Affiliation:
1International Sonoguide Pain School, Tehran 1599813113, Iran
Email: helengharaei@gmail.com
ORCID: https://orcid.org/0000-0001-5379-8667
Affiliation:
2International Sonoguide Pain School, 06250 Alpes Côte d’Azur, France
ORCID: https://orcid.org/0009-0008-2667-048X
Affiliation:
3Emergency Department, King’s College Hospital, SE5 9RS London, UK
ORCID: https://orcid.org/0009-0003-6114-9677
Affiliation:
1International Sonoguide Pain School, Tehran 1599813113, Iran
ORCID: https://orcid.org/0009-0004-7822-5928
Explor Musculoskeletal Dis. 2025;3:1007111 DOI: https://doi.org/10.37349/emd.2025.1007111
Received: August 22, 2025 Accepted: December 01, 2025 Published: December 08, 2025
Academic Editor: Peter Mandl, Medical University of Vienna (MUW), Austria
Scleroderma, also known as systemic sclerosis, is a rare connective tissue disorder with an unclear and poorly understood pathogenesis. While it primarily affects the skin and internal organs through mechanisms involving vascular dysfunction, immune dysregulation, and fibrosis, its effects on the peripheral nervous system may also be substantial. We report the case of a 36-year-old male with a known history of scleroderma who presented with chronic, diffuse burning pain throughout the body. His symptoms included daily asthenia, dizziness, nausea, headaches, and limb pain exacerbated by cold, compression, or stretching. Diagnostic ultrasound confirmed multiple peripheral nerve entrapments, which were treated with ultrasound-guided 5% dextrose hydrodissection. This intervention provided significant relief of pain, paresthesia, and motor symptoms, which improved his quality of life. This case highlights the potential of dextrose hydrodissection as a safe, minimally invasive, and cost-effective symptomatic treatment for peripheral neuropathies in patients with scleroderma. Further studies are warranted to establish its broader therapeutic role in treating scleroderma-related neuropathies.
Scleroderma, or systemic sclerosis (SSc), is a rare connective tissue disorder with an unclear pathogenesis. Its main mechanisms, vascular dysfunction, immune dysregulation, and fibrosis, lead to significant morbidity and mortality. While skin thickening and organ fibrosis are hallmark features, blood vessels and the peripheral nervous system can also be affected. Peripheral nerve involvement in SSc is often underrecognized but clinically important. Neurological complications include polyneuropathy, mononeuropathy, and entrapment syndromes, with recent evidence suggesting a higher prevalence than previously reported. Approximately 16% of patients experience peripheral neuropathy, while trigeminal neuropathy is less common [1–4]. A systematic review reported that peripheral sensorimotor polyneuropathy occurs in approximately 14.25% of scleroderma patients, while carpal tunnel syndrome (CTS) is observed in about 6.56% of cases [1].
We report a case of scleroderma with multiple peripheral nerve entrapments treated with dextrose hydrodissection. Hydrodissection and perineural injection have been discussed as treatments for entrapment neuropathy in various conditions, including rheumatic diseases. To date, only one study has evaluated a combined approach of hydrodissection and corticosteroid injection for managing scleroderma hand dysfunction [5]. However, there is a significant lack of direct evidence regarding hydrodissection for other peripheral nerves in SSc patients, and no studies have explored dextrose hydrodissection in this population. This case report aims to introduce and explore this approach, stimulating further discussion and research in pain management.
A chronological summary of the case is presented to outline the progression from the initial appearance of localized induration in 2015 to the widespread symptoms that developed over the following years. By early 2024, the patient experienced severe paresthesia, mechanical sensitivity, and significant functional decline. A comprehensive consultation in 2024 identified extensive nerve-related findings on examination and ultrasound, leading to the initiation of ultrasound-guided dextrose hydrodissection. Weekly sessions over six weeks resulted in marked improvement, with near-complete symptom resolution by the end of treatment and sustained full recovery at one-year follow-up (Table 1).
Timeline of clinical events and management.
| Date/Period | Visit summary |
|---|---|
| 2015 | First appearance of localized induration over the left arm, described as rough, leathery tissue overlying peripheral nerves. Symptoms are limited at this time. |
| 2015–2024 | Progressive spread of induration to multiple limbs with increasing diffuse burning pain, paresthesia, asthenia, dizziness, and cold-induced exacerbation. Functional decline develops, including impaired hand use and limping from common fibular nerve involvement. |
| Early 2024 | Worsening paresthesia in all extremities, marked mechanical sensitivity along peripheral nerves, and increasing difficulty performing daily tasks such as grasping, walking, and maintaining static positions. Patient reports severe symptoms with nerve compression and stretching. |
| Initial consultation (2024) | Comprehensive evaluation performed. Physical exam identifies widespread nerve-related pain, palpable indurations encasing nerves, trigger points, and mechanical allodynia. Ultrasound confirms nerve thickening, altered echotexture, fascial stiffness, and identifiable trigger points. Diagnosis: diffuse peripheral nerve entrapment due to sclerotic tissue in scleroderma. Patient consents to diagnostic hydrodissection. |
| Week 0 (first procedure) | Ultrasound-guided dextrose hydrodissection was performed on the arms, legs, and right foot nerves. Trigger points treated with 15% dextrose. Immediate mechanical decompression achieved (nerve “floating”). |
| Weeks 1–6 | Weekly ultrasound-guided hydrodissection sessions continued. Progressive reduction in pain, decreased induration, improved limb mobility, and resolution of trigger-point responses were noted. |
| 6 weeks post-treatment | Patient reports near-complete symptom resolution. Palpation no longer elicits pain or paresthesia. Functional abilities in the hands and legs significantly improved. |
| 1-year follow-up | Patient remains asymptomatic. Sustained reduction in induration, normalized nerve glide, and resolution of neuropathic symptoms confirmed clinically and by ultrasound. |
A 36-year-old white male with a known history of scleroderma and a complex medical history—including Raynaud’s syndrome, vitiligo, cardiovascular collapse, hypogammaglobulinemia, anti-Scl-70 antibodies, and micro-angiopathy, presented with chronic, diffuse burning pain throughout the body. Symptoms included daily asthenia, dizziness, nausea, headaches, and limb pain exacerbated by cold, compression, or stretching. He reported frequent paresthesia in all extremities, mild at rest, moderate in static positions, and severe with compression or stretching of peripheral nerves, along with functional deficits in the forearms and hands.
Physical examination revealed pain along peripheral nerve pathways and significant mechanical discomfort with limb use (e.g., flexion/extension during exercise or holding a steering wheel) or compression (e.g., sitting or lying down). Indurations corresponded to peripheral nerves encased in sclerotic tissue, visible under the skin, rough and leathery (initially noted in the left arm in 2015 and now widespread), causing tissue hardness and severe pain on palpation. Paresthesia was elicited in the extremities of the arms and legs, particularly in the little and ring fingers.
The nerves involved in this case included multiple peripheral nerves across the upper and lower limbs. In the arms, the median, ulnar, and medial cutaneous nerves of the forearm were affected. In the legs, the sciatic, common fibular, and superficial fibular nerves demonstrated involvement. Additionally, the dorsal cutaneous nerve of the right foot was also affected, corresponding to the distribution of pain, paresthesia, and functional deficits observed clinically. Burning pain occurred when the affected nerves were palpated along the indurated areas. Trigger points, especially in the mid-left arm, caused sudden involuntary finger retraction during transverse mechanical stimulation of the median, ulnar, and medial cutaneous nerves. The patient experienced significant discomfort and motor limitations, including difficulty with precision tasks, grasping objects, walking, and lifting weights. Major deficits included difficulty moving due to induration of the common fibular nerve and its superficial branch in the left leg (causing limping and abandoning running) and impaired left-arm extension (resulting in cessation of weight-lifting). Pain and paresthesia caused sensory discomfort in all four limbs.
High-frequency ultrasound revealed enlargement and thickening of the affected nerves, along with altered fascicular echotexture. The surrounding fascia appeared thickened with reduced elasticity during probe compression, and nerve glide was notably limited. Several oval or fusiform hypoechoic structures were also observed, consistent with trigger-point-like areas within taut bands. These sonographic findings closely matched the palpable indurations and the patient’s mechanically induced symptoms (Figure 1).
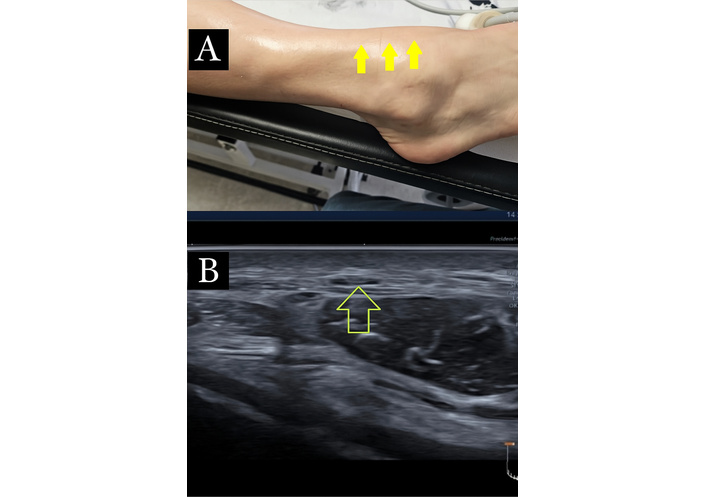
Ultrasound imaging of the medial branch of the superficial peroneal nerve: sclerotic fibrosis, nerve thickening, and fascial changes. (A): Medial branch of the superficial peroneal nerve, with sclerotic fibrosis highlighting a clear passage of the nerve (arrow). (B): Transverse ultrasound at the site of pain, as indicated by the patient, showing the medial branch of the superficial peroneal nerve (arrow). Imaging demonstrates increased cross-sectional area, nerve thickening, altered echotexture, thickening of the overlying fascia, and the presence of fibrosis.
Based on these findings, he consented to diagnostic ultrasound-guided dextrose hydrodissection at sites of sclerotic tissue and trigger points. The patient was positioned supine, with adjustments for nerve location. A high-frequency linear probe was placed transversely and longitudinally over indurated areas to localize entrapped nerves. Diagnostic criteria included increased cross-sectional area, nerve thickening, echotexture changes, and fascial thickening. Trigger points appeared as oval or fusiform hypoechoic regions within muscle, often surrounded by a linear hyperechoic band corresponding to a taut band. Stiffness or restricted elasticity with probe compression or movement supported the diagnosis. For hydrodissection, the needle was introduced in-plane at a high angle under real-time ultrasound guidance. A volume of 3–5 mL of 5% dextrose was injected along the entrapment site, proximal and distal to the nerve, until nerve “floating” was observed (Figure 2). For trigger points, 1–3 mL of 15% dextrose was injected until full release of pressure was achieved.
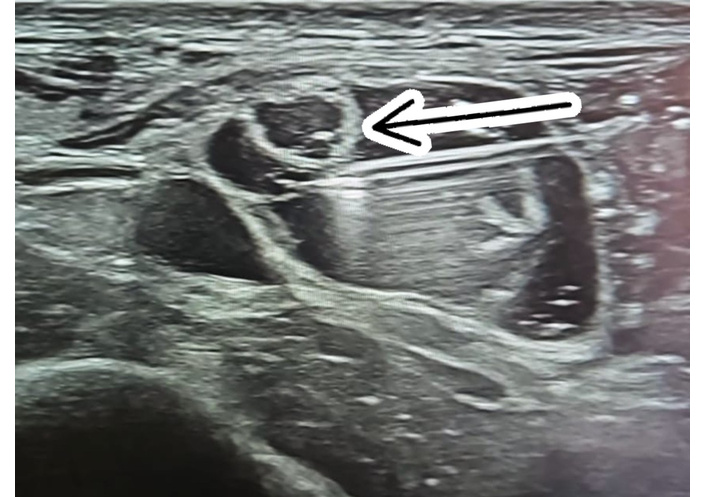
Ultrasound-guided dextrose hydrodissection of the medial branch of the superficial peroneal nerve: in-plane needle approach and complete nerve mobilization. Ultrasound view of dextrose hydrodissection of the medial branch of the superficial peroneal nerve. The needle (arrow) is inserted in-plane, nearly parallel to the skin, with a clear view of its passage. Hydrodissection is performed in an up-and-down motion along the nerve until it is completely separated and floats freely.
The procedure was repeated weekly. After several weeks, the patient reported being asymptomatic. Reduction in induration and muscle spasm was observed, palpation was no longer painful, and significant pain relief persisted over a one-year follow-up (Figure 3).
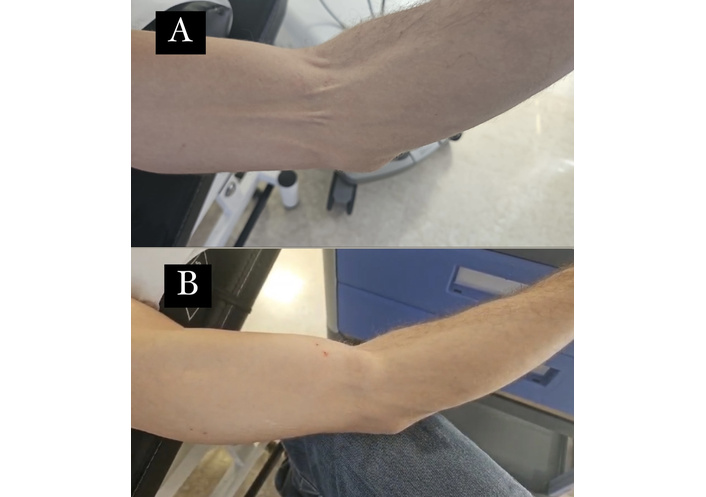
Cutaneous passage of the medial cutaneous nerve of the arm: reduction of sclerotic induration and muscle spasm following weekly treatment sessions. This figure illustrates the cutaneous passage of the medial cutaneous nerve of the arm before (A) and after (B) weekly treatment sessions. It demonstrates a reduction in sclerotic induration and muscle spasm, highlighting the therapeutic effect of the treatment over time.
The patient declined further paraclinical investigations, including electromyography (EMG)/nerve conduction velocity (NCV), magnetic resonance imaging (MRI), and computed tomography (CT), limiting diagnostic evaluation to ultrasound only. Ultrasound performed at symptomatic sites demonstrated findings consistent with entrapment neuropathy, including increased nerve cross-sectional area, nerve thickening, altered echotexture, and surrounding fascial thickening (Table 2).
Diagnostic ultrasound finding.
| Test name | Result | Normal range | Interpretation |
|---|---|---|---|
| Nerve cross-sectional area (CSA) | Increased | Within normal limits for each nerve | Suggestive of entrapment neuropathy |
| Nerve echotexture | Altered; hypoechoic with loss of normal fascicular pattern | Normal fascicular echotexture | Indicates chronic nerve irritation/compression |
| Nerve thickness | Increased | Normal nerve contour and thickness | Consistent with entrapment/structural change |
| Surrounding fascia | Thickened | < 1 mm | Fascial thickening contributing to mechanical compression |
| Dynamic evaluation | Positive for mechanical irritation during movement/compression | Negative | Supports symptomatic entrapment |
| Doppler signal (if assessed) | May show increased perineural vascularity | Minimal or none | Possible inflammation or irritation |
The patient expressed great satisfaction with the outcome of the treatment. He reported being completely pain-free and emphasized a significant improvement in his quality of life, including restored ability to perform daily activities without limitation. He described feeling more energetic, emotionally relieved, and confident in resuming both work and social engagements.
The patient was also very eager to share his experience and to talk about his condition, noting that before treatment, he felt uncertain about the prognosis and frustrated by persistent symptoms. He expressed gratitude that this technique not only relieved his pain but also provided a lasting solution. By sharing his story, he hoped to encourage other patients with similar conditions to seek appropriate evaluation and management, highlighting that timely intervention can make a meaningful difference.
Peripheral neuropathy in SSc may present with numbness, tingling, burning pain, weakness, or autonomic symptoms such as orthostatic hypotension or gastrointestinal dysmotility. It can manifest as symmetric polyneuropathy, mononeuropathy, or plexopathy. Its development is driven by vasculopathy, fibrosis, and immune-mediated nerve injury.
Peripheral neuropathy is more common in SSc than in the general population (2–8%), often appearing within the first decade of disease (average 8.85 years post-diagnosis). Causes include nerve compression from soft tissue swelling, fibrosis, or calcinosis cutis, as well as traumatic injury, medications, metabolic issues, and ischemia. Calcinosis cutis is a key risk factor for compression neuropathy, while non-compressive neuropathies are linked to advanced disease, anticentromere antibodies, vasa nervorum ischemia, iron deficiency, reduced nerve density, medications, and systemic/environmental conditions such as silicosis or uremia.
Electrophysiological studies [nerve conduction study (NCS), EMG] remain the gold standard for assessing nerve function, but cannot visualize nerve morphology or surrounding tissue. Magnetic resonance neurography provides high-resolution imaging of nerve inflammation, fibrosis, and soft tissue changes. Ultrasound and MRI are complementary, with ultrasound demonstrating comparable accuracy for structural changes and early morphological alterations independent of disease duration, autoantibodies, or immunosuppressive therapy [2, 6–9].
Scleroderma hand pain arises from vasospasm, ischemia, tenosynovitis, and nerve entrapment. To date, only one study has evaluated the combined approach of hydrodissection and corticosteroid injection for managing scleroderma hand dysfunction. Ultrasound-guided carpal tunnel hydrodissection followed by corticosteroid injection significantly reduced pain, improved Raynaud’s symptoms, and promoted ulcer healing, with a longer reinjection interval compared to rheumatoid arthritis (RA)/CTS. The effect likely reflects mechanical release of entrapped structures and corticosteroid-mediated reduction of inflammation and vasospasm. This approach appears safe and effective for managing painful scleroderma hand [5]. Surgical intervention can be valuable for improving extremity function, relieving pain, and addressing complications such as calcinosis, tendon adhesions, joint contractures, and severe ischemic changes. However, patients with SSc are high-risk surgical candidates due to vascular compromise, Raynaud phenomenon, tissue fibrosis, impaired wound healing, and potential multiorgan involvement [10].
Perineural injection therapy (PIT) using buffered isotonic dextrose (5% D5W) provides immediate pain relief and, with repeated treatments, sustained improvement. The proposed mechanism includes restoring metabolic homeostasis, reducing compression-induced ischemia, relative hypoglycemia, and local acidity that increase peptidergic C-fiber firing. Hydrodissection mechanically separates nerves from surrounding tissue, alleviating entrapment. Studies indicate efficacy for peripheral nerve entrapments, sometimes reducing the need for surgery [11, 12]. Dextrose may also promote tissue healing and nerve repair, akin to platelet releasate therapy [13].
Ultrasound-guided dextrose hydrodissection provided significant relief of pain, paresthesia, and motor symptoms, enhancing quality of life. This case highlights its potential as a cost-effective, minimally invasive, and safe symptomatic treatment for peripheral neuropathies in scleroderma. Further studies are warranted to establish its broader therapeutic role.
CTS: carpal tunnel syndrome
EMG: electromyography
MRI: magnetic resonance imaging
RA: rheumatoid arthritis
SSc: systemic sclerosis
HG: Conceptualization, Resources, Software, Formal analysis, Supervision, Investigation, Methodology, Writing—original draft, Project administration, Writing—review & editing. PM: Conceptualization, Data curation, Formal analysis, Validation, Methodology, Writing—original draft. NG: Resources, Software, Investigation, Visualization, Writing—original draft. ZB: Writing—original draft, Project administration. All authors read and approved the submitted version.
The authors declare that there are no conflicts of interest.
Ethical approval was not required, as this study describes a single patient case report and does not involve experimental intervention. This observational study was exempted by the International Sonoguide Pain School. All procedures were conducted in accordance with the principles of the Declaration of Helsinki (2013 revision).
Informed consent for participation was obtained from the patient.
Written informed consent was obtained from the patient for publication of anonymized clinical data and accompanying images.
The data supporting the findings of this case report are not publicly available to protect patient confidentiality, but can be obtained from the corresponding author upon reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2068
Download: 106
Times Cited: 0
