs to the special issue Pharmacological and Non-Pharmacological Management of Gout
Open Access
Review
Safety and efficacy of gout treatments in people with renal impairment
Hamish Farquhar ... Lisa K. Stamp
Published: September 02, 2024 Explor Musculoskeletal Dis. 2024;2:360–374
This article belongs to the special issue Pharmacological and Non-Pharmacological Management of Gout

Open Access
Review
Soft tissue sarcoma: clinical recognition and approach to the loneliest cancer
Sujan Shakya ... Xiang Zhou
Published: February 06, 2024 Explor Musculoskeletal Dis. 2024;2:56–68

Open Access
Review
Interstitial lung disease in patients with rheumatoid arthritis: a narrative review
Gloria Candelas Rodríguez, Virginia Villaverde
Published: October 20, 2023 Explor Musculoskeletal Dis. 2023;1:128–142
This article belongs to the special issue Comorbidities in rheumatoid arthritis

Open Access
Review
How should we do in the selection and follow-up of systemic conventional treatments in psoriasis?
Sevgi Akarsu
Published: December 05, 2023 Explor Musculoskeletal Dis. 2023;1:241–256
Open Access
Review
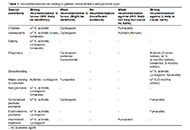
Rheumatoid arthritis and cardiovascular comorbidities
Uğur Özkan ... Murat Birtane
Published: December 06, 2023 Explor Musculoskeletal Dis. 2023;1:264–288
This article belongs to the special issue Comorbidities in rheumatoid arthritis
Open Access
Perspective
Empowering rheumatology through digital health technologies: contributions and barriers
Diego Benavent ... Antonio Gómez-Centeno
Published: April 9, 2024 Explor Musculoskeletal Dis. 2024;2:92–105
This article belongs to the special issue Digital health technologies in rheumatology: emerging evidence and innovation

Open Access
Systematic Review
Role and effectiveness of surface EMG feedback in sports and orthopedic rehabilitation: a systematic review
Thomas Haab ... Paul Burkey
Published: September 12, 2024 Explor Musculoskeletal Dis. 2024;2:391–407

Special Issues
Ongoing Special lssues
Completed Special lssues
Magnetic Resonance Neurography: Advances, Techniques, and Clinical Applications
Guest Editor: Dr. Theodoros Soldatos
Submission Deadline: June 30, 2026
Published Articles: 1
Complementary and Integrative Medicine in Rheumatology: Evidence, Therapies, and Clinical Impact
Guest Editor: Prof. Jozélio Freire de Carvalho
Submission Deadline: April 30, 2026
Published Articles: 1

Innovation in Orthopedics
Guest Editors: Prof. Ashok N. Johari; Prof. Philippe Hernigou
Submission Deadline: March 31, 2026
Published Articles: 3

Evaluation and Outcomes in the Management of Gout
Guest Editor: Prof. Fernando Pérez-Ruiz
Submission Deadline: November 30, 2025
Published Articles: 4

Macrophages and Fibrosis in the Rheumatic Diseases: from Pathophysiology to Treatment
Guest Editor: Dr. Stefano Soldano
Submission Deadline: April 30, 2026
Published Articles: 0

Ultrasound as Outcome Measure in Rheumatic Diseases Trials
Guest Editor: Dr. Andrea Di Matteo
Submission Deadline: August 31, 2025
Published Articles: 0

Multifaceted Imaging in Rheumatic and Musculoskeletal Diseases
Guest Editor: Dr. Peter Mandl
Submission Deadline: December 31, 2025
Published Articles: 5

Pharmacological and Non-Pharmacological Management of Gout
Guest Editor: Prof. George Nuki
Submission Deadline: January 31, 2025
Published Articles: 11

Prevalence and Risk Factors of Work-related Musculoskeletal Disorders
Guest Editor: Prof. Philippe Gorce
Submission Deadline: April 30, 2026
Published Articles: 4

Cell Therapy and Tissue Engineering for Musculoskeletal Conditions: From Pre-clinical Studies to Clinical Trials
Guest Editor: Prof. Elena Jones
Submission Deadline: June 30, 2026
Published Articles: 2
Calcium Pyrophosphate Deposition Disease
Guest Editor: Prof. Jürgen Braun
Submission Deadline: August 31, 2025
Published Articles: 8

Journal Information
Journal Indexing
Journal Metrics


















 Title: Unravelling the interplaybetween #Harmattan wind andbaroreflex functions: implicationon environmental health andcardiovascular #pathophys
Title: Unravelling the interplaybetween #Harmattan wind andbaroreflex functions: implicationon environmental health andcardiovascular #pathophys


