Affiliation:
1Ruhr Universität Bochum, 44801 Bochum, Germany
2Rheumatologisches Versorgungszentrum Steglitz, 12163 Berlin, Germany
Email: juebraun@gmx.de
ORCID: https://orcid.org/0000-0002-9156-5095
Affiliation:
1Ruhr Universität Bochum, 44801 Bochum, Germany
3Cellitinnen-Krankenhaus St. Josef, 42105 Wuppertal, Germany
ORCID: https://orcid.org/0000-0003-3841-624X
Explor Musculoskeletal Dis. 2025;3:1007108 DOI: https://doi.org/10.37349/emd.2025.1007108
Received: September 01, 2025 Accepted: October 21, 2025 Published: November 12, 2025
Academic Editor: Fernando Pérez-Ruiz, Cruces University Hospital, Spain
The article belongs to the special issue Multifaceted Imaging in Rheumatic and Musculoskeletal Diseases
Osteoporosis is a systemic skeletal disease characterized by low bone mineral density (BMD) and poor bone quality, leading to reduced bone strength and increased risk of fracture. In rheumatic and musculoskeletal diseases (RMD), including rheumatoid arthritis (RA) and spondyloarthritides (SpA), such as axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA), generalized bone loss associated with traumatic and non-traumatic fractures, such as insufficiency fractures (IF), is known to occur with the RANK-RANKL-osteoprotegerin axis and the Wnt-β-catenin signalling pathway playing a pathogenetic role. RA has been included in the Fracture Risk Assessment Tool (FRAX) system to be able to more precisely calculate the 10-year fracture risk associated with the disease. Various definitions for IF have been proposed; a recent paper by the EMA has defined fragility fractures as fractures occurring with low trauma at the hip, spine, pelvis, distal femur, proximal tibia, humerus, forearm, and multiple ribs. However, IF of the feet are currently not considered osteoporotic fractures. So-called stress fractures, which are known to often occur in athletes, have a similar MRI appearance. However, these are rather due to repetitive strain than to minor trauma. Also, based on recent data, this manuscript concentrates on the significance of fragility fractures in RMD.
Osteoporosis is a systemic disease of bone that leads to a reduction of bone strength and a higher risk of fracture. Osteoporosis is characterized by poor bone quality (e.g., architecture, turnover, damage accumulation, and mineralization) and low bone mineral density (BMD) [1, 2]. In rheumatic and musculoskeletal diseases (RMD), including rheumatoid arthritis (RA) and spondyloarthritides (SpA) such as axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA), generalized bone loss associated with traumatic and non-traumatic (pathological) fractures is known to occur [3–5]. However, periarticular bone erosions in RA [6] and pathological bone formation are also frequently observed in peripheral and root joints and in the spine of patients with SpA [7]. The Wnt-β-catenin signalling pathway, with its inhibitors sclerostin and Dickkopf (DKK)1, and the Receptor Activator of NF-κB (RANK)-RANK ligand (L)-osteoprotegerin axis are known to play a role in bone loss and bone formation, not only based on inflammation [8, 9]. Targeted anti-inflammatory therapies, including biologic (b-) and synthetic (ts-) disease-modifying anti-rheumatic drugs (DMARDs), have the ability to inhibit radiographic joint damage, stabilize bone turnover, and prevent bone loss [10].
There is strong evidence that patients with RA, PsA, and axSpA have a high prevalence of osteoporosis and an increased fracture risk [3–5]. RA has been included in the Fracture Risk Assessment Tool (FRAX) system to be able to more precisely calculate the 10-year fracture risk associated with the disease [11]. A fracture occurs when the load-bearing capacity of a bone is exceeded by forces applied to it. This is influenced by BMD, but also by bone geometry, microstructure, and quality. Fragility fractures result from low-energy trauma. A patient who had a fragility fracture is at high risk of experiencing secondary fractures. Fragility fractures are usually considered osteoporotic [12]. However, patients with osteoporosis suffer fractures from high-energy trauma more often than healthy individuals [13]. Therefore, sites of osteoporotic fractures are characterised by their association with low BMD and older age [14]. With this definition, the most frequent fractures occur at the hip, spine, and forearm. This, however, does not exclude other sites, where fractures can occur partly due to low BMD. Various definitions for fragility fractures have been proposed, including the European Medicines Agency (EMA), which defines fragility fractures as fractures occurring with low trauma at the hip, spine, pelvis, distal femur, proximal tibia, humerus, forearm, and multiple ribs [15]. Fragility fractures are also known as insufficiency fractures (IF). However, IF of the feet are currently not considered osteoporotic fractures. The so-called stress fractures with a similar magnetic resonance imaging (MRI) appearance also occur in athletes. However, these are rather due to repetitive strain than to minor trauma [16, 17].
Conventional radiography is still the gold standard for the assessment of osteodestructive changes such as erosions in RMD. This technique has also been used to detect IF some decades ago [18]. However, RMD-related inflammatory imaging features such as arthritis, tenosynovitis, and bone marrow edema have been shown to be reliably detected by MRI. MRI also detects erosions with high sensitivity [19, 20]. MRI has also been used to detect fragility fractures in RMD patients [21].
Especially in patients with RA, symptoms related to the feet are rather prevalent. The cause may be due to inflammation, altered mechanics, deformity, secondary skin lesions, and all kinds of combinations. Many RA patients report foot problems, and an impairment of foot function has been frequently observed, suggesting a significant incidence of this health problem [22, 23]. Indeed, foot complaints are the first symptom in about 15% of patients with RA, which usually start in the metatarso-phalangeal (MTP) joints [23]. However, ankle and hindfoot joints are also affected in many patients [22–24]. Comorbidities such as obesity may additionally affect foot health, not only in RA [25].
Involvement of the lower rather than the upper extremities has long been considered characteristic of SpA [26–28], where enthesitis is also a current health problem, not only in PsA [29, 30]. Calcaneal spurs are known to possibly form and cause mechanically induced complaints in patients.
In a recent retrospective study published by our group [31], 1,752 MRI scans of consecutive patients presenting with foot pain were evaluated in two 2-year time periods. The patient group with IF was matched with controls with foot pain but no IF. Conventional radiographs and BMD as assessed by Dual Energy X-ray Absorptiometry (DXA) were available in the majority of patients. In total, 1,145 MRI of patients (median age 59 years, 83% women) with an inflammatory (65.4%) and 607 with a non-inflammatory (34.6%) RMD (median age 58 years, 80.8% female) were available. The most frequent diagnosis was RA (42.2%), while other patients had PsA (22.4%), axSpA (11.1%), or connective tissue disease (CTD; 7.6%) or other RMD such as gout. In 129 MRI scans of patients’ feet, IF was found (7.4%). Examples of IF are shown in Figures 1, 2, and 3. Importantly, there was no difference between time periods. Of interest, in patients with CTD (23%) and RA (11.4%), the prevalence of IF was highest. In comparison, more patients with an inflammatory than a noninflammatory RMD had an IF (9.1% vs. 4.1%, respectively; p < 0.001). Using conventional radiography, IF had only been detected in 25% of patients [30]. Patients with an IF had a low BMD and a history of fractures significantly more often than those without (42.6% vs. 16.2% and 34.9% vs. 8.6%, respectively).
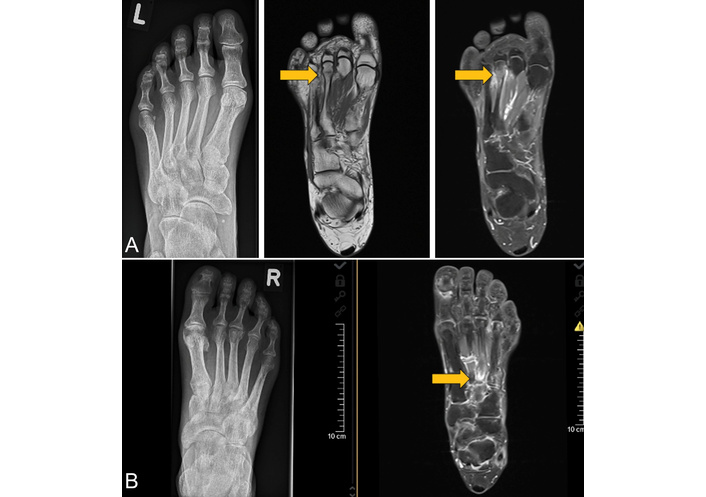
A conventional radiograph (left figure) and MRI of two patients with an IF radiographically not detected. (A) Images of a 56-year-old woman with RA, disease duration 2.5 years. MRI of the left foot showing a subcapital fracture of the 3rd metatarsal head. The T1w MRI sequence (middle figure) shows the fracture as a hypointense line, while the contrast-enhanced fat-saturated T1w MRI sequence (right figure) reveals the reaction of the periosteum and an activation of the BM (gray arrows). This finding is similar in cases of periostitis and osteitis. (B) Images of a 61-year-old male patient with PsA, disease duration 6 years. The MRI shows a fracture of the base of the 3rd metatarsal in the right foot (gray arrow). The anatomy of this area makes the radiographic detection of fractures difficult. Therefore, MRI clearly is the method of choice to find fractures. The contrast-enhanced fat-saturated T1w MRI sequence reveals the fracture as a hypointense line, also with signs of reactive periosteal and BM activation. IF: insufficiency fracture; MRI: magnetic resonance imaging; BM: bone marrow; PsA: psoriatic arthritis; RA: rheumatoid arthritis; T1w: T1-weighted. Reprinted from [31]. © 2023 by the Journal of Rheumatology.
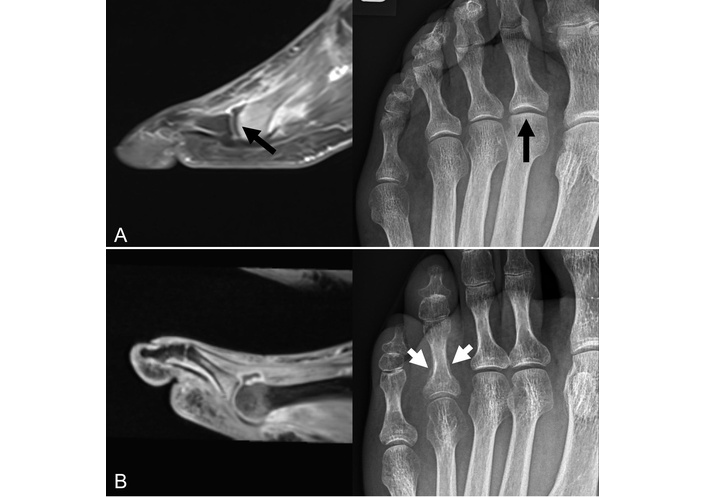
Sagittal contrast-enhanced T1w fat-saturated MRI (left figures) of the forefoot and corresponding X-rays (right figures) of two patients. (A) Images of a 66-year-old female patient presenting with foot pain under suspicion of arthritis. The MRI shows a subchondral fracture of the 2nd metatarsal head. Both radiograph and MRI demonstrate some linear subchondral sclerosis and loss of sphericity of the articular surface due to an infraction (black arrows). (B) Images of a 51-year-old male patient with long-standing axSpA with peripheral involvement, no fracture. The MRI demonstrates dactylitis of the 4th toe with dominant arthritis of the metatarsophalangeal joint, while the X-ray shows a mineralized periostal reaction of the proximal basophalangeal metadiaphysis (white arrows). axSpA: axial spondyloarthritis; MRI: magnetic resonance imaging; T1w: T1-weighted. Reprinted from [31]. © 2023 by the Journal of Rheumatology.
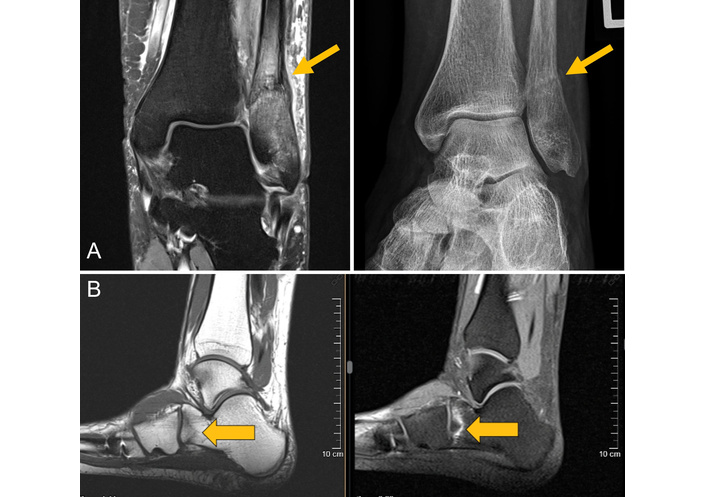
MRI of two and the corresponding radiograph of one patient. (A) Images of a 57-year-old female patient with an osteodestructive course of RA presenting with lateral right ankle pain for about 6 weeks. Both the radiograph and the coronal proton density–weighted turbo spin-echo fat-saturated MRI show a juxtasyndesmal IF of the fibula with reactive thickening of the periosteum and mineralization because of callus formation (gray arrows). (B) MRI of a 42-year-old female PsA patient treated with glucocorticoids and multiple csDMARDs, bDMARDs, and tsDMARDs for approximately 10 years. The T1w-MRI shows a blurred zigzag-shaped hypointense fracture line at the anterior calcaneus with surrounding BME depicted by the STIR MRI sequence. IF: insufficiency fracture; MRI: magnetic resonance imaging; BME: bone marrow edema; T1w: T1-weighted; STIR: short tau inversion recovery; csDMARD: conventional synthetic disease-modifying anti-rheumatic drugs; bDMARD: biologic disease-modifying anti-rheumatic drugs; tsDMARD: targeted synthetic disease-modifying anti-rheumatic drugs. Reprinted from [31]. © 2023 by the Journal of Rheumatology.
In an associated editorial, this paper has received positive comments and was much appreciated by Lems and colleagues [32]. Among the reasons listed, it was first mentioned that IF were frequently diagnosed in referred patients with RMD and foot pain, and especially in those with inflammatory RMD. Second, MRI was found to be superior to conventional radiography in diagnosing IF. The authors further discuss the key issues arising from these data, including the clinical relevance for daily practice, and the research agenda [32].
As pointed out by Lems et al. [32], one major strength of the study by Buehring et al. [31] is the large number of MRIs performed with the clear demonstration of IF in 7.5% of the RMD patients with foot pain. Importantly, risk factors for developing a foot IF were identified, and it was shown that their frequency was double for patients with inflammatory vs. noninflammatory RMD, confirming the elevated fracture risk in patients with inflammatory RMD [3, 20]. The relatively high fracture rate is clinically relevant, given that foot problems occur in up to 90% of RA patients [22, 23]. In contrast, the commonly used disease activity score DAS28 does not include the feet. In addition, when considering the main differential diagnoses in RMD patients with foot pain (arthritis, osteoarthritis, and fracture), it has to be admitted that physical examination is mostly not very helpful, and, thus, sensitive imaging is needed. There is increasing evidence that MRI is superior to conventional radiography, since it better discriminates between RA-related characteristics and other diagnoses, including fractures. This is important because treatment options differ substantially in this regard [31, 32].
The main weakness of the study is the retrospective design with incomplete clinical data. Thus, it remains unclear whether the patients included in the study had any acute pain episode suggestive of fracture [32]. In addition, it would be important to know whether IF occurred after an adequate trauma or even repeated strain or not. Data on disease activity (only yes/no responses for high disease activity) were only available from the records. Whether this was critical in the multivariate analysis, suggesting that high disease activity was associated with a low risk of IF, is unclear [32]. A possible explanation could be that rheumatologists will rather consider high disease activity in patients with polyarticular RA and start with a new DMARD and not perform an MRI, whereas in patients with more or less isolated foot pain, an MRI is performed. That the study was performed in a large, tertiary center is not an argument in this regard because the Rheumazentrum Ruhrgebiet has already been academic for several years, since the head is a full professor at the Ruhr Universität Bochum.
The clinical relevance of the described findings is first, that the reported prevalence in patients with inflammatory and noninflammatory RMDs is substantial and really remarkable. However, many questions remain [31, 32]: were all fractures just IF, related to inappropriate loads, or were they stress fractures, more related to repetitive strain? We actually think that this can also be a mixture. What is the background prevalence of IF in healthy individuals of the same age and gender with complaints of foot pain? Do we need to perform an MRI always in both feet to find out about the percentage of IF in the other and/or the nonpainful foot [32]? Should a prospective MRI study in patients with RMD and painful feet vs. non-painful feet be performed? The higher sensitivity for detecting fractures by MRI than by conventional radiography seems proven, but questions remain about the specificity of imaging abnormalities detected by MRI [32]. In any case, the data presented [31] do not support a 2-step strategy with an initial radiograph. This was similar to an earlier but smaller study from Denmark [21]. We think that MRIs have to be performed in all cases where the rheumatologist is in doubt about the cause of foot pain. This is especially the case when no other joints show signs of synovitis and when anti-inflammatory therapy obviously doesn’t work, and pain persists or worsens. A careful patient history to assess osteoporotic risk factors for IF is mandatory and will help to identify patients with the highest need for MRI [31, 32].
The therapeutic consequences of having diagnosed an IF in an RMD patient, apart from symptomatic treatment for pain relief, are challenging. IF of the feet are usually treated conservatively with analgesics, surgery is usually not indicated. The aims are to relieve pressure on the affected area and at least partly immobilise the foot if possible, with the goal to stabilise the bone fracture and promote the healing process, which tends to take several weeks. Patients with a recent clinical fracture present with heterogeneous patterns of bone-, fall-, and comorbidity-related risks [33]. There are several guidelines out that exclude forefoot fractures from secondary fracture prevention programs [34, 35], and no treatment studies on patients with IF detected by MRI are available. Thus, there is no proof for the treatment effects of anti-osteoporotic drugs to date [36, 37]. Nevertheless, published data [31] have revealed that patients with IF obviously had common risk factors for fractures, such as smoking, low BMD, a history of fractures, drug treatment for osteoporosis and fracture prevention, and use of methotrexate (MTX), which seems to be associated with IF [38].
Initially reported in children treated with high doses for cancer [39], a case of possible ‘MTX osteopathy’ was first reported in a multimorbid patient with psoriasis in 1983 [40]. The potential pathophysiologic mechanisms of MTX-induced bone fragility are multifactorial [41]. As studied in bone biopsies of two patients, MTX may inhibit osteoblast function and promote osteoclastic activity, leading to reduced bone formation and microarchitectural deterioration [42]. In another study on a lupus patient treated with MTX, the bone biopsy revealed osteoporomalacia with pronounced trabecular thinning and increased bone resorption [43]. In a Japanese experimental study, osteoclast formation and expression of osteoclastic genes induced by RANKL were inhibited by MTX. This was based on the inhibition of RANKL-dependent calcium influx into osteoclast progenitors [44].
However, several studies later on didn’t find decreased BMD in patients treated with MTX [45–48], and in a large Danish case-control study [49], the fracture risk associated with use of MTX, azathioprine, and cyclosporine was analysed. Data from several national registers were available to identify all patients with a fracture (n = 124,655) in 2000 to be compared with 3 age- and gender-matched controls randomly drawn from the general population (n = 373,962). The defined exposure was the use of drugs and several covariates. In that study, only the use of azathioprine was associated with an increase in the overall fracture risk, but none of the drugs was significantly associated with an overall fracture risk or the risk of hip, spine, or forearm fracture. Nevertheless, liver and kidney diseases were significantly associated with fracture risk. In contrast, MTX and ciclosporine were not associated with fracture risk [49]. Taken together, IF are increasingly recognized as a clinically relevant phenomenon, particularly in patients with inflammatory RMD such as RA, PsA, and connective tissue diseases [38, 50–52].
A recent systematic review identified nearly 80 published case reports or case series describing MTX-related insufficiency or stress fractures in patients with RMD [38]. These authors have defined ‘MTX osteopathy’ as commonly presenting as a stress or IF of the distal tibia (51.3%), the calcaneus (35.0%), and the proximal tibia (27.5%). Importantly, such IF may well mimick arthritis. Although most patients met the densitometric criteria for osteoporosis (58.1%), typical osteoporotic fractures, such as vertebral fractures, were rarely seen. Importantly, patients frequently suffered from bilateral (55.0%), multiple (71.3%), and recurrent fractures (25.0%) [38].
In the largest case series (excluding oncology cases) published between 2011 and 2019 [50] on 5 patients with IF, the fracture locations were atypical for osteoporotic fractures. All patients improved in the following months with MTX withdrawal. The study included a literature search [50].
A retrospective German study included 34 patients treated with MTX, who reported severe lower extremity pain and immobilization; fractures were detected by MRI and CT [51]. The time between clinical onset and diagnosis was relatively long (17.4 ± 8.6 months). The fractures with a pathognomonic appearance (band-/meander-shaped, along the growth plate) were often found in the distal tibia (53%), the calcaneus (53%), around the knee (62%), and often at multiple sites (68%). Most patients had a low BMD indicative of osteoporosis (62%).
Another German clinical study compared 83 patients diagnosed with ‘MTX osteopathy’ with 89 patients diagnosed with osteoporosis [52]. All patients (mean age about 68 years) underwent an extensive clinical workup, including BMD and high-resolution peripheral quantitative CT and muscular performance tests. Patients with ‘MTX osteopathy’ predominantly had lower extremity fractures and higher pain scores. The mobility restriction potentially caused by MTX was only reduced if the drug was discontinued. This gain in mobility was better achieved by anabolic vs. antiresorptive treatment. The authors concluded that ‘MTX osteopathy’ is characterized by distinct lower extremity stress fractures leading to severe pain and immobility. Discontinuation of MTX and anabolic therapy was critical for treatment success [52].
In a retrospective single-centre case note review of 33 patients with characteristic lower limb IF who had taken a mean MTX dose of 20 mg weekly with an average treatment duration of about 10 years, 21 patients continued MTX after the initial IF. Almost all patients (95.2%) who continued MTX sustained either further IF (67%) or major osteoporotic (33%) fractures. There were significantly fewer fractures (3 out of 11, 27.3%) in the group that stopped MTX after the initial IF [53]. In addition, data from a large longitudinal observational registry revealed that TNF inhibitors had a lower risk of fracture compared to MTX in RA patients [54].
In a very recent French study recruiting between 2012 and 2024, 92 predominantly postmenopausal women with seropositive RA patients treated with MTX were included [55]. While 22% of the patients had a history of major fractures, 56% presented with diagnosed osteoporosis. The localization of fractures was most common in the distal and proximal tibial metaphysis (88%) and the foot bones (49%). In addition, multiple (76%) and frequently repeated (63%) fractures were often reported. Fractures were detected by MRI of the painful sites (84%). Bone scintigraphy was also used (45%). In 79% of the cases, MTX was discontinued [55]. Fracture healing and pain relief were achieved in 77% of cases, with significantly better outcomes for those who discontinued MTX (91%) versus those who continued (29%).
In the study under discussion [31], the multivariable analysis showed an odds ratio of 2.36 (95% CI 1.2–4.66) for MTX use in patients with IF. However, there is a potential for bias for this finding because uncertainty remains whether this association is due to selection bias of patients who receive MTX, as they usually have risk factors for fractures, have active inflammation, are often immobile, and receive glucocorticoids (see below), or a causal relationship of MTX and risk for IF. Although cumulative dose effects are suspected, retrospective analysis limits definitive conclusions regarding dose–response relationships. As mentioned above, coexisting factors such as long-standing inflammation, immobility, or corticosteroid use likely compound the skeletal risk. It is also important to consider that long-term MTX therapy may reflect more severe or difficult-to-treat disease, which itself is linked to osteopenia and fracture risk, and that most patients receiving MTX do not suffer from IF. As such, similar to the observations of bisphosphonate-associated osteonecrosis of the jaw, there remain many open questions on the causal relationship between MTX and bone health. In any case, ‘MTX osteopathy’ seems to be a rather rare disease.
The diagnosis of IF and ‘MTX osteopathy’ can be challenging due to overlapping symptoms with synovitis. Since MRI is clearly superior to conventional radiography [31], it should therefore be strongly considered in RMD patients with unexplained lower extremity pain, including those receiving MTX. Accordingly, laboratory evaluation should include serum vitamin D, calcium, and parathyroid hormone levels, along with exclusion of metabolic bone diseases.
Management strategies need to be individualized. In patients with evidence of MTX-related bone injury, discontinuation seems warranted, particularly if disease control can be sustained using alternative DMARDs. Pharmaceutical osteoporotic therapy is indicated, particularly in patients with a history of multiple fractures. Optimizing vitamin D status, ensuring calcium intake, minimizing glucocorticoid exposure, and promoting weight-bearing activity where feasible are essential components of care.
In addition, an influence of concomitant glucocorticoids on the occurrence of IF seems likely, although we agree that there are many more reasons for the development of osteoporosis than these effective anti-inflammatory drugs with a proven negative effect on bone [56]. Most management recommendations for osteoporosis in inflammatory RMD have focussed on glucocorticoids, considered to be the most important risk factor, but other risk factors have been rather ignored. At this point, it needs to be stressed that several different pathogenetic mechanisms are involved in the development of osteoporosis in inflammatory RMD. This does mainly include the negative effects of local and systemic inflammation on bone due to high disease activity, while general risk factors such as older age and hormonal loss resulting from menopause and other risk factors such as disability, malnutrition, or malabsorption are well known to also play a role [56]. All these established risk factors support the development and presence of osteoporosis and increase the fracture risk. However, osteoporosis, according to the World Health Organisation (WHO) definition, defined as t-score < –2.5, was only found in 43% of patients with an IF, while osteoporosis and/or osteopenia was seen in 67% with an IF vs. 35% without it [31]. This shows that evaluation of an underlying osteoporosis and estimation of the fracture risk is indicated in patients suffering from an IF. The assessment should include a detailed medical history, examination of the BMD by DXA [57] or even the trabecular bone score [58], and vertebral fracture assessment (VFA) [59]. There are well-known therapeutic options not only for patients with RMD after an IF, since effective, relatively safe, and cost-effective anti-osteoporotic drugs, such as bisphosphonates, are available [32, 36, 37]. Furthermore, anabolic drugs such as teriparatide and romosozumab are approved and should be evaluated for the treatment of IF [60].
In summary, in patients with RMD and foot pain, rheumatologists should be aware of identifying IF as an important and not very rare differential diagnosis by using MRI and not conventional radiography in cases under suspicion of IF. The differential diagnosis of foot pain in RMD patients includes inflammatory arthritis, degenerative changes in osteoarthritis, and IF. The treating physician needs to be well aware of the different therapeutic consequences. The diagnosis of an IF should also stimulate secondary fracture prevention in patients with RMD and foot pain, since risk factors for osteoporosis and fractures can be identified [31–33].
MTX seems to be rarely associated with a special form of osteopathy that shows some special clinical features. There is some evidence that discontinuation of MTX is useful to treat this condition.
The study discussed here in detail [31] is hopefully a starting point for a research agenda for patients with and without inflammatory RMD. This agenda should include the prevalence of IF in patients with and without a preceding trauma, and the prevalence of IF in nonpainful feet [31].
axSpA: axial spondyloarthritis
BMD: bone mineral density
DAS: disease activity score
DMARDs: disease-modifying anti-rheumatic drugs
DXA: Dual Energy X-ray Absorptiometry
FRAX: Fracture Risk Assessment Tool
IF: insufficiency fractures
MRI: magnetic resonance imaging
MTX: methotrexate
PsA: psoriatic arthritis
RA: rheumatoid arthritis
RMD: rheumatic and musculoskeletal diseases
SpA: spondyloarthritides
WHO: World Health Organisation
JB: Conceptualization, Writing—original draft, Writing—review & editing. BB: Writing—original draft, Writing—review & editing. Both authors read and approved the submitted version.
Jürgen Braun, who is the Associate Editor of Exploration of Musculoskeletal Diseases, had no involvement in the decision-making or the review process of this manuscript. The other author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 958
Download: 15
Times Cited: 0
Antje L Greenfield, Riti Kanesa-Thasan
Silvia Magni-Manzoni
Janeth Yinh ... Ali Guermazi
Gregory J. Challener ... Minna J. Kohler
